Abstract
Vasculogenesis, the in-situ assembly of angioblast or endothelial progenitor cells (EPCs), may persist into adult life, contributing to new blood vessel formation. However, EPCs are scattered throughout newly developed blood vessels and cannot be solely responsible for vascularization. Here, we identify an endothelial progenitor/stem-like population located at the inner surface of preexisting blood vessels using the Hoechst method in which stem cell populations are identified as side populations. This population is dormant in the steady state but possesses colony-forming ability, produces large numbers of endothelial cells (ECs) and when transplanted into ischaemic lesions, restores blood flow completely and reconstitutes de-novo long-term surviving blood vessels. Moreover, although surface markers of this population are very similar to conventional ECs, and they reside in the capillary endothelium sub-population, the gene expression profile is completely different. Our results suggest that this heterogeneity of stem-like ECs will lead to the identification of new targets for vascular regeneration therapy.
Keywords: angiogenesis, endothelium, side population, somatic stem cell
Introduction
Regeneration of the vasculature in ischaemic-injured organs is essential to ensure their integrity. Postnatal neovascular formation was originally thought to be mediated by angiogenesis, that is, the generation of new endothelial cells (ECs) from preexisting vessels, not by vasculogenesis, a process of blood vessel formation whereby the early vascular plexus forms from mesoderm by differentiation of angioblasts (Risau, 1997). However, accumulating evidence suggests that vasculogenesis persists into adult life, and contributes to the formation of new blood vessels (Asahara et al, 1997). It has been proposed that bone marrow (BM)-derived circulating endothelial progenitor cells (EPCs) are important for promoting vascularization in many pathophysiological situations; several clinical trials are already ongoing based on this concept (Shantsila et al, 2007). However, some reports suggest that the contribution of EPCs to the neovascular ECs itself is not sufficient (Gothert et al, 2004; Peters et al, 2005).
In the peripheral vasculature, there is considerable evidence that although preexisting ECs display many common features, they also represent a heterogeneous population. Transcriptional and antigenic differences in ECs from arteries and veins, and the morphological and functional characteristics referred to as continuous, fenestrated, and discontinuous are widely accepted (Risau, 1995). Recently, it has been shown that in response to angiogenic stimuli, a discrete population of cells, the so-called ‘tip and stalk cells’, lead and guide new sprouts and form additional ECs, respectively (Gerhardt et al, 2003). Furthermore, populations of ECs of another different phenotype, the so-called phalanx cells that generate stable blood vessels, have been reported (Mazzone et al, 2009).
Additionally, the presence of stem/progenitor cells in the vessel wall has been proposed. Investigating adult vessels in mice revealed Sca1+ progenitor cells in the adventitia of large and medium-sized arteries and veins (Hu et al, 2004; Sainz et al, 2006; Passman et al, 2008). Similarly, CD34+ CD31− progenitor cells in the distinct zone between smooth muscle and the adventitial layer of the human adult vascular wall were identified (Zengin et al, 2006). These stem/progenitor cells were reported to have the ability to differentiate into ECs in culture and form capillary-like microvessels in ex-vivo assays. However, during angiogenic growth, microvascular ECs, rather than the ECs of the artery or vein which are completely covered by the vascular wall, are selected for neovascularization (Risau, 1995). Therefore, it is suggested that stem/progenitor cells in the vascular wall of larger blood vessels are not the main source of neovascular ECs.
Haematopoietic cells (HCs) and ECs originate from common progenitors (Choi et al, 1998), with haemogenic ECs generating HCs during development (Nishikawa et al, 1998). Moreover, ECs support self-renewal of haematopoietic stem cells (HSCs; Hooper et al, 2009). We previously reported that HSCs also promote angiogenesis (Takakura et al, 2000), emphasizing the close developmental and functional relationships between HCs and ECs. Most BM HSCs appear dormant, and are characterized as side population (SP) cells effluxing Hoechst 33342 (Goodell et al, 1996). This staining method has been applied to explore stem cells of a wide range of tissues, including skin, lung, heart, mammary gland, muscle and testis (Challen and Little, 2006). It is possible that resident quiescent EC stem/progenitor cells in the preexisting blood vessels are also found within these SP cells. In this study, we examined the ECs residing in preexisting vessels precisely to identify the origin of neovascular ECs.
Results
Identification and characterization of endothelial SP cells
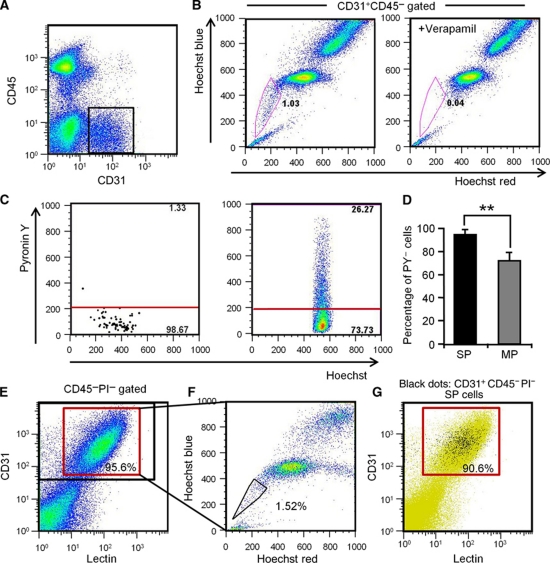
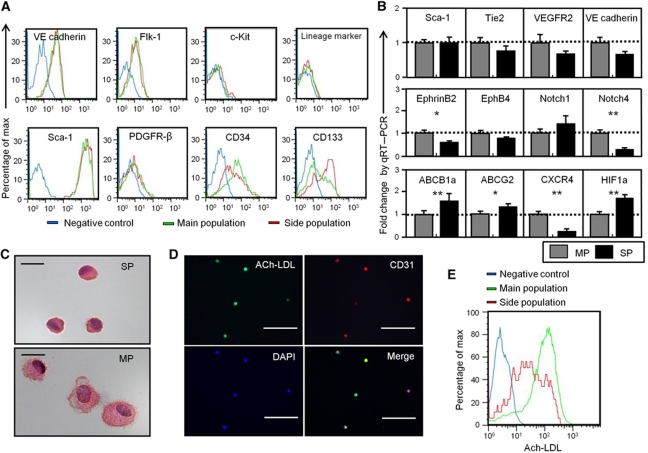
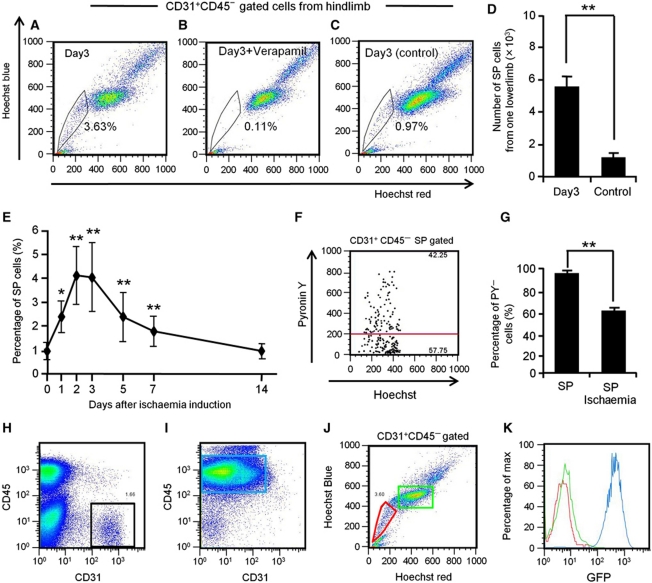
Here, we analysed cells from hind limb muscle to identify endothelial SP cells. Among cells stained by the EC marker CD31, but not the HC marker, CD45 (CD31+ CD45− ECs) (Figure 1A), 1.15±0.14% were in the SP gate, confirmed by their disappearance with the drug efflux pump inhibitor, verapamil. They were distinct from the main population (MP) of cells (Figure 1B). Because the SP phenotype is a marker for quiescence in HSCs (Arai et al, 2004), we applied a method which identifies cells in G0 plus G1 phase by Hoechst 33342 distribution and assigns them to G0 or G1 by Pyronin Y RNA staining (Gothot et al, 1997). As shown in Figure 1C and D, 94.8±2.2% of endothelial SP (EC-SP) cells were in the PY− G0 fraction, clearly different from CD31+CD45− endothelial MP (EC-MP) cells. To confirm that EC-SP cells do reside in the blood vessel, we performed lectin perfusion assays. As shown in Figure 1E, ∼96% of CD31+CD45− cells were lectin positive, indicating that most of them were true ECs residing at the inner surface of vessels. The percentage of SP cells within the lectin+ EC population was approximately the same as the percentage of EC-SP cells identified in Figure 1B (see Figure 1F). On the other hand, ∼91% of EC-SP cells were lectin+, indicating that most of these cells reside at the inner surface of vessels (Figure 1G). Next, we characterized the phenotype of EC-SP cells. These were found to express the EC markers VE-cadherin, Flk-1, and Sca-1, but no haematopoietic lineage markers or the pericyte marker PDGFR-β. This phenotype is identical to the EC-MP cells. However, as with CD34-negative long-term repopulating HSCs (Osawa et al, 1996), EC-SP cells expressed little CD34, but CD133, a stem/progenitor cell marker in several tissues (Mizrak et al, 2008), was strongly expressed (Figure 2A). We confirmed that the EC-SP cell fraction was not contaminated with HCs, pericytes, or fibroblasts, by analysing lineage markers for those cell types in cells from the digested muscle sample (Supplementary Figures S1 and S2). Moreover, Notch4 mRNA levels were significantly lower in EC-SP than in EC-MP cells. In contrast, mRNA expression for ABCB1a (Multiple drug resistance 1a (MDR1a)) and ABCG2, a member of the ABC transporter gene family correlating with SP phenotype (Bunting et al, 2000), was higher in the EC-SP cells (Figure 2B). Furthermore, the expression of several other ABC transporters that are reported to correlate with SP phenotype was higher in the EC-SP cells (Supplementary Figure S3). Morphologically, the nuclear-to-cytoplasm (N/C) ratio of the EC-SP cells was higher than the EC-MP cells (Figure 2C). In addition, acetylated low-density lipoprotein (Ac-LDL) uptake that is functional property of ECs was observed by EC-SP cells but less than by EC-MP cells (Figure 2D and E). Taken together, we conclude that EC-SP cells are not pericytes, fibroblasts, or HCs but are true ECs already committed to the EC lineage and are phenotypically and morphologically different from EC-MP cells.
Figure 1.
Identification of endothelial side population cells. (A) Flow cytometric analysis of hind limb ECs from wild-type mice. (B) Hoechst 33342 staining of CD31+CD45− ECs gated as shown in (A). Note that verapamil selectively prevents Hoechst exclusion from EC-SP cells. (C) Incorporation of Pyronin Y (PY) in EC-SP (left-hand side) and EC-MP (right-hand side) cells. (D) Quantitative evaluation of PY− cells among EC-SP (SP) and EC-MP (MP) cells. Error bars are ±s.e.m. **P<0.01 (n=7). (E) Flow cytometric analysis of mouse hind limb ECs after in-vivo infusion of lectin. Lectin-positive cells among the CD31+CD45− cells are shown in the red gate and total CD31+CD45− cells are shown in the black gate. 95.9±0.2% (n=6) of the CD31+CD45− ECs were lectin positive. (F) Hoechst staining of lectin+ CD31+CD45− cells. (G) Representative flow cytometric plots of EC-SP cells (black dots). The lectin-positive population is shown in the red gate. 90.6±1.4% (n=4) of the EC-SP cells were lectin positive.
Figure 2.
Characterization of endothelial side population cells. (A) Histogram showing expression levels of surface markers in EC-SP, EC-MP cells and the negative control. (B) Quantitative RT–PCR analysis of mRNA as indicated in EC-SP and EC-MP cells, corrected for expression of the control gene GAPDH. Of the endothelial genes, Notch4 was significantly lower in EC-SP cells. Expression levels of the ABC transporter ABCG2 and ABCB1a (MDR1a) were higher in EC-SP cells. Expression of chemokine receptor CXCR4 and hypoxia-inducible factor (HIF1a) was higher in EC-SP cells. Error bars are ±s.e.m. **P<0.01, *P<0.05 (n>6). (C) Haematoxylin and eosin staining of EC-SP and EC-MP cells isolated by FACS. (D) Freshly isolated cells from hind limb were stained with Ac-LDL and then Hoechst staining was performed to detect EC-SP cells. EC-SP cells were cytospun onto slides and Ac-LDL uptake was evaluated. Some of the EC-SP cells showed weak uptake of Ac-LDL, but all were positive. (E) Intensity of Ac-LDL uptake was evaluated by FACS analysis. As indicated, Ac-LDL uptake was observed in EC-SP cells but was lower than in EC-MP cells. Scale bars, 10 μm (C) and 100 μm (D).
EC-SP cells are not derived from BM, are distinct from EPCs, and are distributed in the peripheral vessels
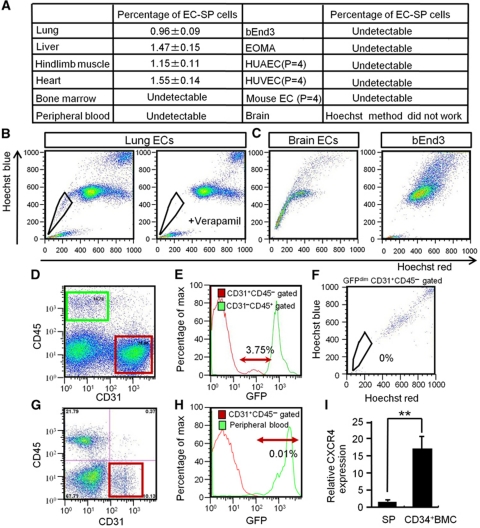
To exclude the possibility that EC-SP cells are only found in the lower limb, we analysed different organs and confirmed that these cells are distributed all over the body, but are not detectable in some organs (Figure 3A–C). For example, we could not identify the EC-SP pattern in the brain, probably due to constitutively high ABC transporter expression (Miller, 2010; Figure 3A and C). In addition, we were unable to detect the SP pattern in cultured ECs (Figure 3A and C). Interestingly, EC-SP cells were also not detectable in peripheral blood or BM, suggesting an origin different from EPCs and that EC-SP cells are present in the peripheral blood vessels. Moreover, EC-SP cells are not present in the lymphatic endothelium (Supplementary Figure S4) and express lower levels of arterial markers but similar levels of venous markers compared with total ECs (Supplementary Figure S5). This indicates that EC-SP cells reside predominantly in veins and capillaries but not in the lymphatics. To confirm that EC-SP cells are not identical to EPCs, we transplanted BM cells from GFP mice into irradiated wild-type mice and assessed the presence of GFP-positive EC-SP cells. Flow cytometry showed that among CD31+CD45− ECs from the hind limb muscle of GFP BM-transplanted mice (Figure 3D), 3.75±0.13% were GFPdim (Figure 3E), but that none of these were EC-SP cells (Figure 3F). This was also confirmed in a BM transplantation model using neonates, in which BM cells were replaced by the injection of BM cells from GFP mice into the liver of wild-type neonates within 12 h after birth. This model allows us to ask whether EPCs derived from BM undergo EC transition at the growing stage and become EC-SP cells. However, among CD31+CD45− ECs from the hind limb muscle of GFP newborn BM-transplanted mice (Figure 3G), we could not detect any GFP-positive or GFP-dim ECs, suggesting that EC-SP cells do not originate from EPCs derived from BM (Figure 3H). It has been reported that EPCs express CXCR4 (Walter et al, 2005); accordingly, the BM CD34+ EPC cell fraction strongly expresses CXCR4. However, EC-SP cells were found to express CXCR4 at significantly lower levels (Figure 3I). Taking these data together, we conclude that EC-SP cells are not identical to EPCs.
Figure 3.
EC-SP cells are present in different organs and are not derived from BM in BM chimeric mice. (A) The ECs from several organs and cultured cell lines as indicated were stained with Hoechst. Percentages of the EC-SP cells are shown in the table. There were few CD31+CD45− ECs in the bone marrow and peripheral blood; SP cells were hardly detected at all. In the EC lines (HUVEC, HUAEC, bEnd3, and EOMA), SP cells were not detected. Of note, the EC-SP phenotype disappeared after culturing primary ECs from hind limbs. (B) One example showing EC-SP cells of lung that disappeared following verapamil treatment. (C) In the brain, a stereotypic EC-SP pattern is not observed and there are no EC-SP cells within the bEnd3 population. (D–F) BM cells from GFP mice were transplanted into lethally irradiated wild-type mice. Four weeks after transplantation, cells from hind limbs were analysed. (D) Representative flow cytometric plots of cells from hind limb muscle. CD31+CD45− EC fraction (red) and CD31−CD45+ peripheral blood fraction (green) are gated. (E) Histogram of CD31+CD45− ECs and CD31−CD45+ peripheral blood cells obtained from hind limbs. Almost all blood cells (green line) after transplantation were GFP positive. Approximately 4% of CD31+CD45− ECs (red line) were weakly GFP positive (GFPdim). GFPdim EC population is shown in arrowed region. (F) Hoechst staining of GFPdim ECs. The SP phenotype was not seen. (G, H) Analysis of hind limb muscle cells from newborn transplantation model. (G) Representative flow cytometric plots of cells from hind limb muscle of BM chimeric mice; CD31+CD45− EC fraction is gated (red). (H) Histogram showing GFP intensity of CD31+CD45− ECs obtained from hind limb and peripheral blood. In this model, GFP-positive CD31+CD45− ECs make up <0.01% of total CD31+CD45− ECs, suggesting no major contribution of BM cells to EC-SP cells. (I) Quantitative evaluation of CXCR4 mRNA expression in EC-SP cells and CD34+ bone marrow mononuclear cells (BMCs) by real-time PCR. Note that CXCR4 expression is 17 times higher in CD34+ BM cells (BMC) than in EC-SP cells (SP). Error bars are ±s.e.m. **P<0.01 (n=7).
Proliferation and colony-forming capacity of EC-SP cells in vitro
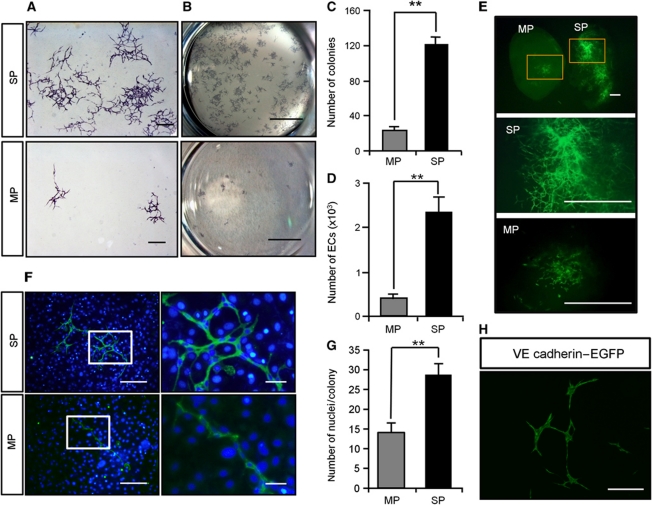
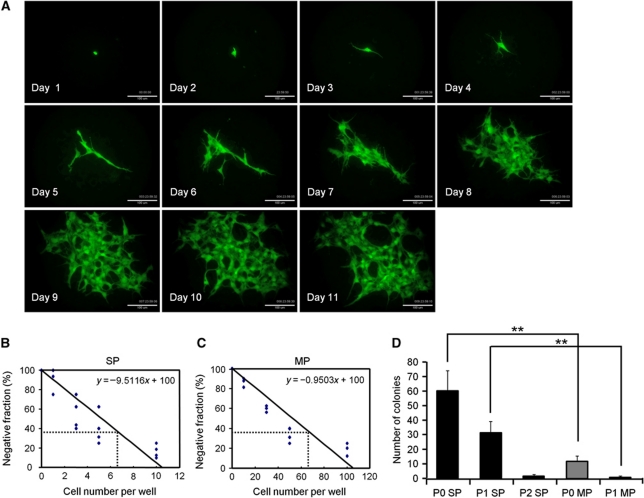
If EC-SP cells are indeed a stem/progenitor population, they must be able to generate large numbers of mature ECs and form colonies originating from a single EC. To explore this issue in vitro, sorted EC-SP cells were cultured on OP9 stromal cells which support EC growth (Takakura et al, 1998). After 10 days, EC-SP cells generated higher numbers of colonies with a ‘cordlike’ structure (Zhang et al, 2001), which formed a fine vascular network, as well as producing higher numbers of ECs than EC-MP cells (Figure 4A–D). It was estimated that 1.2±0.5% of EC-SP cells formed cobblestone-like (sheet-like) colonies (Supplementary Figure S6). To ensure that this degree of colony-forming ability was not a specific property only of ECs from hind limb muscle vessels, EC-SP cells from different organs were cultured on OP9 stromal cells. It was found that they also possessed greater colony-forming ability than EC-MP cells (Supplementary Figure S7). Moreover, we confirmed that these colony-forming cells are indeed ECs, because the colonies were positive for the EC markers CD34, CD105, Flk1, VE-cadherin, vWF, and ZO-1 (Supplementary Figure S8) but negative for the haematopoietic markers B220, CD4, CD8, Gr1, Mac1, Ter119, and CD45 (Supplementary Figure S9A). We excluded the possibility that a contaminating HSC population was giving rise to ECs in our culture system by demonstrating that CD31+ ECs could not be induced from BM-derived c-Kit+Sca-1+Lin− HSC populations (Supplementary Figure S9B). Moreover, VEGF blockade resulted in prevention of colony formation, indicating that expansion of ECs from EC-SP cells depended on VEGF-VEGFR signalling (Supplementary Figure S10). Matrigel plug assays carried out with GFP-positive cells showed that EC-SP cells formed entire vascular networks in the matrigel, but EC-MP cells only formed separate colonies with a small network (Figure 4E). Moreover, to compare the ability of single EC-SP or EC-MP cells to generate EC, numbers of cells in single colonies were counted. It was found that EC-SP cells have a greater capacity to produce ECs than do EC-MP cells (Figure 4F and G). To further clarify whether EC-SP cells are indeed committed to the EC lineage, we crossed endothelial-specific VE-cadherin-Cre-ERT mice with loxP-CAT-EGFP reporter mice and sorted GFP-positive EC-SP cells (Supplementary Figure S11A and B). In the GFP+ (VE-cadherin+) CD31+CD45− fraction, the percentage of EC-SP cells was comparable to wild-type mice. When cultured on OP9 cells for 10 days, GFP+ EC-SP cells generated colonies similar to those from wild-type mice (Figure 4H). Furthermore, EC-SP cells did not give rise to the mesenchymal and haematopoietic lineage in vitro (Supplementary Figures S12 and S13A). Next, to assess clonal expansion of ECs from single cells, we performed time-lapse analysis of EC-SP cells and found that a single EC-SP cell could form a colony (Figure 5A; Supplementary Movie S1). Moreover, to establish whether this EC-SP cell clonal expansion can occur in every colony, sorted EC-SP cells from normal mice and C57BL/6-Tg(CAG-EGFP) mice (EGFP mice) were mixed in equal proportions and cultured on OP9 stromal cells. As expected, colonies with ‘cordlike’ structures were generated from either GFP-positive or -negative ECs (Supplementary Figure S14), suggesting that a single EC-SP cell is able to generate a single colony. Limiting dilution analysis revealed that the frequency of cells with the capacity to form colonies was significantly higher in EC-SP cells than in EC-MP cells by a factor of 10 (1 in 6.6 and 1 in 66, respectively) (Figure 5B and C). Moreover, long-term culture-initiating cell (LTC-IC) assays revealed that ECs having higher proliferative potential were produced from EC-SP cells than could be produced by EC-MP cells (Figure 5D). These findings indicate that cells able to generate EC colonies are enriched within the EC-SP population.
Figure 4.
Endothelial SP cells have EC colony-forming ability. (A) EC-SP cells and EC-MP cells were cultured on OP9 feeder cells and stained with anti-CD31 antibody. (B) Colonies are shown in the low power field. The EC-SP cells form fine CD31-positive networks and many colonies compared with EC-MP cells. (C) The number of colonies stained with anti-CD31 antibody and (D) number of VE-cadherin+ ECs counted by flow cytometry in one well of a 6-well culture dish. Error bars are ±s.e.m. **P<0.01 (n=12). (E) EC-SP and EC-MP cells were sorted from EGFP mice and transplanted to wild-type mice with matrigel. Gated area is shown in higher magnification. (F, G) Nuclear staining of ECs forming colonies on OP9 cells for the evaluation of cell number. Representative image of an EC colony stained with anti-CD31 antibody and Hoechst (F) and quantification of the number of ECs composing one colony (G). (H) EC colonies derived from EC-SP cells from VE-cadherin promoter EGFP mice. Scale bars, 500 μm (A), 1 mm (E), 200 μm (F left panel and H), 50 μm (F right panel), and 5 mm (B).
Figure 5.
Single EC-SP cells form EC colonies. (A) Time-lapse analysis of EC-SP cell from EGFP mice. (B, C) Limiting dilution assay of EC-SP (B) and EC-MP (C) cells. EC-SP and EC-MP cells were cultured on OP9 feeder cells and titrated down to 20, 10, 5, 3, 1, 0 and 200, 100, 50, 30, 10, 0 cells, respectively. The number of colonies was counted after staining with anti-CD31 antibody and the frequency of colony-forming cells was calculated according to Poisson statistics. (D) Results of long-term culture-initiating assays. 5 × 102 primary EC-SP or EC-MP cells were cultured and the number of colonies counted (P0). Cells were harvested and 5 × 102 sorted ECs derived from the first or second rounds of culture were cultured again (P1 and P2, respectively). Note that the P2 assay using ECs from EC-MP cells could not be performed due to insufficient ECs in P1. **P<0.01 (n>5). Scale bar, 100 μm (A).
Angiogenic stimuli induced by ischaemia activate EC-SP cells
To study the potential of the EC-SP cells to facilitate neovascularization in vivo, we first investigated their proliferative capacity using a hind limb ischaemia model (occlusion of the femoral artery). The percentage and absolute number of EC-SP cells increased 1 day after induction of ischaemia, peaked after 3 days at 4.03±1.44% and gradually declined again to the steady state after 2 weeks (Figure 6A, D and E). Addition of verapamil blocked the EC-SP cells, confirming their SP phenotype (Figure 6B). Sham operation on the other hind limb did not have any effect (Figure 6C). Cell-cycle analysis revealed that ∼40% of the EC-SP cells began to divide after induction of ischaemia (Figure 6F and G). The colony-forming ability of the ischaemic EC-SP and EC-MP cells was comparable with that of the same cell types in the steady state (Supplementary Figure S15). Next, we used a BM transplantation model to confirm that the EC-SP cells proliferating after the induction of ischaemia are not derived from BM cells. When hind limb ischaemia was induced in chimeric mice generated by transplanting BM cells from EGFP mice into wild-type mice, all CD31−CD45+ blood cells in the hind limb were positive for GFP, but CD31+CD45− cells within either the EC-MP or EC-SP populations were negative for GFP (Figure 6H–K). This implies that the increased EC-SP cells after induction of ischaemia were not derived from the BM. Taken together, these results suggest that EC-SP cells are quiescent in the steady state but actively proliferate in peripheral vessels when exposed to angiogenic stimuli induced by ischaemia.
Figure 6.
EC-SP cells proliferate under conditions of tissue hypoxia. Flow cytometric analysis of Hoechst 33342 staining of CD31+CD45− ECs from hind limbs in which ischaemia had been induced (A, B) and sham-operated hind limbs from the other side of the animal (C) with (B) or without (A, C) Verapamil treatment. Quantitative evaluation of the number of EC-SP cells (D) and the percentage of EC-SP cells (E) from one hind limb. Control in (D) indicates EC-SP cells in the sham-operated hind limb. Error bars are ±s.e.m. *P<0.05 (n>10), **P<0.01 (n>10). (F) Hoechst and PY emission pattern of EC-SP cells sorted from the hind limb 3 days after induction of ischaemia. (G) Percentage of PY-low G0 EC-SP cells under steady-state conditions or the ischaemic state as observed in (F). Error bars are ±s.e.m. **P<0.01 (n=6). (H–K) Excluding the possibility that proliferating EC-SP cells are derived from BM. Representative FACS analysis of cells from the hind limb (H) and BM (I) 3 days after induction of ischaemia in BM chimeric mice transplanted with BM cells derived from EGFP mice into wild-type mice. (J) Hoechst staining of the CD31+CD45− ECs (black gate in (H)). EC-SP cells (red gate) and EC-MP cells (green gate) are shown. (K) Histogram showing GFP positivity in the gated populations. Colours of lines are the same as the gated colours in (I) and (J). Only CD45+ BM cells (blue gate in (I)) are GFP positive but EC-SP cells and EC-MP cells are GFP negative.
EC-SP cells contribute to the regeneration of vascular endothelium in vivo
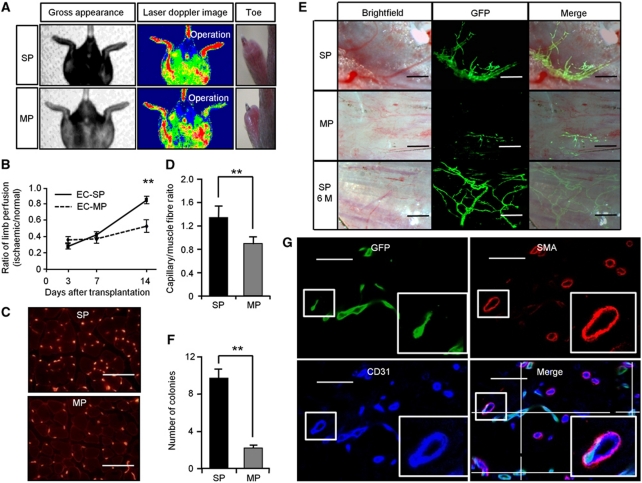
Next, we transplanted EC-SP or EC-MP cells into ischaemic limbs, observed their contribution to the neovasculature, and compared the effectiveness of restoration of the vasculature after ischaemia. To this end, we transplanted 3000 cells from EGFP mice and evaluated blood flow by laser Doppler perfusion image analyzer. After 14 days, the blood flow in the hind limbs of EC-SP-transplanted mice was completely restored, whereas transplantation of EC-MP cells resulted in congestion, with necrosis of the toes (Figure 7A and B). At the site of transplantation, blood vessel density was greater in the animals receiving EC-SP cells (Figure 7C and D). Stereomicroscopic observations on living EC-SP-transplanted mice 14 day after transplantation revealed many GFP-positive vessels on the hind limb muscle surface (Figure 7E). These newly formed GFP-positive vessels were filled with red blood cells, suggesting their connection to the systemic circulation. In contrast, blood vessels originating from EC-MP cells were very small, and even when cordlike, contained no erythrocytes (Figure 7E and F). Immunohistochemical analysis revealed that transplanted GFP-positive EC-SP cells gave rise to CD31-positive ECs but not to smooth muscle actin (SMA)-positive mural cells, connected to GFP-negative CD31-positive host ECs (Figure 7G; Supplementary Figure S16). Furthermore, we investigated the long-term contribution of transplanted EC-SP cells to blood vessel maintenance, and found that they still persisted 6 months after injection. Moreover, complete blood vessels could be generated solely from GFP-positive ECs derived from EC-SP cells, whereas ECs derived from EPCs made only a partial contribution and were unable by themselves to reconstitute vessels in their entirety (Takahashi et al, 1999; Figure 6E). Finally, to confirm that these newly developed blood vessels originate from cells already committed to ECs, we utilized GFP+EC-SP cells derived from VE-cadherin Cre mice crossed with lox-GFP reporter mice, as described in Supplementary Figure S11A and B. This revealed that GFP+EC-SP cells generated fine vascular colonies when transplanted into ischaemic limbs (Supplementary Figure S11C).
Figure 7.
Recovery from ischaemia and long-term incorporation of ECs from EC-SP cells into newly developed blood vessels. (A) Hind limb ischaemia was induced in wild-type mice and EC-SP or EC-MP cells sorted from EGFP mice were transplanted. Gross appearance, Laser Doppler image, and representative photographs of hind limb toes 14 day after transplantation. (B) Blood perfusion ratio of ischaemic hind limb measured by laser Doppler imaging at 3, 7, and 14 days after treatment. Error bars are ±s.e.m. **P<0.01 (n=11). (C) Sections of muscles 14 days after EC-SP or EC-MP cell transplantation stained with anti-CD31 antibody and (D) capillaries per muscle fibre. Error bars are ±s.e.m. **P<0.01 (n>30 random high-power fields). (E) Fluorescent stereomicroscopic image of EC-SP and EC-MP transplanted muscle observed 2 weeks and 6 months after transplantation. ECs derived from EC-SP cells generate fine vascular architecture with large lumens connected to the systemic circulation and filled with blood. These vessels remain functional after 6 months (SP 6M). (F) Quantification of the number of GFP-positive vascular colonies on the whole surface of hind limb muscle. Error bars are ±s.e.m. **P<0.01 (n=10). (G) Confocal microscopic image of a section from hind limb muscle transplanted with EC-SP cells stained with GFP (green), α smooth muscle actin (SMA) (red), and CD31 (blue). Muscle was dissected 2 weeks after transplantation. Insets show high-power view of area indicated by box. Transplanted GFP-positive ECs are connected to the GFP-negative host ECs in the lumen. Scale bars, 100 μm (C), 250 μm (E), and 50 μm (G).
EC-SP cells reside in the peripheral vascular endothelium and show a distinct gene expression profile
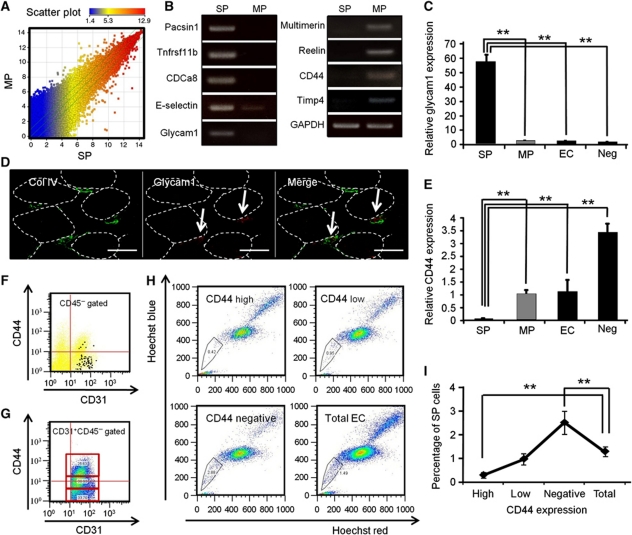
To further characterize EC-SP cells, we employed DNA microarray analysis. We carried out a global survey of mRNA in EC-SP and EC-MP cells. There was a striking difference in the gene expression profiles between these cells (Figure 8A). To confirm the microarray data, several genes specifically expressed in EC-SP cells or EC-MP cells were examined by RT–PCR analysis. It was confirmed that Pacsin1, Tnfrsf11b, and Cdca8 were not expressed in the EC-MP fraction and that E-selectin and Glycam1 expression was higher in EC-SP cells. In contrast, Multimerin and Reelin were not expressed in the EC-SP fraction and CD44 and Timp4 expression was higher in EC-MP cells (Figure 8B). Glycam1 is known to be expressed in the high endothelial venule (HEV) of peripheral lymph nodes and HEV-like islet vessels in areas of tumour infiltration (Onrust et al, 1996). Thus, we investigated the expression of Glycam1 by quantitative RT–PCR analysis. Compared with EC-MP, CD31+CD45− ECs and CD31−CD45− cells sorted from hind limb (negative control), EC-SP cells specifically expressed Glycam1 (Figure 8C). Next, we performed in-situ hybridization experiments to detect the localization of Glycam1-expressing ECs and found scattered distribution in the peripheral vessels surrounded by basement membrane protein collagen type IV (Figure 8D). Moreover, we investigated CD44 expression by quantitative RT–PCR analysis (Figure 8E). The level of CD44 in EC-SP cells was lower than in the other faction. Therefore, we further studied CD44 expression on ECs and EC-SP cells by FACS. First, levels of CD44 expression on EC-SP cells were analysed, with the result that EC-SP cells were found predominantly in the CD44-negative fractions (Figure 8F). Next, we divided the ECs into three equal fractions according to their level of expression of CD44, that is, high, low, and negative (Figure 8G) and then performed Hoechst analysis. As expected, the percentage of EC-SP cells was significantly greater in CD44-negative ECs and lowest in the CD44-high fraction (Figure 8H and I).
Figure 8.
EC-SP cells are scattered in the microvessels and have a distinct gene expression profile. (A) Scatter plots showing comparison of global gene expression between EC-SP cells and EC-MP cells as determined by DNA microarrays. (B) Total RNA was purified from EC-SP and EC-MP cells and several genes as indicated were examined by RT–PCR analysis. (C) Quantitative RT–PCR analysis of Glycam1 using total RNA from EC-SP cells, EC-MP cells, CD31+CD45−ECs (EC), and CD31−CD45− cells (negative control; Neg) isolated from hind limb muscle. Glycam1 expression level of EC-SP cells is >55-fold greater than that of EC-MP cells. Error bars are ±s.e.m. **P<0.01 (n=8). (D) In-situ hybridization for Glycam1 (red) combined with immunohistochemistry for collagen IV (green). Arrow indicates Glycam1 mRNA-expressing ECs; Glycam1-expressing cells do not overlap with collagen type IV, basal membrane protein. Muscle fibres are depicted as dotted line. (E) Quantitative RT–PCR analysis of CD44 using total RNA from EC-SP cells and EC-MP cells. CD44 expression level of EC-MP cells is 20-fold greater than that of EC-SP cells. Cell fractionation is the same as described in (C). Error bars are ±s.e.m. **P<0.01 (n=6). (F) CD44 expression by EC-SP cells. EC-SP cells are plotted in black. Note that most of the EC-SP cells are in the CD44-negative population. (G) FACS plots showing the expression of CD44. CD31+CD45− ECs were subdivided into three equal populations according to their level of expression of CD44. (H) Hoechst staining of ECs fractionated by CD44 expression and total endothelial cells are shown. EC-SP cells are gated. (I) The percentage of EC-SP cells stratified by CD44 intensity. EC-SP cells are significantly higher in the CD44-negative fraction and lower in the CD44-high fraction, compared with the total EC fraction. Error bars are ±s.e.m. **P<0.01 (n=10). Scale bars, 20 μm.
Discussion
In the present study, we have described novel phenotypic heterogeneity of ECs within peripheral blood vessels and documented that EC-SP cells share characteristics with both lineage-committed differentiated ECs and stem/progenitor cells. Previous reports have described the contribution of EPCs to the formation of new vessels in adulthood (Asahara et al, 1999; Nolan et al, 2007), but their pathways of differentiation to vascular ECs had remained undetermined (Purhonen et al, 2008). We now show that this heterogeneity of ECs in the peripheral vasculature reflects the crucial role of small sub-population for angiogenesis, by virtue of producing large numbers of ECs.
The SP assay was first described using mouse BM cells which were shown to be highly enriched for HSCs (Goodell et al, 1996). Thus far, the SP assay has proven to be a valuable approach to isolate putative stem/progenitor populations, particularly in the absence of specific surface markers (Golebiewska et al, 2011). Heterogeneity of ECs has been widely accepted, but the existence of a stem/progenitor cell fraction residing within ECs has not been elucidated. In the present study, we document the existence of SP cells in the CD31+CD45− EC fraction located at the inner surface of the peripheral vascular endothelium (Figure 1). We found that EC-SP cells are quiescent in the steady state, but are driven to cycle upon hypoxic stimuli, and produce a number of ECs and form EC colonies. Even though colony-forming ECs other than EC-SP cells are present in the EC population, they could only give rise to low numbers of small EC colonies in vitro. Moreover, as HSCs are present in the MP fraction (Morita et al, 2006), it is possible that stem/progenitor-like ECs are also contained within the MP fraction. They could then contribute to neovascularization in vivo, although blood vessels thus formed are scanty, seem non-functional and soon regress. On the other hand, cells forming large EC colonies in vitro are highly enriched within the EC-SP fraction and could repopulate long-term surviving functional blood vessels in vivo (Figure 7E). To the best of our knowledge, this is the first report to show that colony-forming stem/progenitor-like EC (namely ‘spEC’; this designation is also derived from abbreviating ‘side population EC’) is present in the peripheral vasculature of adult mice. Moreover, surface marker and functional assays of Ac-LDL uptake, as well as utilization of VE-cadherin Cre mice, revealed that although EC-SP cells are already committed to mature ECs (not due to contamination with HCs, pericytes or fibroblasts), their colony-forming ability, proliferative capacity, ability to regenerate mature blood vessels in vivo, high N/C ratio as evaluated by morphology, and high expression of CD133, all strongly suggest that they also possess important characteristics of stem/progenitor cells.
In terms of heterogeneity, it has been shown that there are at least three cell types of ECs regulating angiogenesis, that is, tip, stalk, and phalanx cells (De Bock et al, 2009). Tip cells develop from preexisting blood vessels and guide stalk cells proliferating and migrating behind the tip cells. In our in-vivo regeneration assay using EC-SP cells, we observed new vessels sprouting from newly developed blood vessels generated by EC-SP cells (Figure 7E). This strongly suggests that EC-SP cells can give rise to both tip and stalk cells and those resident quiescent EC-SP cells are the source of EC sprouts in vivo. It has been recently reported that ECs, so-called phalanx cells, finally emerge during angiogenesis and form mature blood vessels without surface asperity and well covered with mural cells (Mazzone et al, 2009). Neovasculature generated by ECs from EC-SP cells has a highly hierarchical architecture including a variety of caliber sizes ranging from small to large (Figure 7E), and enlarged blood vessels are fully covered with mural cells (Figure 7G). It is reported that ECs can give rise to mesenchymal stem cells (Medici et al, 2010) or HCs (Boisset et al, 2010). However, we failed to induce the endothelial–mesenchymal transition or differentiation to the haematopoietic lineage of EC-SP cells either in vivo or in vitro (Figure 7G; Supplementary Figures S12 and S13). Because immature blood vessels in which ECs are not covered with mural cells must be regressed, ECs derived from EC-SP cells can contribute to mature blood vessel formation. Low expression of PHD2, a sensor of hypoxia, is one of the phenotypic characteristics of phalanx cells (Mazzone et al, 2009). We found that EC-SP cells show low level expression of PHD2 mRNA (Supplementary Figure S17), suggesting that they may overlap with phalanx cells. The relationships between these three phenotypically different ECs and EC-SP cells are of great interest and need to be determined.
Although we did determine that Glycam1-positive ECs are present in the peripheral endothelium by in-situ hybridization (Figure 8D), we failed to detect protein expression by immunohistochemistry. Accurately identifying EC-SP cells in vivo remains an obstacle to progress in understanding their nature. Indeed, the lectin perfusion assay revealed that 91% of EC-SP cells reside in the intra-luminal cavity of the blood vessels (Figure 1E–G). Of course, it is possible that not all intra-luminal ECs are labelled with lectin, but we cannot exclude that a small population of EC-SP cells might reside at a location deeper within the blood vessel wall, or indeed elsewhere, and not in the blood vessels. The localization of the EC-SP cells and their niches, and their relationships with neighbouring cells like pericytes or mural cells, remains of great interest. BrdU labelling assays have been widely assumed to mark stem cells (Cotsarelis et al, 1990; Kalabis et al, 2008), but this method is not applicable to EC-SP cells (Supplementary Figure S18). Thus, specific molecular markers are required for identifying their precise localization and their niches, and for tracking EC-SP cells in vivo. We did determine that EC-SP cells are in the CD44-negative fraction (Figure 8F). It has been suggested that CD44, a cell-surface glycoprotein involved in cell–cell interactions, regulates endothelial networks in blood vessel formation (Cao et al, 2006). As distribution of blood vessels in the embryo may be determined in part by the relative amount of hyaluronic acid contained within tissue (Feinberg and Beebe, 1983), it is reasonable that CD44-low ECs that are surrounded by less hyaluronic acid are more readily responsive to angiogenic stimuli and could represent an angiogenic sprouting point. Although low CD44 expression can mark the endothelial stem/progenitor cells, lack of staining cannot positively identify EC-SP cells in vivo. The results of the microarray analysis indicate that E-selectin could be another candidate surface marker of EC-SP cells (Figure 8B). Combining these markers may more accurately define the EC-SP cells. Further research is required for positive identification of EC-SP cells by their expression of specific molecules.
Additionally, although a role in tumour angiogenesis remains to be elucidated, EC-SP cells are clearly present in tumour vasculature proportional to tumour volume (Supplementary Figure S19). Currently, anti-angiogenic therapy offers great promise for anti-tumor therapy and is often used together with conventional therapies. However, as with all anti-cancer therapies, the tumour commonly acquires resistance. The development of resistance to anti-cancer drugs is often associated with multi-drug efflux pumps; it is possible that EC-SP cells that have high expression of drug pumps may be a cause of drug resistance to anti-angiogenic therapy targeting ECs.
In summary, we have documented the existence of a sub-population of stem/progenitor-like ECs (spEC) with colony-forming ability and vascular regenerating capacity. These data are consistent with the hypothesis that certain ECs retain their original hierarchical stem/progenitor characteristics in the peripheral blood vessels. Therefore, targeting this sub-population may open up new avenues for anti-angiogenic as well as for pro-angiogenic therapy.
Materials and methods
Mice
All experiments were carried out following the guidelines of Osaka University Committee for animal and recombinant DNA experiments. Mice were handled and maintained according to the Osaka University guidelines for animal experimentation. C57BL/6 mice and C57BL/6-Tg (CAG-EGFP) mice (EGFP mice) that express GFP ubiquitously were purchased from Japan SLC. VE-Cadherin-Cre-ERT2 mice (Mahmoud et al, 2010) and Flox-CAT-EGFP mice (Okuno et al, 2011) were provided by Dr Ralf H Adams (Max Planck Institute for Molecular Biomedicine, Munster, Germany) and Dr Toshio Suda (Keio University, Tokyo, Japan), respectively. VE-Cadherin-Cre-ERT2 mice were crossed with the Flox-CAT-EGFP mice and utilized in this study at adult ages (older than 2 months). Recombination was induced by intra-peritoneal injection of tamoxifen (Sigma, St Louis, MO) as described (Mahmoud et al, 2010).
Cell preparation
Mice were euthanized and organs were excised, minced, and digested with Dispase II (Godo Shusei Corp., Chiba, Japan), collagenase (Wako, Osaka, Japan), and type II collagenase (Worthington Biochemical Corp., Lakewood, New Jersey) with continuous shaking at 37°C. The digested tissue was passed through 40-μm filters to yield single cell suspensions. BM cells were collected from the tibiae and femurs, and peripheral blood was collected from the heart using standard methods. Erythrocytes were lysed with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM Na2-EDTA). Mouse EPCs were isolated from BM of ischaemic hind limbs; 3 days after induction of ischaemia, BM mononuclear cells were collected and CD34+ cells sorted by FACS.
Flow cytometry
Hoechst staining was performed as described previously (Goodell et al, 1996). Briefly, cell-surface antigen staining was performed and cell suspensions were incubated with Hoechst 33342 (5 μg/ml) (Sigma) at 37°C for 90 min in DMEM (Sigma) (2% fetal calf serum, 1 mM HEPES) at a concentration of 1 × 106 nucleated cells/ml in the presence or absence of verapamil (50 μmol/l, Sigma). To analyse the cell-cycle status by Pyronin Y (PY) staining, cells were first stained with Hoechst 33342 at 37°C. After 45 min, 1 μg/ml PY was added and cells were incubated at 37°C for 45 min (Summers et al, 2004). Cell-surface antigen staining was performed as described previously (Takakura et al, 1998). The mAbs used in immunofluorescence staining were anti-CD45, -CD44, -CD31, -c-kit, -VEGFR2, -Sca-1, -CD34, -CD133, -VE cadherin, -CD140, and -lineage (a mixture of ter119, Gr-1, Mac-1, B220, CD4, and CD8) (Pharmingen, BD Biosciences). Biotinylated antibodies were visualized with PE-conjugated streptavidin (Pharmingen, BD Biosciences), and purified antibody was visualized with anti-rat IgG Alexa Fluor 546 (Invitrogen). Respective isotype controls (Pharmingen, BD Biosciences) were used as negative controls. Propidium iodide (PI) (2 μg/ml) was added before FACS analysis to exclude dead cells. For lectin staining, 100 μl of fluorescein Lycopersicon Esculentum (Tomato) lectin (Vector Laboratories, Inc. Burlingame, CA, USA) was administered intravenously 30 min before preparation of the cells. The stained cells were analysed and sorted by a JSAN flow cytometer (Bay Bioscience Corp., Kobe, Japan) and data analysed using FlowJo Software (Treestar Software, San Carlos, California, USA).
Cell culture
Mouse brain-derived ECs (bEnd3) were cultured in DMEM with 10% fetal calf serum and 1% penicillin/streptomycin. Human umbilical vascular endothelial cells (HUVECs) were cultured in HuMedia-EG2 (Kurabo, Osaka, Japan) and human umbilical artery endothelial cells (HUAEC) were cultured in growth medium provided by the supplier. Cultured mouse hind limb muscle endothelial cells (MHMECs) were isolated as described above and seeded onto fibronectin-coated 35 mm dishes (Iwaki, Tokyo, Japan) in HuMedia-EG2, supplemented with VEGF (20 ng/ml: Prepro Tech, Rocky Hill, NJ). After the fourth passage, MHMEC was used for SP analysis.
Quantitative reverse-transcription real-time PCR (qRT–PCR)
RNA was extracted from cells using an RNeasy Mini Kit (Qiagen), and cDNA was generated using reverse transcriptase from the ExScript RT reagent Kit (Perfect Real Time) (Takara). Real-time PCR was performed using a Stratagene Mx3000P (Stratagene, La Jolla, CA). PCR was performed on cDNA using specific primers (Supplementary Table S1). Expression level of the target gene was normalized to the GAPDH level in each sample.
Ac-LDL uptake
For analysis of Ac-LDL uptake, freshly isolated cells were incubated with 10 μg/ml Alexa Fluor 488-labelled Ac-LDL (Invitrogen) for 2 h at 37°C and then Hoechst 33342 was added for another 90 min incubation for SP staining.
Immunohistochemistry
The procedure for tissue preparation and staining was as previously reported (Takakura et al, 1998). For immunohistochemistry, biotin-conjugated anti-CD31 antibody (Pharmingen, BD Biosciences), Cy3-conjugated anti-α SMA antibody (Dako, Glostrup, Denmark), and anti-GFP antibody (Invitrogen) were used for staining and alkaline phosphatase (ALP)-conjugated streptavidin (Dako), biotin-conjugated polyclonal anti-rat Ig (Dako), anti-rat IgG Alexa Fluor 546 (Invitrogen) and anti-rabbit IgG Alexa Fluor 488 (Invitrogen) as the secondary antibody. Biotinylated secondary antibodies were developed using ABC kits (Vector Laboratories). 5-Bromo-4-chloro-3-indoxyl phosphate/nitro blue tetrazolium chloride (BCIP/NBT; Boehringer Mannheim, Mannheim, Germany) was used for the ALP colour reaction. Cell nuclei were visualized with Hoechst dye (Sigma). Samples were visualized using an Olympus IX-70 equipped with UPlanFI × 4/0.13 and LCPlanFI × 20/0.04 dry objective lenses, Leica DM5500B equipped with HCX PL FLVOTAR 5/0.15 and HCX PL FLVOTAR 10 × /0.15 dry objective lenses or Leica TCS/SP5 confocal microscopy equipped with HC PLAN APO × 20/0.70 and HCXPLAPO 40/1.25−0.75 oil objective lenses. Images were acquired with a DFC 500 digital camera (Leica) and processed with the Leica application suite (Leica) and Adobe Photoshop CS3 software (Adobe systems). All images shown are representative of >6 independent experiments.
EC colony-forming assay, time-lapse analysis, limiting dilution assay, and LTC-IC assay
In all, 1000 EC-SP or MP cells were seeded onto 24-well plates and co-cultured on OP9 stromal cells in 10% FCS and 10−5 M 2-ME (GIBCO) containing RPMI-1640 (Sigma) and fixed for immunostaining after 10 days. Time-lapse analysis was performed using an Olympus LCV110 (Olympus) and images were processed with Metamorph software (Universal Imaging, West Chester, PA, USA). For limiting dilution assay, cells were titrated to 20, 10, 5, 3, or 1 cells in one well for SP cells and 200, 100, 50, 30, 10 cells for MP cells. Cells were cultured for 10 days and number of colonies counted after immunostaining. For the LTC-IC assay, 500 EC-SP or EC-MP cells collected from EGFP mice were cultured on OP9 cells (P0). After 12 days, GFP-positive cells were counted, and 500 EGFP cells were cultured in the same manner (P1). For EC-SP cells, the same procedure was repeated once more (P2).
In-vivo neovascularization using matrigel
Eight-week-old C57BL/6 mice were injected subcutaneously with 0.5 ml Matrigel (Becton Dickinson) and 60 units of heparin per ml (Sigma), 150 ng/ml VEGF (PeproTech), and 3000 EC-SP or MP cells from the hind limb of an EGFP mouse. Fifteen days later, Matrigel plugs were removed.
Hind limb ischaemia model and EC transplantation
The proximal portion of the right femoral artery and vein including the superficial and the deep branch as well as the distal portion of the saphenous artery and vein were occluded and resected. CD31+CD45− ECs from hind limb were obtained 1, 2, 3, 5, 7, and 14 days after induction of ischaemia. Proportions and numbers of EC-SP cells per ischaemia-induced hind limb were analysed and calculated. Controls were the hind limbs from the other side of the animal that was sham operated. For EC-SP and EC-MP transplantation, CD31+CD45−SP and MP cells were sorted from EGFP mice. The hind limb ischaemia model was prepared and just after occlusion and removal of vessels, 3000 EC-SP cells and 3000 EC-MP cells were injected into the muscle.
Murine BM transplantation model
C57BL/6 mice underwent BM transplantation from EGFP mice. Mice were myeloablated using two different regimens as previously described (Bruscia et al, 2006). Regimen 1: For the adult BM transplantation model, BM cells were obtained by flushing the tibias and femurs of age-matched donor EGFP mouse. The transplantation was performed to C57BL/6 mice lethally irradiated with 10.0 Gy, by intravenous infusion of ∼1 × 107 donor whole BM cells. Four weeks after transplantation, by which time BM of recipient mice was reconstituted, the mice were used for analysis. Regimen 2: Mother mice were myeloablated with busulfan (15 mg/kg, Sigma) on days 17 and 18 of pregnancy. Approximately 12 h after birth, 1 × 107 GFP-positive donor whole BM cells were injected into the livers of the pups. BM chimeric mice were analysed 8 weeks after BM transplantation.
Laser Doppler blood flow analysis
Hind limb blood flow was measured using a laser Doppler blood flow meter (LDBF; MoorLDI, Moor Instrument), as described previously (Kidoya et al, 2010). LDBF analyses over the legs and paws were performed on postoperative days 3, 7, and 14. After scanning, stored images were analysed to quantify blood flow, and mean LDBF values of the ischaemic and non-ischemic limbs were calculated. To avoid data variations because of ambient light and temperature, hind limb blood flow was expressed as the ratio of the left (ischaemic) to right (non-ischemic) hind limb LDBF.
Capillary density analysis
Tissue samples were obtained from the ischaemic skeletal muscles on postoperative day 14. Sections were examined for the presence of capillary ECs, and capillary to muscle fibre ratios were expressed as the ratio of the number of capillaries to the number of myofibres per high-power field (× 400).
In-situ hybridization
Total RNA from mammary glands was isolated and used for cDNA synthesis. The Glycam 1 primers for PCR amplification were forward primer 5′-GTGCCACCATGAAATTCTTC-3′ and reverse primer 5′-TCTTCATGACTTCGTGATAC-3′. A 467-bp PCR fragment of Glycam 1 was subcloned into pGEM-T Easy Vector (Promega). The digoxigenin-labelled RNA probes were made using DIG RNA labeling kits (Roche, Indianapolis, IN). Hind limb muscle sections were processed and hybridization was performed as previously reported (Hou et al, 2000). Hybridized DIG-RNA probes were detected with anti-digoxigenin-rhodamine, Fab fragments (Roche). After in-situ hybridization, sections were stained with polyclonal anti-type IV collagen (Cosmo Bio).
Microarray analysis
Microarray analysis was performed as previously described (Nagahama et al, 2010). Labelled cRNA probes were hybridized to Affymetrix Mouse Genome 430 2.0 array (Affymetrix). Raw data are available for download from Gene Expression Omnibus (GSE28240). Microarray analysis was performed in duplicate from independent RNA preparations and analysed using GeneSpring GX 11.0 (Agilent Technologies).
Statistical analysis
All data are presented as mean±standard error of mean (s.e.m.). For statistical analysis, the statcel 2 software package (OMS) was used with analysis of variance performed on all data followed by Tukey–Kramer multiple comparison testing. When only two groups were compared, a two-sided Student's t-test was used. A probability value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr RH Adams (Max Planck Institute for Molecular Biomedicine, Munster, Germany) and Dr T Suda (Keio University, Tokyo, Japan) for providing us with VE-Cadherin-Cre-ERT mice and Flox-CAT-EGFP mice, respectively. We thank K Fukuhara, C Takeshita, and N Fujimoto for technical assistance. This work was partly supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
Author contributions: HN, HK, SS, and TW conducted in-vivo and in-vitro experiments. HN and NT planed the experiments. HN and NT wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161 [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967 [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C (2010) In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464: 116–120 [DOI] [PubMed] [Google Scholar]
- Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS (2006) Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells 24: 2299–2308 [DOI] [PubMed] [Google Scholar]
- Bunting KD, Zhou S, Lu T, Sorrentino BP (2000) Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood 96: 902–909 [PubMed] [Google Scholar]
- Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, Delisser HM (2006) Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol 169: 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Little MH (2006) A side order of stem cells: the SP phenotype. Stem Cells 24: 3–12 [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998) A common precursor for hematopoietic and endothelial cells. Development 125: 725–732 [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337 [DOI] [PubMed] [Google Scholar]
- De Bock K, De Smet F, Leite De Oliveira R, Anthonis K, Carmeliet P (2009) Endothelial oxygen sensors regulate tumor vessel abnormalization by instructing phalanx endothelial cells. J Mol Med 87: 561–569 [DOI] [PubMed] [Google Scholar]
- Feinberg RN, Beebe DC (1983) Hyaluronate in vasculogenesis. Science 220: 1177–1179 [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska A, Brons NH, Bjerkvig R, Niclou SP (2011) Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 8: 136–147 [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG (2004) Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood 104: 1769–1777 [DOI] [PubMed] [Google Scholar]
- Gothot A, Pyatt R, McMahel J, Rice S, Srour EF (1997) Functional heterogeneity of human CD34(+) cells isolated in subcompartments of the G0/G1 phase of the cell cycle. Blood 90: 4384–4393 [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S (2009) Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Bailey JP, Vomachka AJ, Matsuda M, Lockefeer JA, Horseman ND (2000) Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) is induced by prolactin and suppressed by progesterone in mammary epithelium. Endocrinology 141: 4278–4283 [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q (2004) Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalabis J, Oyama K, Okawa T, Nakagawa H, Michaylira CZ, Stairs DB, Figueiredo JL, Mahmood U, Diehl JA, Herlyn M, Rustgi AK (2008) A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest 118: 3860–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidoya H, Naito H, Takakura N (2010) Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischaemia. Blood 115: 3166–3174 [DOI] [PubMed] [Google Scholar]
- Mahmoud M, Allinson KR, Zhai Z, Oakenfull R, Ghandi P, Adams RH, Fruttiger M, Arthur HM (2010) Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ Res 106: 1425–1433 [DOI] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG et al. (2009) Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136: 839–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR (2010) Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 16: 1400–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 31: 246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR (2008) CD133: molecule of the moment. J Pathol 214: 3–9 [DOI] [PubMed] [Google Scholar]
- Morita Y, Ema H, Yamazaki S, Nakauchi H (2006) Non-side-population hematopoietic stem cells in mouse bone marrow. Blood 108: 2850–2856 [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Ueno M, Miyamoto S, Morii E, Minami T, Mochizuki N, Saya H, Takakura N (2010) PSF1, a DNA replication factor expressed widely in stem and progenitor cells, drives tumorigenic and metastatic properties. Cancer Res 70: 1215–1224 [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y (1998) In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8: 761–769 [DOI] [PubMed] [Google Scholar]
- Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V (2007) Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 21: 1546–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y (2011) Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 117: 5264–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrust SV, Hartl PM, Rosen SD, Hanahan D (1996) Modulation of L-selectin ligand expression during an immune response accompanying tumorigenesis in transgenic mice. J Clin Invest 97: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273: 242–245 [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW (2008) A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 105: 9349–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C (2005) Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med 11: 261–262 [DOI] [PubMed] [Google Scholar]
- Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P (2008) Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA 105: 6620–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W (1995) Differentiation of endothelium. FASEB J 9: 926–933 [PubMed] [Google Scholar]
- Risau W (1997) Mechanisms of angiogenesis. Nature 386: 671–674 [DOI] [PubMed] [Google Scholar]
- Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, Lafont A (2006) Isolation of ‘side population’ progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol 26: 281–286 [DOI] [PubMed] [Google Scholar]
- Shantsila E, Watson T, Lip GY (2007) Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 49: 741–752 [DOI] [PubMed] [Google Scholar]
- Summers YJ, Heyworth CM, de Wynter EA, Hart CA, Chang J, Testa NG (2004) AC133+ G0 cells from cord blood show a high incidence of long-term culture-initiating cells and a capacity for more than 100 million-fold amplification of colony-forming cells in vitro. Stem Cells 22: 704–715 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438 [DOI] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T (1998) Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 9: 677–686 [DOI] [PubMed] [Google Scholar]
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T (2000) A role for hematopoietic stem cells in promoting angiogenesis. Cell 102: 199–209 [DOI] [PubMed] [Google Scholar]
- Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S (2005) Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res 97: 1142–1151 [DOI] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S (2006) Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133: 1543–1551 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Takakura N, Oike Y, Inada T, Gale NW, Yancopoulos GD, Suda T (2001) Stromal cells expressing ephrin-B2 promote the growth and sprouting of ephrin-B2(+) endothelial cells. Blood 98: 1028–1037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.