Abstract
Nature 481, 190–194 (2012); published online December 14 2011
For a cancer to metastasise, it must overcome the physiological mechanisms that normally protect against the dissemination of cells and their lodgement and growth at remote sites. The mechanisms by which invading cancer cells modify and engage with the new environment at a secondary site are poorly understood but provide potential novel avenues for therapeutic intervention. A recent report in Nature (Png et al, 2012) identifies a microRNA, miR-126, whose loss in tumour cells promotes the initiation of metastases in various secondary organs. The key targets of miR-126 in this process are found to be novel regulators of endothelial cell recruitment and angiogenesis.
Over 30 microRNAs have been associated with cancer metastasis since microRNAs emerged on the metastasis research scene just 5 years ago. This might be expected, because microRNAs are pervasive participants in normal intracellular regulatory networks and each of the multiple complex steps in metastasis is likely to be affected by one or more microRNAs. To date, microRNAs have mostly been found to influence the initial stages of metastasis, affecting cell migration and invasion. Prominent among these are miR-10b, miR-21, miR-31, miR-200, miR-335, miR-373, and miR-520 (Bracken et al, 2009). While epithelial–mesenchymal plasticity and the microRNAs that control it, such as miR-200, are likely to also affect events at metastatic sites, the further identification of microRNAs and their targets that influence cellular behaviour at secondary sites is important.
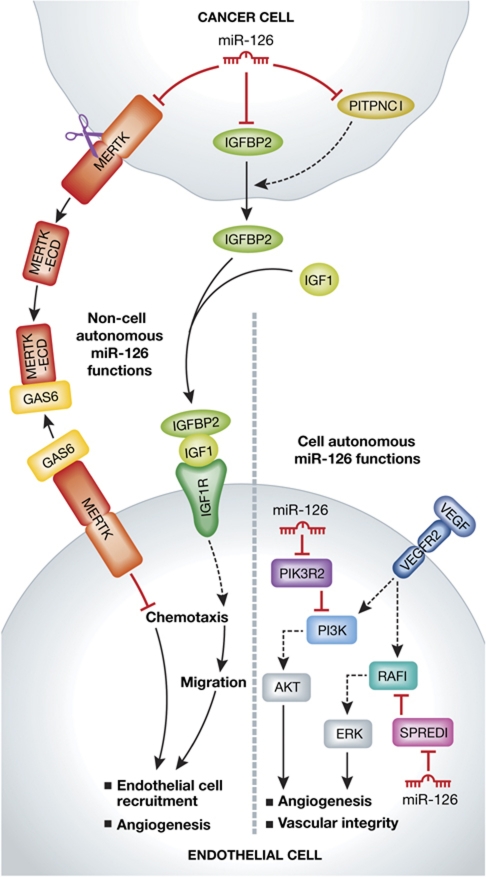
MiR-126 has been previously found to act in endothelial cells to promote angiogenesis by targeting SPRED1, an intracellular inhibitor of the RAS/MAPK pathway and PIK3R2 (also known as PI3K p85β), a negative regulator of the PI3K pathway (Figure 1) (Fish et al, 2008; Wang et al, 2008). Working previously with the Massague group, Tavazoie and colleagues reported in 2008 that several miRNAs, including miR-335 and miR-126, are metastasis suppressors (Tavazoie et al, 2008). These microRNAs were associated with rapid time to metastatic relapse for a cohort of breast cancer patients and in animal models they suppressed metastasis of breast cancer xenografts to lung and bone. MiR-126 also reduced cellular proliferation in vitro and in the xenograft tumours. In the report just published in Nature (Png et al, 2012), Tavazoie and colleagues show that the effect of miR-126 on metastasis occurs primarily at the distal sites, limiting the initiation of metastatic nodules. Using in vivo and in vitro approaches they demonstrate that miR-126 acts in a non-cell autonomous manner, inhibiting endothelial cell recruitment through its effect in the cancer cells on at least three direct targets, IGFBP2, PITPNC1, and MERTK. Individually, these proteins were able to influence endothelial recruitment and metastatic colonisation without significantly affecting the growth of the cancer cells themselves, recapitulating the non-cell autonomous action of miR-126 on tumour metastasis.
Figure 1.
Loss of miR-126 in cancer cells promotes endothelial cell recruitment through a non-cell autonomous mechanism involving its direct targets. MiR-126 in the cancer cell directly targets MERTK, IGFBP2, and PITPNC1. PITPNC1 promotes secretion of IGFBP2, which binds to IGF1 to facilitate its binding to IGF1R on endothelial cells. MERTK is cleaved and its extracellular domain (ECD) binds GAS6, a MERTK ligand that inhibits endothelial cell recruitment, thereby acting as a GAS6 antagonist to promote endothelial recruitment. MiR-126 in endothelial cells acts in a cell autonomous manner to promote angiogenesis through activation of the PI3K-Akt and RAF-1-ERK pathways. The miR-126 endothelial cell autonomous functions are adapted from Fish et al (2008).
The mode of action deduced for MERTK is an interesting story in itself. MERTK is a cell surface receptor expressed on both epithelial and endothelial cells. Endothelial MERTK activates cell migration, but its activity is inhibited by the extracellular protein GAS6. However, the MERTK expressed on the cancer cells is cleaved to release the extracellular domain as a soluble protein, which binds and neutralises extracellular GAS6. Thus, MERTK acts as an antagonist of an inhibitor. On the other hand, IGFBP2, whose secretion is augmented by PITPNC1, induces angiogenesis by promoting recruitment of endothelial cells. Hence miR-126-deficient cancer cells have a 2-pronged action on endothelial cells. They simultaneously promote endothelial activation with one factor while relieving inhibition with another, and together cooperate to promote endothelial recruitment. The work provides yet another example of the networking action of a microRNA.
It might be expected that the resulting angiogenesis would enhance metastatic development by providing a source of nutrient and oxygen to the nascent growing tumours. Consistent with this, the authors show that metastases derived from miR-126-deficient cells have enhanced vascularisation. However, the authors propose that the primary effector of increased metastatic colonisation is through endothelial–cancer cell interactions, presumably via cell–cell interactions or paracrine factors provided by the endothelial cells. The evidence for this proposal is based on the ability of co-injected endothelial cells (HUVEC) to rescue the metastatic colonisation defect of miR-126-overexpressing cells. Although the nature of the interaction was not explored, this is an interesting concept, suggesting that tumour cells may have hijacked a normal developmental process to further their progression, and is consistent with emerging evidence for perfusion-independent activities of endothelial cells in other scenarios (Butler et al, 2010). During embryonic development, endothelial cells invade into newly formed organs and provide paracrine factors and adhesion molecules that prime the tissue for organogenesis (Butler et al, 2010).
Through elucidation of the mechanism of tumour suppression by miR-126, the authors have identified this microRNA as a novel inhibitor of endothelial cell recruitment. Furthermore, through identification of its direct targets, they have identified three new pro-angiogenic proteins whose levels are increased in stages III and IV patients and which provide potential new therapeutic targets for anti-angiogenic therapy.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bracken CP, Gregory PA, Khew-Goodall Y, Goodall GJ (2009) The role of microRNAs in metastasis and epithelial-mesenchymal transition. Cell Mol Life Sci 66: 1682–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Kobayashi H, Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev 10: 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15: 272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png KJ, Halberg N, Yoshida M, Tavazoie SF (2012) A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481: 190–194 [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J (2008) Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15: 261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]