Abstract
Cell motility and adhesion involves dynamic microtubule (MT) acetylation/deacetylation, a process regulated by enzymes as HDAC6, a major cytoplasmic α-tubulin deacetylase. We identify G protein-coupled receptor kinase 2 (GRK2) as a key novel stimulator of HDAC6. GRK2, which levels inversely correlate with the extent of α-tubulin acetylation in epithelial cells and fibroblasts, directly associates with and phosphorylates HDAC6 to stimulate α-tubulin deacetylase activity. Remarkably, phosphorylation of GRK2 itself at S670 specifically potentiates its ability to regulate HDAC6. GRK2 and HDAC6 colocalize in the lamellipodia of migrating cells, leading to local tubulin deacetylation and enhanced motility. Consistently, cells expressing GRK2-K220R or GRK2-S670A mutants, unable to phosphorylate HDAC6, exhibit highly acetylated cortical MTs and display impaired migration and protrusive activity. Finally, we find that a balanced, GRK2/HDAC6-mediated regulation of tubulin acetylation differentially modulates the early and late stages of cellular spreading. This novel GRK2/HDAC6 functional interaction may have important implications in pathological contexts.
Keywords: GRK2, HDAC6, microtubules, migration, spreading
Introduction
Cell chemotaxis involves the projection of organelle-free extensions (termed pseudopodia or lamellipodia depending on the cell type) in the direction of the chemoattractant source. These extensions establish new adhesions to the substratum and create centripetal contractile tension, leading to the detachment and retraction of the cell tail, thus allowing the cell body to translocate forward (Kay et al, 2008; Berzat and Hall, 2010). In fibroblasts or epithelial cells, locomotion is initiated by chemoattractant binding to a variety of membrane sensors, as G protein-coupled receptors (GPCRs) or tyrosine-kinase receptors (Cotton and Claing, 2009) which trigger downstream signals responsible for polarized and stable cell protrusion during migration (Berzat and Hall, 2010). Such protrusive activity is mainly driven by enhanced actin polymerization adjacent to the leading edge membrane (Insall and Machesky, 2009), mediated by factors like Arp2/3, m-Dia2 or cofilin (Kay et al, 2008). In addition, microtubules (MTs) may also play a significant role in cell protrusion formation depending on the cell type and physiological context (Watanabe et al, 2005). MTs may be directly involved in the generation of a protrusive activity, counteracting the contractile action of the actin-myosin cortex (Levina et al, 2001), or stimulate cortical F-actin nucleation via local delivery to the MT plus-ends in protruding regions of several small G proteins GEFs (Fukata et al, 2002; Krendel et al, 2002; Nalbant et al, 2009). The MT network can also provide polarized routes for kinesin-dependent trafficking of intracellular vesicles, thus supporting membrane extension (Reed et al, 2006; Kay et al, 2008). Finally, dynamically growing MTs would favour focal adhesion (FA) disassembly and directional motility, whereas depolymerization or the presence of less-dynamic MTs would favour the formation of stress fibres and large FA, thus compromising migration (Kaverina et al, 1999; Wagner et al, 2002). Interestingly, MT dynamics is different in protruding and retracting regions of polarized, motile cells (Salaycik et al, 2005), strengthening an essential role for MT modulation in this process.
MT acetylation–deacetylation cycling at the amino-terminus of α-tubulin subunits has been suggested to play a prominent role in cell migration and adhesion (Zhang et al, 2003; Watanabe et al, 2005; Creppe et al, 2009), although the underlying mechanisms linking such events are controversial, with evidences in both favour and against tubulin acetylation modulating MT stability and dynamics (Hubbert et al, 2002; Matsuyama et al, 2002; Palazzo et al, 2003). Tubulin acetylation levels are finely regulated by the opposite action of acetyltransferases (Creppe et al, 2009) and of the major deacetylases SIRT2 and HDAC6 (Hubbert et al, 2002; North et al, 2003; Zhang et al, 2003). Increased chemotaxis is observed upon overexpression of HDAC6 in different cell types either in a deacetylase activity-dependent (fibroblast and epithelial tumour cells) or in an independent manner (lymphocytes) (Valenzuela-Fernandez et al, 2008). Conversely, inhibition of HDAC6 markedly enhances MT acetylation and decreases cell migration (Hubbert et al, 2002; Haggarty et al, 2003). Besides tubulin, HDAC6 triggers deacetylation of other substrates as diverse as cortactin, Hsp90 or β-catenin, and also interacts with a broad spectrum of signalling partners, which underlies its role not only in migration but also in other cellular processes as well (Boyault et al, 2007; Valenzuela-Fernandez et al, 2008). Despite its functional relevance, very little is known about the mechanisms that regulate HDAC6 functionality in the cell migration context.
GPCR stimulation can modulate tubulin polymerization by altering the functionality of different proteins that regulate the overall dynamics of MTs as a result of its binding to soluble and polymerized tubulin, association with the plus-ends of MTs (+TIPs proteins) or promotion of tubulin post-translational modifications (Westermann and Weber, 2003; Etienne-Manneville, 2010). Most GPCRs are regulated by GPCR kinases (GRKs), which phosphorylate agonist-occupied receptors, allowing the subsequent binding of β-arrestins, which in turn blocks G protein-dependent receptor signalling and promote receptor internalization (Moore et al, 2007). Besides such regulatory role, the ubiquitous GRK2 isoform has been shown to modulate a growing number of signalling sensors, switchers and effectors (some of them related to cell migration) in a phosphorylation-dependent or -independent way (Ribas et al, 2007; Penela et al, 2010). Consistently, changes in GRK2 expression and/or activity have been reported to alter chemotactic motility in a cell type-specific manner (Vroon et al, 2006; Penela et al, 2009). We have recently shown that GRK2 positively regulates integrin-dependent motility in epithelial cell types and fibroblasts (Penela et al, 2008). Such effect involves the GRK2-dependent modulation of the scaffold function of GIT-1 in the activation of the Rac/PAK/MEK/ERK1/2 pathway. The positive effects of GRK2 on cell motility seem to involve the promotion of F-actin remodelling at the cell periphery and FA turnover (Cant and Pitcher, 2005; Penela et al, 2008). Interestingly, GRK2 interacts with and phosphorylates β-tubulin subunits (Pitcher et al, 1998), but whether this kinase could also affect cell migration by means of the modulation of MT has not been investigated.
We report herein that GRK2 modulates MT acetylation in an HDAC6-dependent manner in order to regulate key cellular processes relying on cytoskeletal rearrangements such as migration, polarity and cell spreading.
Results
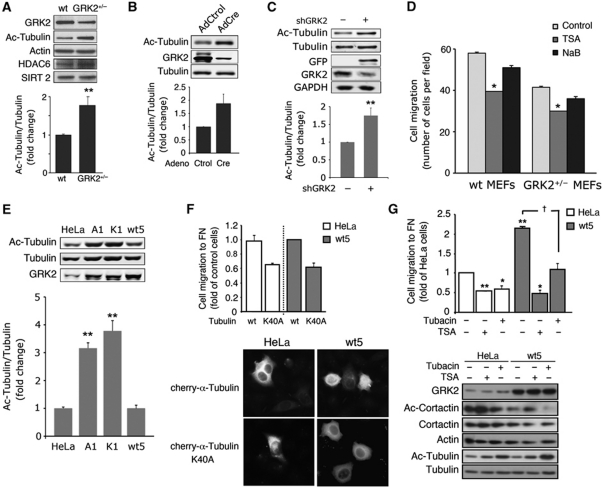
Effect of GRK2 expression levels on α-tubulin acetylation
As directional locomotion requires both the dynamic reorganization of MTs and proper regulation of tubulin acetylation in different cell types (Hubbert et al, 2002; Watanabe et al, 2005; Azuma et al, 2009), and GRK2 levels are critically involved in the modulation of the chemotactic migration of murine embryonic fibroblasts (MEFs) and epithelial cells (Penela et al, 2008), we decided to analyse the extent of tubulin acetylation in cells with different GRK2 expression levels. Decreased expression of GRK2 in MEFs derived from hemizygous GRK2 (+/−) mice (which have 40–50% less kinase protein as compared with wild-type (wt) animals) clearly enhanced (1.8-fold) tubulin acetylation compared with MEFs from wt animals (Figure 1A). Such effect was not accompanied by global changes in the expression of either HDAC6 or SIRT2 deacetylases. Moreover, either conditional ablation of the GRK2 gene in MEFs using Cre-Lox technology (Figure 1B) or GRK2 downregulation triggered by RNA interference in wt or GRK2+/− MEFs (Supplementary Figure S1A–C) or in HeLa cells (Figure 1C), lead to a higher accumulation of acetylated tubulin in parallel with the reduced motility caused by such decrease in GRK2 levels (Supplementary Figure S1B–E; Penela et al, 2008). Consistent with such inverse correlation between tubulin acetylation and cell migration, fibronectin (FN)-induced chemotaxis was reduced in +/−MEFs compared with wt (Figure 1D, control conditions) and treatment with the general HDAC inhibitor trichostatin A (TSA) (Hubbert et al, 2002; Matsuyama et al, 2002), but not with sodium butyrate (a compound that inhibits other HDAC family members but not HDAC6) inhibited migration of both +/− and wt MEFs (Figure 1D).
Figure 1.
GRK2 expression levels modulate the extent of tubulin acetylation in MEFs and HeLa cells in a kinase activity-dependent manner. (A–C) Downregulation of GRK2 expression enhances tubulin acetylation. MEFs derived from wt or hemizygous GRK2 mice (A), as well as from GRK2-floxed mice infected with control or Cre-recombinase expressing adenovirus (B) or HeLa cells transfected with either a control or a GRK2 silencing construct (C) were lysed and levels of HDAC6, SIRT2, GRK2, tubulin, acetylated tubulin (Ac-tubulin) and actin or GADPH (as loading controls) were determined by western blot analysis. Data of normalized Ac-tubulin levels are mean±s.e.m. from three independent experiments. (D) Motility of MEFs depends on GRK2 expression levels and HDAC6 activity. Cells as in (A) were seeded on Transwell filters precoated with FN (20 μg/ml) in the presence of HDAC inhibitors (TSA, NaB) or vehicle (control). Cell migration was assessed as detailed in Materials and methods. Data are mean±s.e.m. of three independent experiments performed in duplicate. (E) Ac-tubulin markedly accumulates in HeLa cells upon expression of catalytically inactive GRK2 or of the S670A mutant. Parental HeLa cells or cells stably expressing GRK2-wt (wt5), GRK2-S670A (A1) or GRK2-K220R (K1) mutants were analysed for GRK2 levels and the extent of tubulin acetylation determined as above. (F, G) Dynamic deacetylation/acetylation of α-tubulin is involved in the effect of GRK2 on cell migration. Parental and HeLa cells with extra GRK2 were co-transfected with cherry-α-tubulin-wt or cherry-α-tubulin-K40A and the CD-8 antigen (F) or treated (G) with either the general HDAC inhibitor TSA (1 mM) or the HDAC6-specific inhibitor tubacin (15 μM). Transfected tubulin constructs were expressed at similar levels as detected by confocal microscopy (F) and cells positive for co-transfected CD8 were sorted for migration assays by using microbeads precoated with anti-CD8 antibody. Chemotactic motility to FN was assessed as in (D). Data are mean±s.e.m. of three independent experiments performed in duplicate. Total and acetylated levels of α-tubulin and cortactin were measured by western blot (G). Total α-tubulin and actin serve as loading controls. Representative blots are shown in most panels. †P<0.05, *P<0.05, **P<0.01, compared with parental, control transfected or infected cells or with vehicle-treated cells, unpaired two-tailed t-test. Figure source data can be found in Supplementary data.
Notably, acetylated tubulin markedly accumulated in HeLa cells that stably overexpress either a catalytically inactive mutant of GRK2 (GRK2-K220R; HeLa-K1 cells) or a mutant at the S670 regulatory site (GRK2-S670A; HeLa-A1 cells) (Figure 1E). Such increased tubulin acetylation takes place in the absence of changes in HDAC6 protein expression (Supplementary Figure S1F) or in the extent of other tubulin post-translational modifications (Supplementary Figure S1G) and is coincidental with the impaired ability of HeLa-A1 to migrate towards both mechanical and chemotactic cues (reported in Penela et al, 2008). A similar trend was noted in wound-healing experiments. Increased expression of wt GRK2 enhanced wound-healing closure, whereas this process was blocked in HeLa-K1 cells (Supplementary Figure S1H), as also observed upon GRK2 silencing (Penela et al, 2008). In agreement with a dependency of GRK2-mediated enhanced motility on α-tubulin acetylation–deacetylation cycling, migration of both parental and HeLa-wt5 cells was clearly inhibited in the presence of α-tubulin-K40A mutant (Figure 1F), a construct that enforces permanent hypoacetylation of MTs (Creppe et al, 2009). Similarly, the presence of the general HDAC inhibitor TSA or the HDAC6-specific inhibitor tubacin counteracted the enhancing effect of GRK2 levels in cell motility (Figure 1G). Interestingly, although both TSA and tubacin cause hyperacetylation of α-tubulin, only TSA alters the acetylation state of cortactin (Figure 1G), which deacetylation is mandatory for actin binding and branching and also potentiates migration (Zhang et al, 2007; Kaluza et al, 2011). These data suggest that tubulin is the relevant target of HDAC6 underlying GRK2-induced migration. Further stressing this point, neither downregulation nor overexpression of wt or mutant GRK2 proteins promoted differences in deacetylation of endogenous or overexpressed cortactin (Supplementary Figure S2A and B). Interestingly, expression of extra cortactin-wt or cortactin-K9R (which mimics the deacetylation state) stimulated migration of HeLa cells, whereas they failed to increase further the higher motility of HeLa-wt5 cells (Supplementary Figure S2C and D). Moreover, migration of HeLa but not of HeLa-wt5 cells was inhibited in the presence of cortactin-K9Q (which mimics acetylation) (Supplementary Figure S2D), demonstrating that GRK2 regulates migration independently of the deacetylation status of cortactin. Overall, GRK2 seems to enhance cell migration by mechanisms involving specifically the control of the deacetylation extent of tubulin and not of other potential HDAC6 targets related to motility. Intriguingly, expression of extra wt GRK2 in HeLa-wt5 cells did not alter the steady-state levels of global tubulin acetylation, despite its chemotactic response and motility was clearly enhanced (see Penela et al, 2008 and Supplementary Figure S1H). This might reflect that either endogenous kinase is sufficient to maximally modulate tubulin deacetylation or localized increases in deacetylation elicited by an activated pool of GRK2 are involved in such effects.
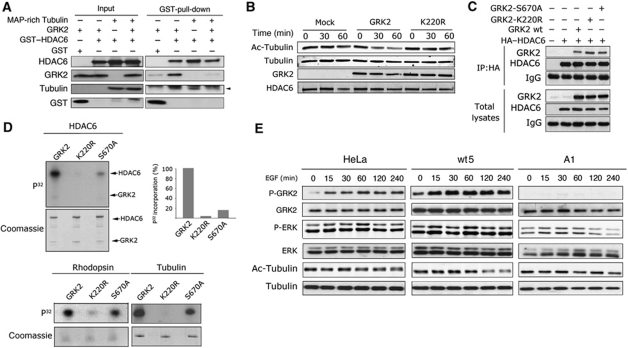
GRK2 associates with and phosphorylates HDAC6 to stimulate tubulin deactetylase activity
We next explored the potential functional interactions between GRK2 and HDAC6. A significant fraction of GRK2 co-immunoprecipitated with an HA-tagged construct of HDAC6 in cells transiently transfected with these proteins (Figure 2A). Furthermore, co-immunoprecipitation of endogenous HDAC6 and GRK2 was detected in cytoplasmic extracts from HeLa cells (Figure 2B), indicating a specific association of these proteins at steady-state physiological conditions. Moreover, such association does not require other protein intermediates as indicated by the direct binding of recombinant GRK2 and GST-HDAC6 proteins (Figure 2C). To identify the GRK2-binding region in HDAC6, a battery of HA-tagged HDAC6 truncated constructs was used (Figure 2D). Only deletion mutants containing at least one hdac catalytic domain (DD1 or DD2) appear to be able to co-immunoprecipitate with GRK2, although the more critical determinants of the interaction reside in the N-terminal half of the protein encompassing the first hdac domain, since its removal strongly reduced HDAC6/GRK2 association (Figure 2D).
Figure 2.
GRK2 associates with and phosphorylates HDAC6 to stimulate tubulin deactetylase activity. (A) HDAC6 co-immunoprecipitates with GRK2. HEK293 cells were transfected with GRK2 alone or together with HA-tagged HDAC6. Protein association was analysed by HA immunoprecipitation followed by immunobloting using anti-HA or anti-GRK2 antibodies. The same antibodies were used to check GRK2 and HDAC6 expression in cell lysates. (B) Association of endogenous HDAC6 and GRK2. Cytoplasmic extracts obtained from HeLa cells were incubated with anti-HDAC6 or IgG antibodies as indicated. Immunoprecipitates (IP) or total cell extracts (input) were analysed by western blot. (C) GRK2 can directly interact with HDAC6. Recombinant GRK2 was incubated with GST alone or with GST-HDAC6 fusion proteins. Proteins bound to Glutathione-sepharose beads were detected with specific anti-GRK2 and anti-GST antibodies. Binding experiments were performed four times with similar results. (D) Identification of the GRK2-binding region in HDAC6. HEK293 cells were transfected with the indicated HA–tagged HDAC6 constructs and co-immunoprecipitation assays performed as above. The expression of HDAC6 constructs or GRK2 in cell lysates was verified as above. Quantification of one of two independent co-immunoprecipitation experiments is shown. (E) HDAC6 is a GRK2 substrate. GRK2 (50 nM) and GST-HDAC6 (50–500 nM) were incubated in the presence of [γ-32P]-ATP as detailed in Materials and methods. The kinetic parameters of the reaction (Vmax and Km) were estimated by double-reciprocal plot analysis. Data are the mean from four independent experiments. (F) A region bearing the second deacetylase catalytic domain of HDAC6 is the main target of GRK2 phosphorylation. HEK293 cells were transfected with the indicated HA-tagged HDAC6 deletion mutants. HA immunoprecipitates were incubated under phosphorylation conditions with recombinant GRK2 (100 nM), followed by SDS–PAGE and autoradiography (upper panel). Overexpression of HDAC6 constructs was monitored by immunoblot. (G) Phosphorylation of HDAC6 by GRK2 enhances tubulin deacetylation. GST-HDAC6 was preincubated with GRK2 or vehicle under phosphorylation conditions, followed by addition of tubulin isolated from TSA-treated HeLa cells. Deacetylase activity was monitored for the indicated times with an anti-acetylated and anti-α-tubulin antibodies. Acetylated band densities were normalized to total tubulin values. A blot representative of two independent experiments is shown. Figure source data can be found in Supplementary data.
In-vitro kinase assays revealed that purified GST-HDAC6 was readily phosphorylated by recombinant GRK2 with an apparent Km of ∼45 nM (Figure 2E). The ability of GRK2 to phosphorylate HDAC6 but not HDAC5 (Martini et al, 2008) strongly suggested that such modification was specific. Notably, we found that a construct encompassing the second hdac domain and the C-terminal portion of HDAC6 (C+DD2) was phosphorylated by GRK2 as efficiently as the full-length protein (Figure 2F), whereas the truncated protein N+DD1 (able to efficiently interact with GRK2) was not. Overall, our data indicated that whereas several HDAC6 domains are involved in a multisite interaction with GRK2, the main phospho-acceptor site(s) is located in or near to the second catalytic domain of HDAC6. Interestingly, this domain is the only one that invariably shows impaired α-tubulin deacetylation activity when mutated (Kaluza et al, 2011 and references therein) and is the target of HDAC6 inhibitors specific for tubulin deacetylase (TDAC) activity (Haggarty et al, 2003). Therefore, we performed in-vitro deacetylation assays to test whether GRK2-mediated phosphorylation could alter HDAC6 activity. Preincubation with GRK2 clearly enhanced both the extent and kinetics of HDAC6-mediated α-tubulin deacetylation (Figure 2G), indicating that GRK2 was a direct positive modulator of HDAC6 activity.
Since both hdac domains of HDAC6 interact with GRK2, and the first one has been proposed to serve as a α-tubulin-anchoring domain, the possibility that GRK2 was favouring HDAC6 activity by acting as a scaffold protein could not be ruled out. However, binding of HDAC6 to polymerized MAP-enriched tubulin was not facilitated but rather decreased in the presence of GRK2, suggesting that a ternary complex of GRK2/HDAC6/tubulin might not be feasible (Figure 3A). Moreover, the kinase-deficient GRK2-K220R mutant (that would preserve scaffolding functions) did not stimulate the ability of GST-HDAC6 to deacetylate brain tubulin in vitro (Figure 3B), consistent with increased tubulin acetylation levels in cells expressing this mutant (see Figure 1F). Intriguingly, tubulin acetylation was also augmented in cells expressing GRK2-S670A, a mutant described to keep unaltered its catalytic function towards GPCR or tubulin (Pitcher et al, 1999). The inability of these mutants to modulate HDAC6 is not due to a binding defect, since the amount of GRK2-S670A or GRK2-K220R protein associated with HA-tagged HDAC6 was undistinguishable from that of wt GRK2 (Figure 3C). Likewise, association of tubulin with HDAC6 was not altered in the presence of GRK2-S670A or GRK2-K220R compared with wt protein (Supplementary Figure S2E) arguing against the possibility that such mutants could impair the potential bridging role of β-tubulin, with which GRK2 can interact (Pitcher et al, 1998), in such association (Zhang et al, 2003).
Figure 3.
Regulation of HDAC6 activity by GRK2 is strictly dependent on its kinase activity and is modulated by GRK2 phosphorylation status. (A) Competition between GRK2 and tubulin for HDAC6 association in vitro. Pull-down assays were performed as in Figure 2C in the presence or absence of purified tubulin. Free and proteins bound to the Glutathione-sepharose beads were immunodetected with specific antibodies. Gels are representative of three independent assays. (B) A catalytically inactive GRK2 mutant is unable to stimulate HDAC6-mediated deacetylation. GST-HDAC6 was preincubated with GRK2, GRK2-K220R or vehicle under phosphorylation conditions, followed by analysis of deacetylation activity as in Figure 2G. GRK2 and HDAC6 levels were monitored to confirm equal loading. (C) GRK2-K220R and GRK2-S670A mutants interact normally with HDAC6. HEK293 cells were co-transfected with HA-tagged HDAC6 in the presence or absence of GRK2-wt or the indicated mutants. GRK2/HADC6 interaction was analysed by co-immunoprecipitation as described in Figure 2A. (D) The GRK2-S670A mutant displays a markedly reduced ability to phosphorylate HDAC6, but not other GRK2 substrates. Phosphorylation of GST-HDAC6 (100 nM), rhodopsin (25 nM) or Tubulin (100 nM) was performed in the presence of [γ-32P]-ATP using recombinant GRK2-wt, GRK2-S670A or GRK2-K220R proteins as described in Materials and methods and Figure 2D. Intensity of 32P-bands was quantified by densitometry and plotted as percentage of wt GRK2-triggered 32P incorporation. Data representative of 2–3 independent experiments are shown. (E) Increased phosphorylation of GRK2 at S670 in response to chemotactic stimuli correlates with active deacetylation of α-tubulin. Parental and HeLa cells stably overexpressing GRK2-wt (wt5) or GRK2-S670A (A1) were challenged with EGF for the indicated times. Levels of acetylated α-tubulin, ERK1/2 activation and the phosphorylation status of GRK2 at S670 were analysed by using specific antibodies as detailed in Materials and methods. Gels are representative of three independent experiments. Figure source data can be found in Supplementary data.
Taken together, these data supported that regulation of HDAC6 activity by GRK2 was strictly dependent on its kinase activity. Remarkably, we observed that recombinant GRK2-S670A showed a markedly reduced ability to phosphorylate HDAC6 compared with wt GRK2, despite phosphorylation of other established GRK2 substrates was not significantly affected in this mutant (Figure 3D). These data indicated that phosphorylation of GRK2 at the S670 regulatory site acts as a key switch that specifically modulates its ability to phosphorylate HDAC6 and thus to affect its activity. Strengthening the significance of such regulatory event, we found that GRK2 is robustly phosphorylated at S670 in response to pro-migratory stimuli, and that such enhanced phosphorylation correlates with tubulin deacetylation (Figure 3E).
GRK2 activity towards HDAC6 and tubulin deacetylation promote efficient pseudopodia extension in response to chemotactic cues
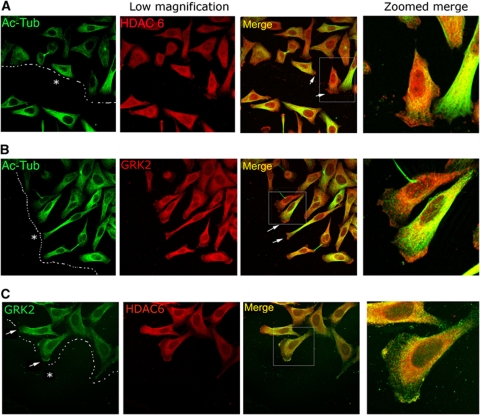
To determine the physiological implications of HDAC6 regulation by GRK2, we analysed their subcellular localization in HeLa-wt5 cells directionally migrating to close an in-vitro scratch. In such polarized cells, endogenous HDAC6 showed a broad cytoplasmic distribution, although a clear and reliable accumulation was noted in the leading edge (Figure 4A; Supplementary Figure S3A). Acetylated MTs displayed an asymmetric distribution, with an increased density towards the wound, but were excluded from the lamellipodium at the leading edge (zoomed images in Figure 4A and B, and Supplementary Figure S3A), in agreement with earlier reports (Hubbert et al, 2002; Salaycik et al, 2005; Gao et al, 2007). Such non-overlapping distribution at the cell border is consistent with a role for HDAC6-mediated deacetylation in motility and with an active tubulin deacetylation taking place at the dynamic leading edge. Remarkably, total and pS670-GRK2 were also enriched at the leading edge of migrating cells (Figure 4B and Supplementary Figure S3A and B), displaying a marked colocalization with HDAC6 in the lamellipodial region devoid of acetylated tubulin (Figure 4C; Supplementary Figure S3B). These results strongly suggested that GRK2 would interact with HDAC6 at the cell periphery to positively regulate its activity to promote local tubulin deacetylation in a GRK2 phosphorylation-dependent manner, which would help to maintain a gradient of MT instability that seems to be critical for migration (Salaycik et al, 2005; Siegrist and Doe, 2007; Zilberman et al, 2009). To further substantiate this point, we performed pseudopodia purification assays, which allow the isolation of both lamellipodia and adjacent lamellae structures (Cho and Klemke, 2002), in HeLa cells stably expressing either extra wt GRK2 or mutant GRK2-S670A and GRK2-K220R proteins. In response to serum, both parental and HeLa-wt5 cells extended pseudopodia through 3.0 μm porous membranes as determined by protein recovery on the underside of the membrane (Figure 5A). Such parameter was clearly impaired in cells expressing GRK2 mutants kinase defective towards HDAC6 (pseudopodial protein content 0.29-fold and 0.27-fold in HeLa-A1 and -K1 cells, respectively, compared with parental cells), consistent with inefficient protruding activity and defective locomotion in these cell lines (Supplementary Figure S1H; Penela et al, 2008).
Figure 4.
HDAC6 and GRK2 colocalize in the leading edge of migrating cells. HeLa cells stably expressing GRK2-wt were plated in FN (10 μg/ml)-coated dishes and scratched to promote wound healing as indicated in Materials and methods. After 16 h of migration, cells were fixed and potential colocalization of acetylated α-Tubulin with HDAC6 (A) or GRK2 (B) and of HDAC6 with GRK2 (C) was determined by confocal microscopy upon staining with specific antibodies. Arrows indicate the leading edge of migrating cells and dotted lines the margin and direction of the wound. Asterisk denotes wounded area.
Figure 5.
GRK2-stimulated HDAC activity is relevant for pseudopodia formation in response to chemotactic cues. (A) Expression of GRK2 mutants unable to phosphorylate HDAC6 inhibits pseudopodia formation. Parental, wt5, A1 or K1 HeLa cells were serum starved for 16 h and subjected to transwell migration assays as detailed in Materials and methods and in the absence or presence of serum in the bottom chamber. Levels of pseudopodia protein recovered on the underside of porous filters were analysed using the Bradford method. Data are mean±s.e.m. of three independent experiments. (B, C) Accumulation of both HDAC6 and GRK2 phosphorylated at S670 at pseudopodia correlates with local deacetylation of tubulin. Cells as in (A) were allowed to migrate in the absence or presence of a serum gradient, and 2 h later purified pseudopodia were collected and the levels of α-Tubulin acetylation (B) or of GRK2, its phosphorylation at S670 and HDAC6 (C) were determined by immunoblot. Data in (B) are mean±s.e.m. from 3 to 4 experiments. Representative blots are shown. *P<0.05, **P<0.01, compared with control, untreated HeLa cells; †P<0.05, ††P<0.01 compared with serum-stimulated HeLa cells, unpaired two-tailed t-test. Figure source data can be found in Supplementary data.
We also observed that serum-induced pseudopodia extension was accompanied by local tubulin deacetylation in cells that expresses either endogenous GRK2 or extra wt protein, while relative acetylation levels remained unaltered in HeLa-A1 and -K1 cells lamellipodia (Figure 5B). In addition, a local increase in HDAC6 and GRK2 protein levels was detected biochemically in the cortical edge of parental and HeLa-wt5 cells but not in HeLa-A1 or -K1 cells (Figure 5C). Interestingly, a marked upregulation of GRK2 phosphorylation at S670 was specifically noted in parallel in the pseudopodia of the former cells (Figure 5C), probably driven by the enhanced ERK activity in the protruding membrane (data not shown). As we have found that a GRK2 protein unable to be phosphorylated at S670 does not efficiently phosphorylate HDAC6, our data strongly suggest that dynamic GRK2 post-translational modification at this residue would take place at the leading edge and thus favour the kinase activity of GRK2 towards its colocalized substrate HDAC6.
GRK2 regulates cell adhesion and cellular spreading by promoting tubulin deacetylation
We next investigated the potential involvement of GRK2-mediated HDAC6 modulation in a distinct cellular process affected by changes in MT dynamics. Cell spreading is a multiphase process in which spherical cells in suspension initially contact with the extracellular matrix (phase P0), rapidly increase the contact area with a continuous membrane protrusion (phase P1) and finally undergo periodic contractions and FA stabilization (phase P2), leading to a maximal spread area before cells become polarized and adopt a final morphology (Dubin-Thaler et al, 2008). The implication of MTs and MT acetylation in cell spreading has been established in different cell types (Rhee et al, 2007; Tran et al, 2007).
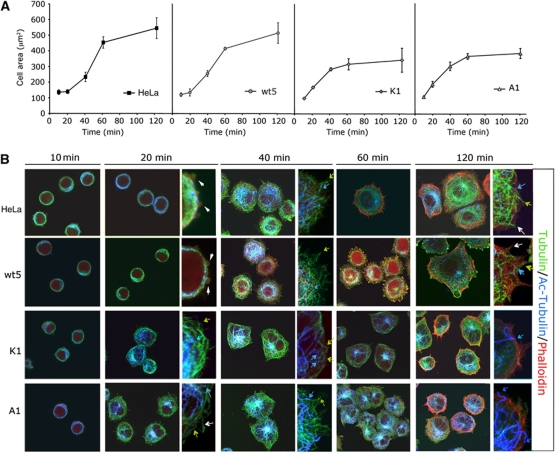
We monitored by confocal microscopy the isotropic spreading of HeLa cells with either silenced, endogenous or extra wt GRK2 or mutant proteins S670A and K220R on FN-coated surfaces. The pattern and kinetics of spreading of parental and HeLa-wt5 cells was similar to that reported for HeLa and other adherent cells (Cuvelier et al, 2007; Dubin-Thaler et al, 2008). Interestingly, downregulation of GRK2 or expression of either GRK2-S670A or GRK2-K220R notably altered such normal spreading pattern. The initial phase of cell spreading proceeded in an exponential way, but despite such initial enhanced spreading, shGRK2, A1 and K1 cells did not attain a larger final cellular area (Figure 6A and Supplementary Figure S4A, see detailed description in Supplementary data). HeLa-A1 and -K1 cells spreading for 60 min on FN display a low number of (aberrant) FAs as compared with HeLa or wt GRK2 cells (Supplementary Figure S5A; Supplementary data), suggesting that this defect in adhesion could compromise the maintenance of a fully spread area at these later stages, when cell expansion becomes mostly dependent on FA formation and substrate traction forces (Dubin-Thaler et al, 2008).
Figure 6.
Expression of GRK2 mutants defective in HDAC6 regulation results in an altered cell spreading pattern. (A, B) Parental, wt5, A1 or K1 HeLa cells were plated on coverslips coated with FN (10 μg/ml), fixed at the indicated times and analysed by confocal microscopy. The spreading area was quantified by morphometric analysis (A) and cells were triple stained (B) for acetylated α-Tubulin (blue), α-Tubulin (green) and F-actin (Phalloidin, red) as described in Materials and methods. Zoomed images are shown at 20, 40 and 120 min of spreading. Blue, green and white arrows and white arrowheads indicate acetylated MTs, non-acetylated MTs, pioneer MTs, and blebs, respectively.
We also investigated the qualitative features of cell spreading in the aforementioned cells by analysing the organization of the actin and tubulin cytoskeleton. Interestingly, the organization of the MT and actin cytoskeleton was quite different in cells expressing extra GRK2-S670A or K220R (Figure 6B) or upon GRK2 downregulation (Supplementary Figure S4B). Expansion of the MT network initiated earlier in these cells, with a higher proportion of acetylated MTs compared with controls. After 20 min of plating onto FN, a well-organized net of MTs was formed around the nucleus, with longer filaments lining the edge of the cell and covering the more extended area that the shGRK2, K1 and A1 cells occupy at this time point (compare the surface of the different cells at 20 min of spreading), while such level of organization is not noted until much more later in HeLa cells, suggesting that modulation of HDAC6-mediated MT acetylation by GRK2 regulates the early phase of cell spreading. On the other hand, at later times there were markedly less central stress fibres and actin transverse arcs in shGRK2, A1 and K1 lines compared with control, while F-actin at the cortical border and filopodia was detected as in HeLa cells (compare panels at 120 min in Figure 6B and Supplementary Figures S4B and S5B). Such failure to develop F-actin bundles correlates with the phase of impaired spreading in HeLa-shGRK2, A1 and K1 cells, pointing again to defects in adhesion/contractility.
Interestingly, many MTs were found into the newly formed membrane protrusions at the cell periphery of the different stable HeLa cells (Figure 6B, inserts), resembling the highly dynamic pioneer MTs that are extended towards the lamellipodium in motile cells (Salaycik et al, 2005). We observed that such leading MTs are less acetylated at their distal than at their proximal ends (see insert images in Figure 6B) during both early and late spreading of parental and HeLa-wt5 cells, but only at early spreading in HeLa-A1 and -K1 cells. Indeed, 2 h after plating onto FN most MTs are acetylated in these cells, even those that reach the cellular edge (Figure 6B, inserts). A similar trend is noted in HeLa-shGRK2 cells (Supplementary Figure S4B). Since wt, GRK2-S670A or K220R is colocalizing with cortical F-actin during spreading (Supplementary Figure S6), but tubulin deacetylation is only detected at later spreading times in cells expressing wt GRK2 (Figure 6B), maintenance of deacetylated MTs in the edge of spread cells at this stage seems to be dependent on the recruitment to this region of GRK2 catalytically competent towards HDAC6.
To further analyse the modulatory role of GRK2 in cell spreading, real-time resistance measurements were performed on these cells spread over a FN-coated gold electrode sensor plate using the XCELLigence system (Roche Applied Science). Cellular impedance was continuously recorded and converted to a cell index (CI) that allows for the assessment of attached cells on the electrodes (see Supplementary data). This analysis confirmed that both HeLa-A1 and -K1 cells spread more rapidly than HeLa-wt5 control cells (Supplementary Figure S7A). Consistently, the period of time required to achieve steady-state CI values was significantly lower in these cells (Figure 7A). Downregulation of GRK2 levels in both HeLa-shGRK2 and Cre-infected GRK2-floxed MEFs also promoted enhanced cell spreading (Figure 7B; Supplementary Figure S7B and C). Noteworthy, the spreading time of both HeLa-wt5 and control cells can be similarly lowered upon treatment with an HDAC6 inhibitor (Figure 7A). These results indicate that either pharmacological inhibition of HDAC6 or functional downregulation of the positive HDAC6 modulator GRK2 might accelerate the rate of spreading by increasing the global acetylation of tubulin. Indeed, global levels of acetylated tubulin underwent a modest increase within the first 20–30 min of spreading in parental and HeLa-wt5 cells and remained stable thereafter. As expected, Hela-A1 and -K1 cells accumulated much more acetylated tubulin before substrate attachment and during spreading in comparison with control cells (Figure 7C). Remarkably, the burst in the extent of tubulin acetylation in HeLa-A1 and -K1 cells was paralleled by a lack of GRK2 phosphorylation at S670, while in HeLa-wt5 and control cells such modification increased up to 60 min of spreading (Figure 7D). This event could propitiate the stimulation of HDAC6 activity and help to keep a balanced acetylation of tubulin during early spreading of cells.
Figure 7.
Enhanced tubulin deacetylation caused by GRK2-mediated HDAC6 phosphorylation modulates cell spreading kinetics and motility. (A) Impairment of GRK2-mediated HDAC6 phosphorylation or pharmacological inhibition of HDAC6 accelerates spreading. The spreading kinetics of parental and HeLa-wt5 pretreated or not with TSA (1 mM) or HeLa-A1 or -K1 cells was analysed using the XCELLigence system as detailed in Materials and methods. (B) Gene-targeted inactivation of GRK2 increases the rate of fibroblast spreading. Primary MEFs derived from GRK2-floxed mice were infected with control or Cre-recombinase expressing adenovirus and their spreading was analysed as above. Total time needed to achieve a maximum cell index during spreading and the extent of tubulin acetylation at this stage was determined for each cell line. Data are mean±s.e.m. of three independent experiments. *P<0.05, **P<0.01, compared with HeLa parental cells or control infected MEFs, unpaired two-tailed t-test. (C) Both the extent and time course of tubulin acetylation during cellular spreading are altered in the presence of GRK2 mutants defective in HDAC6 phosphorylation. Parental and HeLa-wt5, -A1 and -K1 cells were kept in suspension for 2 h and then allowed to adhere and spread into FN-coated plates for the indicated times. Acetylated α-tubulin and total α-tubulin levels were immunodetected with specific antibodies. A representative blot and quantification of tubulin acetylation are shown. (D) Levels of GRK2-pS670 are differentially regulated during spreading and inversely correlate with the spreading rate. Cells were serum starved and collected after kept in suspension (S) or allowed to adhere and spread for 1 h (A) onto FN-coated plates. The extent of GRK2 phosphorylation at S670 and total GRK2 levels were analysed by western blot. A representative blot from two independent experiments is shown. (E) HDAC6-induced migration requires phosphorylation of C-terminal residues on HDCA6 by GRK2. HeLa cells were co-transfected with the CD-8 antigen in the presence of HDAC6-wt or HDAC6-S1060,1062A or S1060,1062,1069A mutants and sorted using microbeads precoated with anti-CD8 antibody. Cell migration was assessed as detailed in Materials and methods. *P<0.05, compared with HDAC6-wt transfected cells (unpaired two-tailed t-test). (F) Expression of a HDAC6 mutant defective in GRK2 phosphorylation accelerates cell spreading kinetics. HeLa cells transfected with GFP-HDAC6-wt or mutant GFP-HDAC6-S1060,1062,1069A were plated on coverslips coated with FN (10 μg/ml), fixed at the indicated times and analysed by confocal microscopy. The spreading area of GFP positive (green labelled) and negative cells was quantified by morphometric analysis and cells were double stained for acetylated α-Tubulin (blue) and F-actin (Phalloidin, red) as in Figure 6. Plotted data are mean±s.e.m. from 10 to 30 cells for each time point and cellular condition. Figure source data can be found in Supplementary data.
Overall, our data indicated that GRK2-mediated phosphorylation of HDAC6 enhanced its deacetylase activity towards tubulin and lead to changes in cell migration and spreading patterns. To further support this notion, we set to identify the site(s) of HDAC6 phosphorylation by GRK2 using a battery of single, double or triple mutations to alanine to target potential serine/threonine residues within the second half of HDAC6, considering that GRK2 prefers acidic amino acids N-terminal to the phosphorylated residue and hydrophilic residues at P+1. In-vitro phosphorylation assays revealed serines 1060, 1062 and 1069 as important phospho-acceptor sites for GRK2 (Supplementary Figure S8A). Cellular assays using these mutants showed that phosphorylation of HDAC6 at these residues is necessary for full TDAC activity (Supplementary Figure S8B). Moreover, expression of such phosphorylation-deficient mutants failed to mimic the enhanced cell migration promoted by wt HDAC6, similarly to what is noted with the catalytically inefficient HDAC6-DD (Figure 7E), and promoted a cell spreading pattern (Figure 7F) similarly to that observed in the presence of the GRK2 mutants unable to phosphorylate HDAC6 (K220R, S670A) or upon GRK2 downregulation.
Discussion
We describe a novel functional interaction between GRK2 and HDAC6 that plays a key role in the dynamic modulation of MTs taking place during oriented migration of fibroblasts and epithelial cells and in cell spreading. HDAC6 is increasingly being characterized as a relevant molecular sensor and effector that modulates diverse cellular responses in ways either dependent or independent of its catalytic activity (Boyault et al, 2007; Valenzuela-Fernandez et al, 2008). Highly dynamic MTs are present at specific sites of the cell cortex of motile cells, thereby reinforcing cell polarization and enabling directional migration. MT acetylation promotes the interaction of molecular motors with MTs (Reed et al, 2006) and the inhibition of MT dynamics (Westermann and Weber, 2003; Tran et al, 2007; Zilberman et al, 2009). Consistently, HDAC6 upregulation and decreased tubulin acetylation enhance the motility of different cell types including fibroblasts (Hubbert et al, 2002) and breast cancer cells (Saji et al, 2005; Azuma et al, 2009).
Several lines of evidence support GRK2 as a new endogenous stimulator of HDAC6 TDAC activity in motile cells. First, GRK2 and HDAC6 can be found in the same protein complex in cells, directly interact in vitro, are colocalized in the leading front of polarized, motile wound-edge cells, and both proteins are specifically co-recruited to chemoattractant-induced pseudopodia. Second, recombinant GRK2 protein stimulates the TDAC activity of HDAC6 in vitro, in a process involving direct phosphorylation of HDAC6, since neither a catalytically inactive protein (GRK2-K220R) nor a GRK2 mutant that specifically fails to phosphorylate HDAC6 (GRK2-S670A) can promote this effect. Third, downregulation of GRK2 levels in HeLa cells and primary fibroblasts or functional silencing of endogenous GRK2 by means of overexpression of such kinase mutants with impaired activity towards HDAC6 markedly increase the extent of whole-cell acetylated tubulin. Moreover, the extent of integrin- or serum-directed cell migration as well as pseudopodia extension positively correlates with enhanced total and pS670-GRK2 levels whereas these processes are strongly inhibited in the presence of the GRK2-K220R or GRK2-S670A mutants. Noteworthy, MTs present in the leading pseudopodia of motile cells that express endogenous or extra wt GRK2 show low levels of α-tubulin acetylation, whereas cortical MTs are highly acetylated in K220R or S670A-expressing cells. Such cells seem to be unable to establish or maintain polarized, stable protrusions as evidenced by lack of persistent lamellae towards the wound edge (Supplementary Figure S9). Consistently, disturbance of tubulin acetylation/deacetylation gradients by expression of a non-acetylated dominant-negative form of tubulin (tubulin-K40A) also abrogates GRK2-induced migration as previously described in other cells types (Creppe et al, 2009), thereby suggesting that dynamic and localized tubulin deacetylation events underlie the effect of GRK2 and HDAC6 phosphorylation in cell motility. Further stressing the relevance of HDAC6 phosphorylation by GRK2 in such processes, expression of HDAC6 mutants with impaired phosphorylation by GRK2 failed to mimic the enhanced chemotactic motility promoted by wt HDAC6 in HeLa cells, similarly to the effect of a tubulin-deacetylase inactive mutant (HDAC6DD).
Modulation of HDAC6 TDAC activity has been reported as a result of phosphorylation events either within or out of the second catalytic domain (DD2) (Deribe et al, 2009; Watabe and Nakaki, 2011). The novel GRK2-phosphorylated sites localize in the region between DD2 and the ubiquitin binding domain of HDAC6, suggesting an indirect regulation of the catalytic activity through allosteric conformational changes or altered responsiveness to HDAC6 activator/inhibitors. Independently of the mechanisms involved, GRK2 seems to specifically stimulate HDCA6 deacetylase activity towards defined substrates, since cortactin acetylation is not altered by GRK2 levels. Consistently, tubacin, a specific inhibitor of HDAC6-triggered tubulin deacetylation, counteracts the effect of GRK2 in migration in the absence of changes in cortactin deacetylation.
Our results suggest that chemoattractant-induced translocation of GRK2 to the plasma membrane (Penela et al, 2008) might help to recruit HDAC6 to the lamellipodium and that phosphorylation of HDAC6 by GRK2 at such specific location would allow full stimulation of its activity thus enhancing local tubulin deacetylation. Importantly, we also uncover that the modulatory effect of GRK2 on HDAC6 can be dynamically regulated. The marked inability of GRK2-S670A to phosphorylate HDAC6 but not other substrates constitutes the first evidence that phosphorylation at S670 would confer a novel layer of GRK2 regulation by switching its substrate repertoire. As phosphorylation of GRK2 at S670 is rapidly upregulated by EGF and other chemotactic cues (Penela et al, 2008) and we find it specifically increased in pseudopodia of motile cells, such modification might be instrumental in enhancing localized phosphorylation of HDAC6 in situ.
We propose that GRK2-triggered, HDAC6-mediated dynamic deacetylation of tubulin at the plus-ends of MTs would be important for maintaining the cortical polarization underlying pseudopodia extension and directed migration (Figure 8A). Deacetylation might favour the dynamic anchoring of pioneering MTs to the cell cortex, propitiating actin polymerization through local recruitment of different Rac activators, such as IQGAP1 via the +TIP protein CLIP-170 (Fukata et al, 2002). It is also possible that GRK2-mediated modulation of HDAC6 activity may trigger the deacetylation of substrates other than tubulin also related to migration, such as Hsp90 (Gao et al, 2007). Alternatively, scaffolding functions of HDAC6 could be altered by GRK2. In this regard, changes in both expression and deacetylase activity of HDAC6 have been shown to affect localization of +TIP proteins and MT regulatory functions (Zilberman et al, 2009), and HDAC6 interacts with EB1 and p150glued, which are key in MT nucleation and growth (Valenzuela-Fernandez et al, 2008; Conde and Caceres, 2009; Zilberman et al, 2009), leading to cell polarization and migration (Li et al, 2011). Whether GRK2 can alter such potential alternative HDAC6 activities remains to be addressed in future studies.
Figure 8.
Models depicting the intertwinement of GRK2 and HDAC6-mediated tubulin deacetylation in directed cell motility and cellular spreading. (A) Chemotactic movement of cells involves the projection of a dominant cell protrusion in the direction of the chemoattractant source, as a result of localized actin polymerization and the establishment of new adhesions to the substratum. The leading edge is dominated by interrelated structures such as lamella and the organelle-free lamellipodia, which are characterized by different actin and MT networks and distinct extent of focal adhesion (FA) maturation. Association of actin bundles with adhesion sites creates centripetal contractile tension that leads to detachment and retraction of the cell at the rear edge, allowing cell body translocation forward. An increased gradient of MT acetylation is present from the rear to the lamella. This would support cell polarity by facilitating the targeting and dissolution of FA at the rear edge and the delivery of regulatory and structural components to the leading edge. In the lamellipodium, GRK2 would be recruited in a Gβγ-dependent manner to sites of the plasma membrane wherein chemotactic activation is taking place. At such specific locations, chemokine receptor stimulation would promote the phosphorylation of GRK2 at S670 by MAPK, which would in turn switch on the ability of GRK2 to phosphorylate colocalized HDAC6. Phosphorylated HDAC6 would display a higher deacetylase activity towards tubulin at such location, contributing to keep down MT acetylation specifically at the lamellipodium. The presence of highly dynamic, hypoacetylated MTs would stimulate cortical F-actin polymerization by helping to recruit at their plus-ends different small G proteins-GEF activities (that are directly recruited by tubulin or indirectly by the microtubule-interacting +TIP proteins). (B) In the early phase of spreading, hyperacetylation of MTs would increase the rate of spreading as a result of ‘pushing forces’ generated by the sustained growth of stable MT that extend the membrane forward and facilitate the trafficking processes that drive protein cargo to the cell periphery and bring back membrane rafts that were endocytosed during the non-attached, rounded-state of cells before spreading. The extent of bulk MT acetylation would be counterbalanced by the action of HDAC6 in a GRK2-dependent manner. At this stage, local assembly of actin filaments occurs rapidly at the leading membrane edge as integrin contacts with substratum are taking place. MTs entering into this region seem to play a role akin to that of MTs in lamellipodium, displaying lower acetylation levels even in the absence of HDAC6 regulation by GRK2. At later spreading phases, the process turns to rely on FA assembly and actin stress fibres that connect FAs to generate myosin II-dependent traction forces on the substratum. At this phase, hyperacetylation of MTs increases the spreading area by means of stabilization of FA and enhancement of actomyosin contractility, while deacetylated MTs in the lamellipodium contribute to membrane protrusion. In late spreading, the extent of tubulin acetylation of both cortical and non-cortical MTs is determined by the functional interaction of GRK2 with HDAC6.
It has been shown that extrinsic polarity cues derived from GPCR activation can translocate GRK2 to the plasma membrane (Penela et al, 2009), while pioneering MTs act as cell-intrinsic components of the polarization machinery of the cell (Siegrist and Doe, 2007). Therefore, it is tempting to speculate that GRK2, through regulation of tubulin-deacetylase activity and/or localization of HDAC6 to the leading edge, acts as a signalling node to engage and coordinate both extrinsic and intrinsic pathways relevant in controlling polarity.
GRK2 regulation of MT acetylation might also affect motility by modulating cellular adhesion. Targeting of dynamic, hypoacetylated MTs to FA promotes their disassembly, which lessens the strength of cellular adhesion and increases motility (Kaverina et al, 1999). Consistently, a higher turnover of FA has been found in cells upon GRK2 upregulation (Penela et al, 2008), which would reduce acetylation. Strikingly, cells expressing GRK2-K220R or GRK2-S670A proteins, unable to stimulate HDAC6 and thus displaying MT hyperacetylation, show a retracted morphology with reduced actin stress fibres and aberrant FA rather than the expected FA stabilization. Thus, these mutants must be interfering with the activity of other factors engaged in FA assembly/disassembly (Penela et al, 2008), resulting in impaired cell migration.
Our data uncover as well that GRK2 may modulate the rate of cell spreading by regulating tubulin acetylation. Furthermore, our results also suggest that the mechanisms by which MT acetylation impacts such process are more complex than previously anticipated (Figure 8B). Our data support the notion that in the initial P1 phase, which progresses in the absence of stress fibres and mature FA (Dubin-Thaler et al, 2008) acetylated MTs would stimulate membrane protrusion and area growth in an adhesion-independent way. During such continuous spreading, protrusive forces are generated by the formation of F-actin and exocytosis at the plasma membrane, in a way resembling lamellipodium extension (Kay et al, 2008). Hyperacetylation of MTs underneath the cell periphery would improve the delivery of regulatory and structural surface components in a kinesin-dependent manner (Reed et al, 2006), thus enabling membrane extension. Consistent with this model, global tubulin acetylation levels increase at this early stage in HeLa cells, and hyperacetylation in those expressing GRK2-K220R or GRK2-S670A, HDAC6 mutants with impaired phosphorylation by GRK2, or in the absence of GRK2 expression results in enhanced rate of area growth during early spreading, in line with data showing that loss of HDAC6 activity increases the rate of the initial phase of cell spreading (Tran et al, 2007). After early spreading, actomyosin contraction is activated and cell extension starts to proceed with actin filaments pulling integrins and assembling new adhesions. At this adhesion-dependent stage, acetylated MTs would help to promote an increase in cellular area by inhibiting turnover of FAs (Tran et al, 2007). Despite higher levels of tubulin acetylation, this process is neither efficient in K1 or A1 cells due to their intrinsic defects in FA dynamics, nor is in HeLa-shGRK2 cells or conditional GRK2 null MEFs (see above). Finally, at later spreading times, an active process of tubulin deacetylation seems to take place at the cell edge, in a process that requires the presence of GRK2 catalytically competent towards HDAC6, since it is not detected in the A1 or K1 lines or in GRK2-depleted cells, and that may help to attain full surface growth. Therefore, balanced tubulin acetylation might serve cell extension and adhesion by different independent, overlapping mechanisms, which particular contribution would vary as cell transits through different phases of the spreading process (Rhee et al, 2007; Dubin-Thaler et al, 2008).
It is worth noting that HDAC6 activity has been associated with malignant transformation and invasive motility in breast cancer (Azuma et al, 2009). Moreover, both HDAC6 expression and tubulin deacetylation are required for a complete TGFβ-induced epithelial–mesenchymal transition in a lung adenocarcinoma cell line (Shan et al, 2008), and reduced tubulin acetylation was associated with malignant breast cancer progression (Suzuki et al, 2009). Conversely, inactivation of HDAC6 confers cell resistance to oncogenic transformation and tumour formation (Lee et al, 2008). Notably, GRK2 is upregulated in different malignant mammary cell lines compared with normal cells (Salcedo et al, 2006), and enhanced GRK2 levels not only increase epithelial cell motility upon integrin and GPCR engagement (Penela et al, 2008) but also lead to reduced DNA damage-induced p53 responsiveness (Penela et al, 2010). In the light of the results reported herein, it is tempting to suggest that GRK2 upregulation in human tumour malignancies by increasing the deacetylase activity of HDAC6 towards α-tubulin (and perhaps towards other substrates or scaffolding functions) would favour aberrant cell motility, adhesion and transformation The potential role of this novel GRK2/HDAC6 interaction in cancer via the modulation of cellular processes related to cellular growth, motility or stress surveillance opens an interesting field for future research.
Materials and methods
Cell culture and treatments
HeLa and HEK-293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) at 37°C in a humidified 5% CO2 atmosphere. HeLa cell lines stably overexpressing GRK2-wt (HeLa-wt5) or mutant constructs GRK2-K220R (HeLa-K1) and GRK2-S670A (HeLa-A1) were previously described (Penela et al, 2008). Mouse embryonic wt, GRK2-flox/flox and GRK2 (+/−) fibroblasts (MEFs) were obtained as described (Penela et al, 2008). HeLa or HEK-293 cells (70–80% confluent monolayers in 60 or 100 mm dishes) were transiently transfected with the chosen combinations of cDNA or shRNAi constructs using the Lipofectamine/Plus method, following the manufacturer's instructions, and, when desired, co-transfected with cDNA encoding the CD8 antigen for subsequent cell selection by using polystyrene microbeads precoated with anti-CD8 antibody (Dynabeads M450, Dynal Biotech, Oslo, Norway). The cells were serum starved for 12–16 h and stimulated either with immobilized FN in serum-free DMEM (20 μg/ml of FN for transwell migration assays and 10 μg/ml for motility scratch assays) or with 10% FBS (pseudopodia purification experiments). When indicated, cells were treated with the histone deacetylase inhibitors TSA (0.4 mM), tubacin (15 μM) or NaB (0.4 mM).
TDAC assay
TDAC assays were developed as described (Hubbert et al, 2002). Briefly, recombinant HDAC6 protein (12.5 nM) was incubated in the presence or absence of GRK2-wt or GRK2-K220R (25 nM) for 30 min at 30°C in kinase buffer (10 μl). Then, deacetylase activity of both phosphorylated and unphosphorylated HDAC6 proteins was assessed towards either 1 μg of α/β tubulin dimers (Cytoskeleton, Inc.) or immunoprecipitated tubulin from TSA-treated HeLa cells in 100 μl of deacetylation buffer (10 mM Tris–HCl pH 8, 10 mM NaCl). Reactions were allowed to proceed at 37°C for the indicated times and then stopped on ice for 10 min. Levels of whole and acetylated α-tubulin were analysed by western blot.
Cellular adhesion and spreading assays
Cells were detached and kept in suspension on 150 mm Petri dishes precoated with 1% BSA (lipid free) for 2 h in serum-free medium. Cells were then either immediately lysed (cells in suspension (S)) or allowed to adhere and spread for the indicated time periods to culture dishes coated with 10 μg/ml FN, followed by lysis in RIPA buffer to quantitate by immunoblot analysis the expression levels of GRK2 and tubulin and their extent of phosphorylation and acetylation, respectively.
To monitor the process of spreading in live cells at real time, we measured in parallel changes in cellular electrical impedance using the xCELLigence system (Roche Applied Science) as detailed in Supplementary data.
Pseudopodia purification
Purification of pseudopodia was assessed as described (Matsuyama et al, 2002). Briefly, 1–1.5 × 106 cells were serum starved for 16 h and then seeded onto 1 μg/ml of FN-coated 24 mm Transwell filters with 3.0 μm pores (Costar) in the presence of serum-free DMEM media in both upper and bottom chambers. After 2 h, cells were induced or not to form pseudopodia for 1 h by adding 10% FBS or vehicle (control), respectively, to the bottom chamber. Cells were rinsed in excess cold PBS and rapidly fixed in 100% ice-cold methanol. Cell bodies on the upper side of the membrane filter were scraped into PBS, and lysed with RIPA buffer. Remaining debris on the upper membrane were manually removed with a cotton swab and pseudopodia on the under surface scrapped into loading buffer for immunoblot analysis.
Other detailed methods are available in the Supplementary data.
Supplementary Material
Acknowledgments
Our laboratory is funded by grants from Ministerio de Educación y Ciencia (SAF2008-00552), Fundación Ramón Areces, The Cardiovascular Network (RECAVA) of Ministerio Sanidad y Consumo-Instituto Carlos III (RD06-0014/0037), Comunidad de Madrid (S-SAL-0159-2006) to FM and Comunidad de Madrid and Universidad Autónoma de Madrid (CCG08-UAM/BIO-4452) to PP. We thank Drs J Tesmer, I Dikic, S Khochbin, T Nakaki, E Seto, F Saudou, and WJ Koch for the indicated reagents and tools; Dr MT Berciano for key advice in confocal microscopy experiments; Dr F Sánchez Madrid for tools and critical reading of the manuscript; and Dr A Ruiz-Gomez and S Rojo and P Ramos for recombinant GRK2 and helpful technical assistance, respectively.
Author contributions: VL planned and performed most of the experimental work and participated in writing the manuscript. IA characterized the relationship between cellular GRK2 levels and tubulin acetylation. OT performed some of the immunofluorescence experiments. FM and PP coordinated the project, planned experiments and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Azuma K, Urano T, Horie-Inoue K, Hayashi S, Sakai R, Ouchi Y, Inoue S (2009) Association of estrogen receptor alpha and histone deacetylase 6 causes rapid deacetylation of tubulin in breast cancer cells. Cancer Res 69: 2935–2940 [DOI] [PubMed] [Google Scholar]
- Berzat A, Hall A (2010) Cellular responses to extracellular guidance cues. EMBO J 29: 2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyault C, Sadoul K, Pabion M, Khochbin S (2007) HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene 26: 5468–5476 [DOI] [PubMed] [Google Scholar]
- Cant SH, Pitcher JA (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell 16: 3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Klemke RL (2002) Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol 156: 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10: 319–332 [DOI] [PubMed] [Google Scholar]
- Cotton M, Claing A (2009) G protein-coupled receptors stimulation and the control of cell migration. Cell Signal 21: 1045–1053 [DOI] [PubMed] [Google Scholar]
- Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L (2009) Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136: 551–564 [DOI] [PubMed] [Google Scholar]
- Cuvelier D, Thery M, Chu YS, Dufour S, Thiery JP, Bornens M, Nassoy P, Mahadevan L (2007) The universal dynamics of cell spreading. Curr Biol 17: 694–699 [DOI] [PubMed] [Google Scholar]
- Deribe YL, Wild P, Chandrashaker A, Curak J, Schmidt MH, Kalaidzidis Y, Milutinovic N, Kratchmarova I, Buerkle L, Fetchko MJ, Schmidt P, Kittanakom S, Brown KR, Jurisica I, Blagoev B, Zerial M, Stagljar I, Dikic I (2009) Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci Signal 2: ra84. [DOI] [PubMed] [Google Scholar]
- Dubin-Thaler BJ, Hofman JM, Cai Y, Xenias H, Spielman I, Shneidman AV, David LA, Dobereiner HG, Wiggins CH, Sheetz MP (2008) Quantification of cell edge velocities and traction forces reveals distinct motility modules during cell spreading. PLoS One 3: e3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S (2010) From signaling pathways to microtubule dynamics: the key players. Curr Opin Cell Biol 22: 104–111 [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K (2002) Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109: 873–885 [DOI] [PubMed] [Google Scholar]
- Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY, Yao TP (2007) Histone deacetylase 6 regulates growth factor-induced actin remodeling and endocytosis. Mol Cell Biol 27: 8637–8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 100: 4389–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458 [DOI] [PubMed] [Google Scholar]
- Insall RH, Machesky LM (2009) Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 17: 310–322 [DOI] [PubMed] [Google Scholar]
- Kaluza D, Kroll J, Gesierich S, Yao TP, Boon RA, Hergenreider E, Tjwa M, Rossig L, Seto E, Augustin HG, Zeiher AM, Dimmeler S, Urbich C (2011) Class IIb HDAC6 regulates endothelial cell migration and angiogenesis by deacetylation of cortactin. EMBO J 30: 4142–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV (1999) Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol 146: 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RR, Langridge P, Traynor D, Hoeller O (2008) Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol 9: 455–463 [DOI] [PubMed] [Google Scholar]
- Krendel M, Zenke FT, Bokoch GM (2002) Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol 4: 294–301 [DOI] [PubMed] [Google Scholar]
- Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68: 7561–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina EM, Kharitonova MA, Rovensky YA, Vasiliev JM (2001) Cytoskeletal control of fibroblast length: experiments with linear strips of substrate. J Cell Sci 114: 4335–4341 [DOI] [PubMed] [Google Scholar]
- Li D, Xie S, Ren Y, Huo L, Gao J, Cui D, Liu M, Zhou J (2011) Microtubule-associated deacetylase HDAC6 promotes angiogenesis by regulating cell migration in an EB1-dependent manner. Protein Cell 2: 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini JS, Raake P, Vinge LE, DeGeorge BR Jr, Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ (2008) Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci USA 105: 12457–12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21: 6820–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL (2007) Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482 [DOI] [PubMed] [Google Scholar]
- Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM (2009) Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell 20: 4070–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11: 437–444 [DOI] [PubMed] [Google Scholar]
- Palazzo A, Ackerman B, Gundersen GG (2003) Cell biology: Tubulin acetylation and cell motility. Nature 421: 230. [DOI] [PubMed] [Google Scholar]
- Penela P, Murga C, Ribas C, Lafarga V, Mayor F Jr (2010) The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol 160: 821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, Kavelaars A, Sanchez-Madrid F, Mayor F Jr (2008) G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J 27: 1206–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penela P, Ribas C, Aymerich I, Mayor F Jr (2009) New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. Cell Adh Migr 3: 19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS (1998) The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem 273: 12316–12324 [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ (1999) Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem 274: 34531–34534 [DOI] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ (2006) Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 16: 2166–2172 [DOI] [PubMed] [Google Scholar]
- Rhee S, Jiang H, Ho CH, Grinnell F (2007) Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci USA 104: 5425–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F Jr (2007) The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta 1768: 913–922 [DOI] [PubMed] [Google Scholar]
- Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, Itoh A, Funata N, Schreiber SL, Yoshida M, Toi M (2005) Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene 24: 4531–4539 [DOI] [PubMed] [Google Scholar]
- Salaycik KJ, Fagerstrom CJ, Murthy K, Tulu US, Wadsworth P (2005) Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J Cell Sci 118: 4113–4122 [DOI] [PubMed] [Google Scholar]
- Salcedo A, Mayor F Jr, Penela P (2006) Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J 25: 4752–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA (2008) Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem 283: 21065–21073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ (2007) Microtubule-induced cortical cell polarity. Genes Dev 21: 483–496 [DOI] [PubMed] [Google Scholar]
- Suzuki J, Chen YY, Scott GK, Devries S, Chin K, Benz CC, Waldman FM, Hwang ES (2009) Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res 15: 3163–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AD, Marmo TP, Salam AA, Che S, Finkelstein E, Kabarriti R, Xenias HS, Mazitschek R, Hubbert C, Kawaguchi Y, Sheetz MP, Yao TP, Bulinski JC (2007) HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J Cell Sci 120: 1469–1479 [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Cabrero JR, Serrador JM, Sanchez-Madrid F (2008) HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol 18: 291–297 [DOI] [PubMed] [Google Scholar]
- Vroon A, Heijnen CJ, Kavelaars A (2006) GRKs and arrestins: regulators of migration and inflammation. J Leukoc Biol 80: 1214–1221 [DOI] [PubMed] [Google Scholar]
- Wagner S, Flood TA, O′Reilly P, Hume K, Sabourin LA (2002) Association of the Ste20-like kinase (SLK) with the microtubule. Role in Rac1-mediated regulation of actin dynamics during cell adhesion and spreading. J Biol Chem 277: 37685–37692 [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T (2011) Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J Cell Sci 124: 1519–1532 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K (2005) Regulation of microtubules in cell migration. Trends Cell Biol 15: 76–83 [DOI] [PubMed] [Google Scholar]
- Westermann S, Weber K (2003) Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol 4: 938–947 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E (2007) HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell 27: 197–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A (2009) Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci 122: 3531–3541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.