Abstract
Glucocorticoids are secreted into the systemic circulation from the adrenal cortex and initiate a broad range of actions throughout the organism that regulate the function of multiple organ systems, including the liver, muscle, the immune system, the pancreas, fat tissue, and the brain. Delayed glucocorticoid effects are mediated by classical steroid mechanisms involving transcriptional regulation. Relatively rapid effects of glucocorticoids also occur that are incompatible with genomic regulation and invoke a noncanonical mode of steroid action. Studies conducted in several labs and on different species suggest that the rapid effects of glucocorticoids are mediated by the activation of one or more membrane-associated receptors. Here, we provide a brief review focused on multiple lines of evidence suggesting that rapid glucocorticoid actions are triggered by, or at least dependent on, membrane-associated G protein-coupled receptors and activation of downstream signaling cascades. We also discuss the possibility that membrane-initiated actions of glucocorticoids may provide an additional mechanism for the regulation of gene transcription.
Glucocorticoids have an extensive range of actions in target tissues throughout the organism, and these actions have long been recognized to elicit both rapid and delayed effects on physiological and behavioral responses (1). The delayed glucocorticoid actions are mediated, for the most part, by activation of known cytosolic receptors belonging to the nuclear receptor superfamily, the corticosteroid type I, or mineralocorticoid, and corticosteroid type II, or glucocorticoid, receptors (2). These intracellular corticosteroid receptors initiate transcriptional activation or repression by the translocation of the ligand-bound receptor to the nucleus and binding to a glucocorticoid response element sequence in the promoter region of different glucocorticoid-regulated genes (3, 4). Glucocorticoids can also regulate transcription without binding directly to the DNA but by associating with other transcription factors to regulate their transcriptional activity (5). There is a rapidly growing body of evidence suggesting that acute physiological and behavioral effects of glucocorticoids are mediated by actions associated with the plasma membrane and independent of gene transcription, as has been surmised by the incompatibility of the rapid effects with genomic regulation. Here, we present an overview of the evidence for the rapid effects of glucocorticoids being mediated by one or more membrane-associated glucocorticoid receptors coupled to downstream G protein-dependent signaling cascades. We touch upon findings suggesting an interaction of the glucocorticoid membrane receptor signaling with other receptor signaling cascades and discuss the possibility of an alternative mechanism of glucocorticoid transcriptional regulation via membrane glucocorticoid receptor signaling.
Rapid Glucocorticoid Actions
Glucocorticoids have been shown to exert a vast array of rapid functional effects on different cells and tissues as well as on behavioral responses in different vertebrate species. Among the tissues and systems targeted for rapid glucocorticoid effects are muscle, pancreas, heart, adipose tissue, the immune system, and the brain. We will concentrate in this review primarily on the rapid cellular effects of glucocorticoids on the vertebrate brain, although here we mention briefly some of the rapid, transcription-independent cell/molecular actions of glucocorticoids in other tissues. For example, glucocorticoids have been shown to inhibit smooth muscle contraction in the trachea via rapid, nongenomic actions that are not blocked by the intracellular glucocorticoid receptor antagonist mifepristone (6). Glucocorticoids also have been reported to cause a rapid suppression of the stimulated release of insulin from pancreatic β-cells, whereas they have little or no effect on resting insulin levels in vivo and in vitro (7); this rapid decrease in insulin, interestingly, is opposite to the delayed increase in insulin levels caused by slow glucocorticoid actions. In the heart, glucocorticoids induce endothelial nitric oxide release by stimulating nitric oxide synthase via activation of phosphatidylinositol-3 kinase and protein kinase Akt, which contributes to the acute cardiovascular protective effects of the steroid (8). Glucocorticoids stimulate fat cell production in adipose tissue by facilitating the differentiation of preadipocyte precursors into adipocytes via a nontranscriptional suppression of a histone deacetylase complex (9), representing an epigenetic action of the glucocorticoids on adipose tissue. The well-known antiinflammatory action of glucocorticoids in the immune response was shown to be mediated by glucocorticoid actions that are in part independent of glucocorticoid receptor binding to the DNA, also indicating a nontranscriptional action of the steroid (10). Thus, as one might expect from blood-borne steroids with such widespread access to tissues, multiple target organs throughout the organism mount rapid responses to glucocorticoids that are independent of, or parallel to, the transcriptional regulatory function of the steroids. Interestingly, rapid actions of glucocorticoids are seen in lower vertebrate species and represent an evolutionarily conserved mechanism of steroid action, to the extent that it has been posited that the membrane glucocorticoid receptor and its rapid actions may represent the more evolutionarily ancient of the forms of glucocorticoid receptor activity (11). It is worth noting that the rapid glucocorticoid actions are extremely varied in nature and probably represent the actions of multiple receptor mechanisms, including possibly the classical intracellular receptor(s) acting at the membrane through nongenomic signaling mechanisms. Although it has generally been assumed that glucocorticoids access their cognate intracellular receptors by passive diffusion through the membrane because of their lipophilicity, there are recent reports that even the transcriptional effects of glucocorticoids may require transport across the plasma membrane by a membrane transporter (12).
Rapid Glucocorticoid Regulation of Brain Function
Glucocorticoids have been found to exert effects on neuronal activity that can occur within seconds to minutes of the exposure of the cells to the steroid, precluding the involvement of genomic regulation in these effects. The transcription-independent glucocorticoid actions have been reported mainly in different limbic and brain stem structures of the brain, where they control functions ranging from learning to neuroendocrine function to reproductive behaviors. Thus, systemic glucocorticoids have been reported to increase locomotor activity of rats placed in a novel environment but not in rats previously exposed to the same environment (13). This glucocorticoid effect is dependent on nitric oxide release, because it was blocked by nitric oxide synthase antagonists and rescued by the nitric oxide precursor l-arginine (14). Moore and colleagues (15, 16) have published several probing studies using the rough-skinned newt as a model in which they have shown that stress levels of corticosterone suppress male reproductive behavior in these animals via rapid actions at a putative membrane corticosteroid receptor. This behavioral effect is caused by the rapid glucocorticoid inhibition of brain stem neural circuits that control motor outputs responsible for limb clasping during reproduction (17), and this appears to be mediated by the glucocorticoid-induced synthesis and release of endogenous cannabinoids in these circuits (18). This rapid action of glucocorticoids via endocannabinoid release is interesting in light of recent findings of an endocannabinoid intermediate in the rapid effects of glucocorticoids on synaptic transmission in the hypothalamus (see below) (19–21).
Several studies have been performed to determine the effects of glucocorticoids on learning and memory based on the empirical notion that stress can have both positive and negative effects on one’s ability to process information and form memories (e.g. see Ref. 22 for review). Briefly, although the long-term effects of elevated circulating glucocorticoid levels have generally been found to be detrimental to memory (23), acute glucocorticoid actions have been reported to enhance memory formation. Thus, learning is enhanced by glucocorticoids applied during or immediately after training sessions via actions requiring an intact amygdala (24). Similarly, adrenalectomy and blocking corticosterone synthesis inhibits the consolidation of emotional memories in rats, and this memory impairment is rescued by acute glucocorticoid application (25–27). The glucocorticoid effects on memory appear to a large degree to result from genomic modifications of the response properties of hippocampal and amygdalar neurons by changing the intrinsic and/or synaptic excitability of these cells. Long-term exposure to glucocorticoids has deleterious effects on the morphological structure and synaptic innervation of hippocampal neurons (28). Chronically elevated glucocorticoids by chronic stress or repeated corticosterone administration and chronically low glucocorticoid levels after adrenalectomy have been shown to cause changes in the excitability of hippocampal CA1 pyramidal neurons in rats by altering their intrinsic electrical properties and their synaptic inputs (29, 30), and these effects generally have been attributed to genomic actions of the glucocorticoids. However, it was shown recently that acute glucocorticoid application in hippocampal slices enhances long-term potentiation of Schaffer collateral inputs to CA1 pyramidal neurons (31), suggesting facilitation of a probable cellular mechanism for hippocampus-dependent learning. Thus, although the transcriptional actions of glucocorticoids undoubtedly regulate how hippocampal neurons respond to afferent inputs over the long run, the rapid, nontranscriptional effects of glucocorticoids in the hippocampus may provide a possible mechanism for the acute facilitatory effects of glucocorticoids on some forms of learning and memory.
Much of the attention on the glucocorticoid effects in the brain over the years has been focused on the negative feedback effects of glucocorticoids on the stress activation of the hypothalamic-pituitary-adrenal (HPA) axis, the neuroendocrine system responsible for triggering the stress-induced elevations in blood glucocorticoid levels (see Ref. 1 for review). It has long been recognized that glucocorticoids exert a negative feedback regulation of the HPA axis that is mediated by both rapid and protracted glucocorticoid actions at the levels of the anterior pituitary, where the ACTH cells are located, the hypothalamic paraventricular nucleus (PVN), where the hypophysiotropic (CRH and vasopressin) neurons are located, and the hippocampus, an upstream regulator of the HPA axis. We will review briefly what is known about the glucocorticoid actions at each of these relays in the regulation of the HPA axis.
Glucocorticoid Feedback Regulation of the HPA Axis
Slow, transcription-dependent actions of glucocorticoids occur at each of the glucocorticoid feedback target sites of the HPA axis. At the pituitary, glucocorticoids regulate the expression of proopiomelanocorticotropic hormones, including ACTH, as well as the expression of CRH receptors and vasopressin receptors by the pituitary adrenocorticotrophs, thus controlling the amount of ACTH secreted into the blood in response to portal CRH and vasopressin (32–34). Glucocorticoids also regulate the levels of expression of the peptides CRH and vasopressin in the CRH neurons in the hypothalamus (35, 36), as well as the GABAergic inhibitory synaptic inputs to the CRH neurons of the PVN (37–40). The combined effects of lower pituitary ACTH levels, reduced expression of pituitary CRH and vasopressin receptors, and lower CRH and vasopressin expression in the PVN contribute to a suppression of the HPA hormone response to stress. Finally, it has also been shown that chronically elevated glucocorticoids and chronic stress cause long-term changes in the excitatory synaptic response properties of the upstream hippocampal CA1 neurons, exerting a slow enhancement of glutamate excitation (29) but suppressing N-methyl-d-aspartate receptor-mediated long-term potentiation (41). How these long-term changes in hippocampal synaptic responses impact the regulation of the HPA axis remains to be determined.
Conveniently, the acute effects of glucocorticoids on the main glucocorticoid feedback target sites tend predominantly toward the rapid suppression of activation of the HPA axis, as would be predicted for a negative feedback regulation. Thus, at the pituitary, glucocorticoids suppress CRH-induced ACTH secretion within minutes via a rapid, transcription-independent mechanism (42, 43). The rapid glucocorticoid effect on the pituitary accounts for about half of the total rapid feedback inhibition of ACTH release in vivo (1, 44), the remaining half occurring within the brain. At the level of the PVN, glucocorticoids have been reported to exert rapid effects that were mainly, although not exclusively, inhibitory on the spiking activity of median eminence-projecting PVN neurons recorded in vivo (45–47). This predominantly inhibitory effect on spiking activity was also seen in PVN neurons recorded in slices of hypothalamus in vitro (47), although these effects were blocked by the intracellular glucocorticoid receptor antagonist mifepristone. In another series of studies in hypothalamic slices, cortisol generally did not affect the spontaneous spike frequency of PVN neurons recorded in the parvocellular subdivision (48), although it blocked spike activation induced in these neurons by norepinephrine (49), suggesting an indirect effect on the noradrenergic modulation of synaptic inputs. Additionally, glucocorticoids have been found to suppress stimulated vasopressin release from hypothalamic slices/explants via a transcription-independent mechanism (50, 51). It should be noted that glucocorticoids also have been reported to suppress voltage-activated potassium currents (52) and to enhance the spiking activity of some PVN neurons (47, 52), suggesting a possible excitatory effect on some cells in the PVN.
The rapid inhibitory effects of glucocorticoids on PVN neurons are consistent with a suppression of excitatory synaptic drive and/or a facilitation of inhibitory synaptic input to the PVN. We reported recently that glucocorticoids inhibit glutamatergic excitatory postsynaptic currents in PVN neuroendocrine cells within minutes of application by stimulating the release of endocannabinoids (19), retrograde messengers that act to suppress the presynaptic release of glutamate and GABA (53, 54). Thus, glucocorticoids stimulate the synthesis and release of the endocannabinoids anandamide and 2-arachidonoylglycerol in the PVN, which inhibit glutamate release onto PVN neuroendocrine cells via the activation of CB1 receptors on presynaptic glutamate terminals (Fig. 1) (19, 55). We also have found that glucocorticoids rapidly facilitate the release of GABA inhibitory synaptic inputs to a subset of neuroendocrine cells, the magnocellular neurons (20), whereas there was no effect on GABA inputs to parvocellular neuroendocrine cells (19). The lack of effect onGABAinputs to PVN parvocellular neurons and the rapid facilitatory effect on GABA inputs to magnocellular neurons is in stark contrast to the suppression of GABA inputs to PVN parvocellular neurons caused by chronic stress and sustained high corticosterone (38) and suggests that there is a differential regulation of GABA inputs under conditions of acute vs. sustained rises in glucocorticoids. It is not yet clear whether the rapid facilitatory glucocorticoid effect on GABA release is also caused by the retrograde action of endocannabinoids at GABA terminals or by some other retrograde messenger, although preliminary evidence suggests that, unlike the rapid glucocorticoid effect on glutamate release (21), the rapid glucocorticoid effect on GABA release is not blocked by the adipocyte hormone leptin (Di, S., and J. G. Tasker, unpublished observation). The effect of suppression of excitatory synaptic inputs combined, in some cells (i.e. oxytocin and vasopressin neurons), with facilitation of inhibitory inputs to PVN neurons would be expected to strongly inhibit the PVN outputs that control the HPA axis. It is worth noting that vasopressin secretion from the magnocellular neuroendocrine cells, like that from the parvocellular neuroendocrine cells, facilitates ACTH release at the anterior pituitary (56).
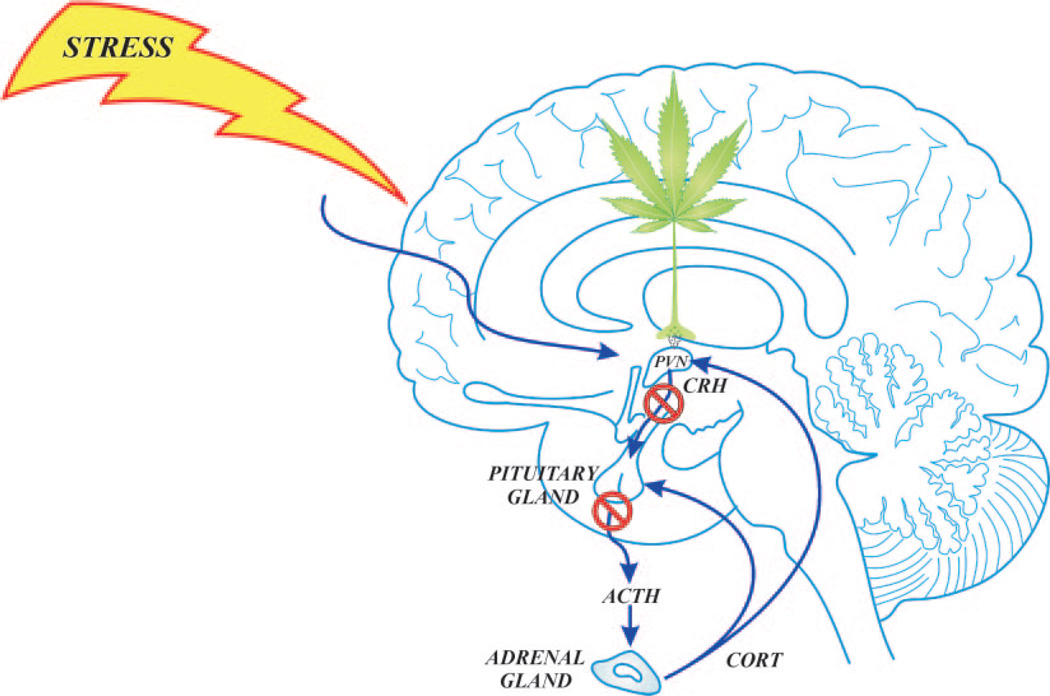
FIG. 1.
Rapid glucocorticoid feedback inhibition of the HPA axis. Glucocorticoids (CORT) are secreted into the blood from the adrenal glands in response to stress activation of the HPA axis, and the circulating glucocorticoids feed back to the anterior lobe of the pituitary gland, the hypothalamic PVN, and the hippocampus (not shown). Glucocorticoids inhibit CRH neuron activity via endocannabinoid release in the PVN and curtail HPA hormone release within minutes of reaching the PVN.
The rapid effects of glucocorticoids in the hippocampus are also consistent with inhibition of the HPA axis, although, paradoxically, these represent primarily excitatory effects on the principal cells of the hippocampus. Thus, glucocorticoids cause a rapid, transcription-independent increase in the probability of glutamate release onto CA1 pyramidal neurons, resulting in an increase in excitatory synaptic inputs to these cells (57). Interestingly, although independent of transcriptional mechanisms, this glucocorticoid effect is nevertheless sensitive to blocking, or knocking out, the intracellular type I corticosteroid (mineralocorticoid) receptor, suggesting a role for this receptor in the rapid glucocorticoid signaling. Glucocorticoids have also been shown to enhance the functional expression of long-term potentiation in the CA1 pyramidal cell layer, a rapid effect that is independent of the activation of both the classical glucocorticoid and mineralocorticoid receptors (31). Thus, glucocorticoids exert rapid modulatory effects on excitatory synaptic transmission in the hippocampal CA1 subfield that lead to an increase in the excitability of the CA1 pyramidal neurons, principal output neurons of the hippocampus. Despite the predominantly excitatory glutamatergic projection from the hippocampus/subiculum to the PVN, the hippocampal regulation of the HPA axis is primarily inhibitory (58). The transformation of an excitatory hippocampal output to an inhibitory hypothalamic input is thought to be mediated by the hippocampal projections relaying through GABAergic inhibitory neurons located in the perifornical region surrounding the PVN (59–61). Therefore, a rapid increase in the hippocampal excitatory output to the hypothalamus by glucocorticoids would result in an increased inhibition of hypothalamic neuroendocrine cells, including presumably the CRH neurons, and could contribute to the fast glucocorticoid feedback inhibition of the HPA axis.
Membrane Glucocorticoid Receptor Signaling via Intracellular Second Messengers
There is a growing body of evidence that at least some of the rapid glucocorticoid actions are mediated by G protein-coupled receptors (GPCRs) and intracellular signaling cascades downstream from GPCRs. For example, the binding of radiolabeled glucocorticoids to the membrane fraction of newt brain stem tissue is suppressed by GTP-γ-S and enhanced by magnesium, suggesting that the steroids bind to one or more GPCRs (15, 62). Glucocorticoids have been found to rapidly inhibit high-voltage-activated calcium currents in several different cell types, including in dissociated hippocampal neurons, dorsal root ganglion neurons, and PC12 cells, and this is blocked by suppressing G protein activity (63–65). Additionally, the transcription-independent glucocorticoid inhibition of ACTH release from AtT20 pituitary tumor-derived cells is blocked by pertussis toxin, suggesting it is mediated by activation of a receptor coupled to an inhibitory G protein (Gi) (66). Interestingly, although the Gi dependence suggests that the rapid glucocorticoid effect is mediated by the negative regulation of cAMP activity, the rapid effect of glucocorticoids upstream in the PVN is dependent on activation of a stimulatory G protein (Gs), implying a positive regulation of cAMP production by glucocorticoids (21). Thus, recent findings from our lab suggest that the rapid glucocorticoid-induced synthesis and release of endocannabinoids from neuroendocrine cells in the PVN is mediated by the activation of a Gs-coupled membrane receptor (Fig. 2) (19, 21).
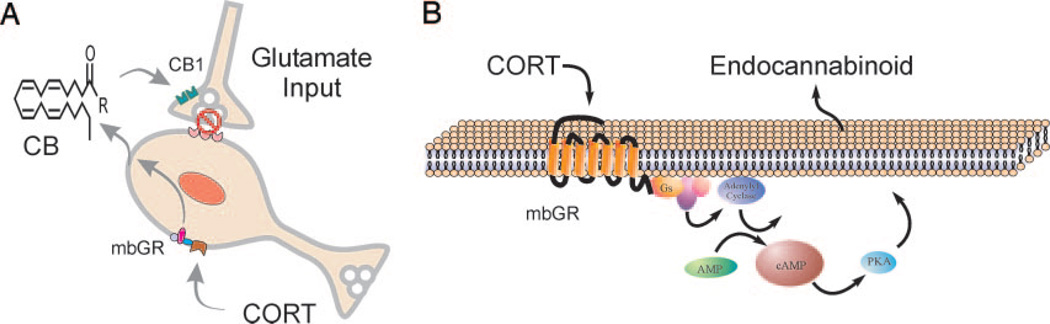
FIG. 2.
Model of rapid glucocorticoid signaling in PVN parvocellular neuroendocrine cells. A, Glucocorticoids (CORT) bind to a membrane-associated receptor (mbGR) and activate a G protein-dependent intracellular signaling pathway that leads to endocannabinoid (CB) synthesis and retrograde release. Endocannabinoids bind to presynaptic CB1 cannabinoid receptors and suppress glutamate release from glutamate synapses onto the PVN neuron. B, Proposed membrane glucocorticoid receptor signaling pathway. In this model, the membrane glucocorticoid receptor (mbGR) is a stimulatory G protein-coupled receptor (Gs) that, upon binding corticosterone (CORT), triggers the activation of adenylyl cyclase and the production of cAMP, resulting in increased PKA activity, which leads downstream to endocannabinoid synthesis from membrane lipid precursors followed by release.
Downstream from the putative membrane glucocorticoid receptors, various signaling pathways have been implicated in the rapid regulation by glucocorticoids of cell signaling in different cell types/systems. Chen and colleagues (64, 67–70) have reported on the rapid glucocorticoid inhibition of voltage-gated and transmitter-evoked calcium transients in PC12 cells and different neuroblastoma-derived cell lines via either protein kinase C (PKC)- or PKA-dependent mechanisms, depending on the cell type. Similarly, the inhibitory effect of glucocorticoids on high-voltage-activated calcium currents in dissociated hippocampal neurons and dorsal root ganglion neurons was attenuated with PKC inhibitors (63, 65).
We recently reported that the rapid glucocorticoid inhibition of excitatory synaptic inputs to neuroendocrine cells of the hypothalamic PVN is dependent on the activation of a cAMP signaling cascade. Thus, the synthesis and retrograde release of endocannabinoids responsible for the glucocorticoid-induced suppression of glutamate release onto PVN neurons was blocked with agents that prevent cAMP formation and PKA activation (21). The membrane-associated glucocorticoid receptor in PVN neuroendocrine neurons, therefore, signals via a mechanism that is dependent on the activation of a Gs-cAMP-PKA cascade (Fig. 2). It should be noted, however, that this does not necessarily mean that the putative membrane glucocorticoid receptor is coupled directly to the Gs-cAMP-PKA pathway, because in vitro blockade of PKA activity before glucocorticoid application caused a reduction in PVN 2-arachidonoylglycerol levels, suggesting the existence of a PKA-mediated, glucocorticoid-independent basal release of endocannabinoid in PVN slices (21). Therefore, it is still not clear whether the rapid glucocorticoid-induced stimulation of endocannabinoid release is mediated by a direct activation of adenylyl cyclase, by an indirect mechanism leading to increased cAMP levels, or, less likely, by a mechanism that depends on basal cAMP synthesis. Indeed, it would appear that the signaling mechanism responsible for the rapid synthesis and release of endocannabinoids in PVN neuroendocrine cells by glucocorticoids is more complex than a simple activation of the Gs-cAMP-PKA signaling pathway, because the rapid glucocorticoid effect on synaptic glutamate release is also blocked by a PKC inhibitor (19), although this does not involve activation of the Gq subtype of G protein because inactivating Gq did not block the rapid glucocorticoid effect (21). Additionally, endocannabinoid synthesis in hypothalamic neuroendocrine cells is also stimulated by electrical activation (55, 71), suggesting a convergence on endocannabinoid synthetic pathways of glucocorticoid signaling and activity-dependent mechanisms.
Rapid glucocorticoid signaling via intracellular corticosteroid receptors
It is also possible that rapid, nongenomic effects of glucocorticoids are mediated by the classical intracellular corticosteroid receptors associating with the membrane. For example, there is evidence that the nuclear estrogen receptors (ERs), ERα and ERβ, are capable of localizing to both the intracellular and membrane compartments (72) and that the intracellular receptors can associate with the membrane of nonneuronal cells via tethering to membrane calveolae (73). In ERα-expressing pituitary tumor cells (GH3 cells), ERα is located in the plasma membrane and mediates the estrogen-induced rapid release of prolactin, because it is blocked or stimulated directly by antibodies directed to different amino acid sequences of ERα (74). The involvement of the classical nuclear ERs in the rapid membrane response is not consistent, however, with the dependence of rapid estrogen effects on G proteins and PKC and PKA signaling in hypothalamic neurons and with a selective agonist that targets the membrane receptor and not the nuclear receptors (75, 76). Indeed, a recent report suggests that the membrane ER may actually be a separate G protein-coupled receptor that, unexpectedly, is localized to the intracellular endoplasmic reticulum membrane (77). Nevertheless, the issue of whether the rapid estrogen effects are mediated by a separate G protein-coupled receptor or by the intracellular receptors associating with the membrane is controversial (78), with recent evidence suggesting that the rapid estrogen effects may be mediated by an intermediate between the two, membrane association of intracellular ERs via linkage with other neuronal membrane G protein-coupled receptors (i.e. metabotropic glutamate receptors) (79, 80).
The classical corticosteroid receptors, or isoforms thereof, have also been found localized to the membrane of neuronal and nonneuronal cells with antibodies directed against the intracellular glucocorticoid receptor (81, 82), suggesting the possibility that rapid effects of glucocorticoids may be mediated by the classical intracellular receptors associated with the membrane. Consistent with this possibility, the rapid glucocorticoid-induced increase in glutamate excitatory postsynaptic currents in hippocampal CA1 pyramidal neurons is blocked with an antagonist to the intracellular mineralocorticoid receptor and is not seen in mice in which mineralocorticoid receptors have been knocked out (57). Although the different affinities of the putative membrane receptor and the intracellular receptor to corticosteroid agonists (83, 84), the frequent lack of sensitivity of the membrane receptor actions to the intracellular receptor antagonist mifepristone (85), and the G protein dependence of the membrane receptor-mediated effects argue against the identity of the intracellular receptor and the membrane receptor, this cannot be ruled out because the same protein may assume different properties in the two different subcellular environments.
Signaling to the nucleus via the membrane glucocorticoid receptor
Glucocorticoids also have been reported to cause the rapid activation of several MAPKs in both immortalized cell lines and in hippocampal neurons. Thus, glucocorticoids phosphorylate the MAPKs p38, c-Jun NH2-terminal kinase (JNK), and ERK1 and -2 in cultured hippocampal neurons (86, 87) and in PC12 cells (88) via a PKC-dependent signaling mechanism. Activation of these downstream messengers that signal to the nucleus suggest that the membrane glucocorticoid receptor signaling cascade may provide an alternative mechanism of transcriptional regulation that runs in parallel to the intracellular corticosteroid receptor regulation of gene transcription (85). A rise in phosphorylated ERK MAPKs was seen in AtT20 cells only between 1 and 3 h after corticosterone treatment, which is not consistent with a rapid glucocorticoid effect (89). In this same study, mice subjected to restraint stress showed an activation of ERK in hippocampal extracts only after 60–120 min from the onset of the stress, whereas circulating corticosterone levels peaked within 30 min, and ERK activation was abolished in mice with a knockout of the intracellular glucocorticoid receptor. Together, these findings point to ERK activation via the intracellular glucocorticoid receptor and not the membrane receptor. However, signals upstream of ERK (e.g. Raf-1) showed peak activation within 30 min of the stress onset, providing a possible rapid substrate for the activation of other MAPKs. Regardless, the activation and nuclear translocation of MAPKs by either, or both, transcriptional and nontranscriptional mechanisms provide the potential for epigenetic regulation of gene expression, because MAPKs can cause histone phosphorylation that can lead to modification of chromatin structure (90, 91). It will be interesting in the future to see how these potentially distinct mechanisms of accessing the genome might interact, either positively or negatively, to formulate the resulting net regulation of gene expression by glucocorticoids.
Conclusion
Thus, rapid glucocorticoid actions point to membrane-associated mechanisms of glucocorticoid signaling that are distinct from the transcriptional actions of glucocorticoids mediated by activation of the classical intracellular receptors. These rapid actions can occur within minutes of exposure of the target cells to the glucocorticoids, which suggests a rate of cellular regulation that approaches that of neurotransmitters acting at metabotropic receptors. Although the identity of the membrane-associated glucocorticoid receptor(s) has not yet been discovered, there is a growing body of data that suggest that the receptors signal via G protein-dependent mechanisms and downstream kinases. Evidence for membrane progestin and estrogen receptors coupled to G protein signaling pathways has recently been reported (77, 92, 93), which suggests that analogous receptors for glucocorticoids exist. It remains to be determined whether the rapid actions of glucocorticoids are mediated by one or more membrane-associated receptors and whether these receptors are separate G protein-coupled receptors or the intracellular receptors that associate with the membrane. It is also possible that the rapid effects of glucocorticoids are mediated by allosteric actions at known or unknown receptors of another transmitter or hormone, as has been reported for the neurosteroids and GABAA receptors (94) and for progesterone and oxytocin receptors (95). Although relatively little is known currently about the putative membrane glucocorticoid receptor(s) (16, 83), the recent advances in molecular biological techniques and sequencing of the mouse genome hold promise for the future identification of the receptor(s) responsible for the widespread rapid actions of glucocorticoids.
Acknowledgments
This work was supported by National Institutes of Health Grants MH066958 and MH069879.
Abbreviations
- ER
Estrogen receptor
- GPCR
G protein-coupled receptor
- HPA
hypothalamic-pituitary-adrenal
- PKC
protein kinase C
- PVN
paraventricular nucleus
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones: a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 5.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun HW, Miao CY, Liu L, Zhou J, Su DF, Wang YX, Jiang CL. Rapid inhibitory effect of glucocorticoids on airway smooth muscle contractions in guinea pigs. Steroids. 2006;71:154–159. doi: 10.1016/j.steroids.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67:77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 8.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Hache RJ. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J. 2003;22:2135–2145. doi: 10.1093/emboj/cdg218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20:7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Daufeldt S, Lanz R, Allera A. Membrane-initiated steroid signaling (MISS): genomic steroid action starts at the plasma membrane. J Steroid Biochem Mol Biol. 2003;85:9–23. doi: 10.1016/s0960-0760(03)00141-9. [DOI] [PubMed] [Google Scholar]
- 13.Sandi C, Venero C, Guaza C. Novelty-related rapid locomotor effects of corticosterone in rats. Eur J Neurosci. 1996;8:794–800. doi: 10.1111/j.1460-9568.1996.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandi C, Venero C, Guaza C. Nitric oxide synthesis inhibitors prevent rapid behavioral effects of corticosterone in rats. Neuroendocrinology. 1996;63:446–453. doi: 10.1159/000127070. [DOI] [PubMed] [Google Scholar]
- 15.Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 16.Evans SJ, Murray TF, Moore FL. Partial purification and biochemical characterization of a membrane glucocorticoid receptor from an amphibian brain. J Steroid Biochem Mol Biol. 2000;72:209–221. doi: 10.1016/s0960-0760(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 17.Rose JD, Moore FL, Orchinik M. Rapid neurophysiological effects of corticosterone on medullary neurons: relationship to stress-induced suppression of courtship clasping in an amphibian. Neuroendocrinology. 1993;57:815–824. doi: 10.1159/000126440. [DOI] [PubMed] [Google Scholar]
- 18.Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 19.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- 21.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 23.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 24.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Roozendaal B, McGaugh JL. The memory-modulatory effects of glucocorticoids depend on an intact stria terminalis. Brain Res. 1996;709:243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Res. 1999;821:134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 29.Karst H, Joels M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. J Neurophysiol. 2005;94:3479–3486. doi: 10.1152/jn.00143.2005. [DOI] [PubMed] [Google Scholar]
- 30.Joels M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 31.Wiegert O, Joels M, Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn Mem. 2006;13:110–113. doi: 10.1101/lm.87706. [DOI] [PubMed] [Google Scholar]
- 32.Birnberg NC, Lissitzky JC, Hinman M, Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci USA. 1983;80:6982–6986. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino S, Schulkin J, Smith MA, Pacak K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- 34.Aguilera G, Rabadan-Diehl C. Regulation of vasopressin V1b receptors in the anterior pituitary gland of the rat. Exp Physiol. 2000;85(Spec No):19S–26S. doi: 10.1111/j.1469-445x.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 35.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc. 1985;44:221–227. [PubMed] [Google Scholar]
- 36.Sawchenko PE. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res. 1987;437:253–263. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- 37.Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–1673. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- 38.Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- 39.Cullinan WE, Wolfe TJ. Chronic stress regulates levels of mRNA transcripts encoding β-subunits of the GABAA receptor in the rat stress axis. Brain Res. 2000;887:118–124. doi: 10.1016/s0006-8993(00)03000-6. [DOI] [PubMed] [Google Scholar]
- 40.Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 41.Krugers HJ, Alfarez DN, Karst H, Parashkouhi K, van Gemert N, Joels M. Corticosterone shifts different forms of synaptic potentiation in opposite directions. Hippocampus. 2005;15:697–703. doi: 10.1002/hipo.20092. [DOI] [PubMed] [Google Scholar]
- 42.Widmaier EP, Dallman MF. The effects of corticotropin-releasing factor on adrenocorticotropin secretion from perifused pituitaries in vitro: rapid inhibition by glucocorticoids. Endocrinology. 1984;115:2368–2374. doi: 10.1210/endo-115-6-2368. [DOI] [PubMed] [Google Scholar]
- 43.Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- 44.Jones MT, Hillhouse EW, Burden JL. Structure-activity relationships of corticosteroid feedback at the hypothalamic level. J Endocrinol. 1977;74:415–424. doi: 10.1677/joe.0.0740415. [DOI] [PubMed] [Google Scholar]
- 45.Kasai M, Kannan H, Ueta Y, Osaka T, Inenaga K, Yamashita H. Effects of iontophoretically applied cortisol on tuberoinfundibular neurons in hypothalamic paraventricular nucleus of anesthetized rats. Neurosci Lett. 1988;87:35–40. doi: 10.1016/0304-3940(88)90141-3. [DOI] [PubMed] [Google Scholar]
- 46.Saphier D, Feldman S. Iontophoretic application of glucocorticoids inhibits identified neurones in the rat paraventricular nucleus. Brain Res. 1988;453:183–190. doi: 10.1016/0006-8993(88)90157-6. [DOI] [PubMed] [Google Scholar]
- 47.Chen YZ, Hua SY, Wang CA, Wu LG, Gu Q, Xing BR. An electrophysiological study on the membrane receptor-mediated action of glucocorticoids in mammalian neurons. Neuroendocrinology. 1991;53 Suppl 1:25–30. doi: 10.1159/000125791. [DOI] [PubMed] [Google Scholar]
- 48.Kasai M, Yamashita H. Inhibition by cortisol of neurons in the paraventricular nucleus of the hypothalamus in adrenalectomized rats: an in vitro study. Neurosci Lett. 1988;91:59–64. doi: 10.1016/0304-3940(88)90249-2. [DOI] [PubMed] [Google Scholar]
- 49.Kasai M, Yamashita H. Cortisol suppresses noradrenaline-induced excitatory responses of neurons in the paraventricular nucleus: an in vitro study. Neurosci Lett. 1988;91:65–70. doi: 10.1016/0304-3940(88)90250-9. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Wang CA, Chen YZ. Nongenomic effect of glucocorticoid on the release of arginine vasopressin from hypothalamic slices in rats. Neuroendocrinology. 1995;62:628–633. doi: 10.1159/000127059. [DOI] [PubMed] [Google Scholar]
- 51.Papanek PE, Sladek CD, Raff H. Corticosterone inhibition of osmotically stimulated vasopressin from hypothalamic-neurohypophysial explants. Am J Physiol. 1997;272:R158–R162. doi: 10.1152/ajpregu.1997.272.1.R158. [DOI] [PubMed] [Google Scholar]
- 52.Zaki A, Barrett-Jolley R. Rapid neuromodulation by cortisol in the rat paraventricular nucleus: an in vitro study. Br J Pharmacol. 2002;137:87–97. doi: 10.1038/sj.bjp.0704832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 54.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 55.Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol. 2005;569:751–760. doi: 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wotjak CT, Ludwig M, Ebner K, Russell JA, Singewald N, Landgraf R, Engelmann M. Vasopressin from hypothalamic magnocellular neurons has opposite actions at the adenohypophysis and in the supraoptic nucleus on ACTH secretion. Eur J Neurosci. 2002;16:477–485. doi: 10.1046/j.1460-9568.2002.02101.x. [DOI] [PubMed] [Google Scholar]
- 57.Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 59.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 60.Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- 62.Orchinik M, Murray TF, Franklin PH, Moore FL. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc Natl Acad Sci USA. 1992;89:3830–3834. doi: 10.1073/pnas.89.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ffrench-Mullen JM. Cortisol inhibition of calcium currents in guinea pig hippocampal CA1 neurons via G-protein-coupled activation of protein kinase C. J Neurosci. 1995;15:903–911. doi: 10.1523/JNEUROSCI.15-01-00903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lou SJ, Chen YZ. The rapid inhibitory effect of glucocorticoid on cytosolic free Ca2+ increment induced by high extracellular K+ and its underlying mechanism in PC12 cells. Biochem Biophys Res Commun. 1998;244:403–407. doi: 10.1006/bbrc.1998.8280. [DOI] [PubMed] [Google Scholar]
- 65.He LM, Zhang CG, Zhou Z, Xu T. Rapid inhibitory effects of corticosterone on calcium influx in rat dorsal root ganglion neurons. Neuroscience. 2003;116:325–333. doi: 10.1016/s0306-4522(02)00568-7. [DOI] [PubMed] [Google Scholar]
- 66.Iwasaki Y, Aoki Y, Katahira M, Oiso Y, Saito H. Non-genomic mechanisms of glucocorticoid inhibition of adrenocorticotropin secretion: possible involvement of GTP-binding protein. Biochem Biophys Res Commun. 1997;235:295–299. doi: 10.1006/bbrc.1997.6785. [DOI] [PubMed] [Google Scholar]
- 67.Qiu J, Lou LG, Huang XY, Lou SJ, Pei G, Chen YZ. Nongenomic mechanisms of glucocorticoid inhibition of nicotine-induced calcium influx in PC12 cells: involvement of protein kinase C. Endocrinology. 1998;139:5103–5108. doi: 10.1210/endo.139.12.6376. [DOI] [PubMed] [Google Scholar]
- 68.Han JZ, Lin W, Lou SJ, Qiu J, Chen YZ. A rapid, nongenomic action of glucocorticoids in rat B103 neuroblastoma cells. Biochim Biophys Acta. 2002;1591:21–27. doi: 10.1016/s0167-4889(02)00242-2. [DOI] [PubMed] [Google Scholar]
- 69.Qiu J, Wang CG, Huang XY, Chen YZ. Nongenomic mechanism of glucocorticoid inhibition of bradykinin-induced calcium influx in PC12 cells: possible involvement of protein kinase C. Life Sci. 2003;72:2533–2542. doi: 10.1016/s0024-3205(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 70.Han JZ, Lin W, Chen YZ. Inhibition of ATP-induced calcium influx in HT4 cells by glucocorticoids: involvement of protein kinase A. Acta Pharmacol Sin. 2005;26:199–204. doi: 10.1111/j.1745-7254.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- 71.Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 73.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 74.Norfleet AM, Clarke CH, Gametchu B, Watson CS. Antibodies to the estrogen receptor-α modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly MJ, Wagner EJ. Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 76.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 78.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 79.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boulware MI, Mermelstein PG. The influence of estradiol on nervous system function. Drug News Perspect. 2005;18:631–637. doi: 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- 81.Liposits Z, Bohn MC. Association of glucocorticoid receptor immunoreactivity with cell membrane and transport vesicles in hippocampal and hypothalamic neurons of the rat. J Neurosci Res. 1993;35:14–19. doi: 10.1002/jnr.490350103. [DOI] [PubMed] [Google Scholar]
- 82.Gametchu B, Watson CS, Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J. 1993;7:1283–1292. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- 83.Guo Z, Chen YZ, Xu RB, Fu H. Binding characteristics of glucocorticoid receptor in synaptic plasma membrane from rat brain. Funct Neurol. 1995;10:183–194. [PubMed] [Google Scholar]
- 84.Orchinik M, Matthews L, Gasser PJ. Distinct specificity for corticosteroid binding sites in amphibian cytosol, neuronal membranes, and plasma. Gen Comp Endocrinol. 2000;118:284–301. doi: 10.1006/gcen.2000.7462. [DOI] [PubMed] [Google Scholar]
- 85.Chen YZ, Qiu J. Possible genomic consequence of nongenomic action of glucocorticoids in neural cells. News Physiol Sci. 2001;16:292–296. doi: 10.1152/physiologyonline.2001.16.6.292. [DOI] [PubMed] [Google Scholar]
- 86.Qi AQ, Qiu J, Xiao L, Chen YZ. Rapid activation of JNK and p38 by glucocorticoids in primary cultured hippocampal cells. J Neurosci Res. 2005;80:510–517. doi: 10.1002/jnr.20491. [DOI] [PubMed] [Google Scholar]
- 87.Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- 88.Li X, Qiu J, Wang J, Zhong Y, Zhu J, Chen Y. Corticosterone-induced rapid phosphorylation of p38 and JNK mitogen-activated protein kinases in PC12 cells. FEBS Lett. 2001;492:210–214. doi: 10.1016/s0014-5793(01)02254-2. [DOI] [PubMed] [Google Scholar]
- 89.Revest JM, Di Blasi F, Kitchener P, Rouge-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 90.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 91.Chwang WB, O’riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 94.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 95.Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]