Abstract
Background aims
A phase I trial examined the ability of immunotherapy to mobilize progenitor and activated T cells.
Methods
Interleukin (IL)-2 was administered subcutaneously for 11 days, with granulocyte (G)-colony-stimulating factor (CSF) (5 mcg/kg/day) and granulocyte–macrophage (GM)-CSF (7.5 mcg/kg/day) added for the last 5 days. Leukapheresis was initiated on day 11. Thirteen patients were treated (myeloma n = 11, non-Hodgkin’s lymphoma n = 2).
Results
Toxicities were minimal. IL-2 was stopped in two patients because of capillary leak (n = 1) and diarrhea (n = 1). Each patient required 2.5 leukaphereses (median; range 1–3) to collect 3.2 × 106 CD34+ cells/kg (median; range 1.9–6.6 × 106/kg). Immune mobilization increased the number of CD3+ CD8+ T cells (P = 0.002), CD56+ natural killer (NK) cells (P = 0.0001), CD8+ CD56+ T cells (P = 0.002) and CD4+ CD25+ cells (P = 0.0001) compared with cancer patients mobilized with G-CSF alone. There was increased lysis of myeloma cells after 7 days (P = 0.03) or 11 days (P = 0.02). The maximum tolerated dose of IL-2 was 1 × 106 IU/m2/day.
Conclusions
Immune mobilization is well tolerated with normal subsequent marrow engraftment. As cells within the graft influence lymphocyte recovery, an increased number of functional lymphocytes may result in more rapid immune reconstitution.
Keywords: effector cells, immune mobilization, interlukin-2, myeloma
Introduction
The use of peripheral blood progenitor cells (PBPC) has surpassed the use of marrow as a source of stem cells in the autologous transplant setting (1). Cellular subsets within the stem cell product vary greatly, depending upon how the cells are mobilized (2–7). A common regimen utilizes granulocyte (G)-colony-stimulating factor (CSF) with cyclophosphamide. Unfortunately, both G-CSF and cyclophosphamide suppress the immune system and thus may influence the cellular subsets mobilized and collected (6,8). The early recovery of an increased number of lymphocytes by day 15 following autologous transplant correlates with improved survival in patients with myeloma, non-Hodgkin’s lymphoma (NHL) and acute myelogenous leukemia (AML) (9–13). As the cellular composition of the stem cell graft influences lymphocyte recovery, we postulated that an ‘improved’ stem cell product with an increased number of highly functional lymphocytes may result in more rapid immune reconstitution following autologous progenitor cell infusion (14,15). If the stem cell product contains activated effector cells, such as natural killer (NK) cells, cytotoxic T cells (CTL) and cytokine-induced killer cells (CIK), their presence may improve immune recovery, and possibly outcome, following transplant (16,17).
In an attempt to mobilize effector cell populations along with progenitor cells, a novel regimen combining interleukin (IL)-2 and growth factors was employed during a phase I clinical trial. We had previously evaluated IL-2 mobilization of hematopoietic progenitor cells in a mouse model. C57Bl/6 mice were administered cyclophosphamide (200 mg/kg) followed by either G-CSF (5 mcg/kg/day) or IL-2 (6000 IU Bi-daily (2×/day)) from day 2 to 6 (6). In contrast to the use of G-CSF, following mobilization with IL-2 the recovering lymphocytes demonstrated increased cytotoxicity against AML (K562) and NHL (Daudi) tumor cell lines. These results suggested an enhanced immune potential of progenitor cells mobilized with IL-2 compared with the G-CSF-mobilized cells.
Based on this mouse model, we designed a clinical trial using IL-2 in combination with G-CSF and granulocyte–macrophage (GM)-CSF for mobilization of autologous progenitor cells. We postulated that immunotherapy would provide efficient mobilization of hematopoietic progenitor cells and immune effector cells. We hypothesized that the increased number and function of the activated immune effector cells would improve the immune function of the graft without compromising hematopoietic recovery post-transplant.
Methods
Patient population and eligibility criteria
Patients were required to have a pathologically confirmed hematologic malignancy with therapy-sensitive disease, either in first remission or relapsed disease following initial remission. Patients between the ages of 17 and 70 years, with a Karnofsky status of ≥80%, were eligible. Adequate cardiopulmonary function was required, including a systolic cardiac ejection fraction ≥45%, Difusion Lung Capacity for CO (DLCO) ≥ 60% and Forced Expiratory Volume (FEV1) and Forced Vital Capacity (FVC) both ≥70% of predicted. Creatinine was required to be <3.0 mg/dL with a measured creatinine clearance of >50 mL/min. The patients needed to have adequate BM funcion to be eligible, exceptions were made if BM function was noted inadequate as a result of characheristic underlying malignancy. The protocol was approved by Dartmouth Medical School’s (Lebanon, NH, USA) Committee for the Protection of Human Subjects. All patients signed an informed consent.
Treatment plan
Treatment schema
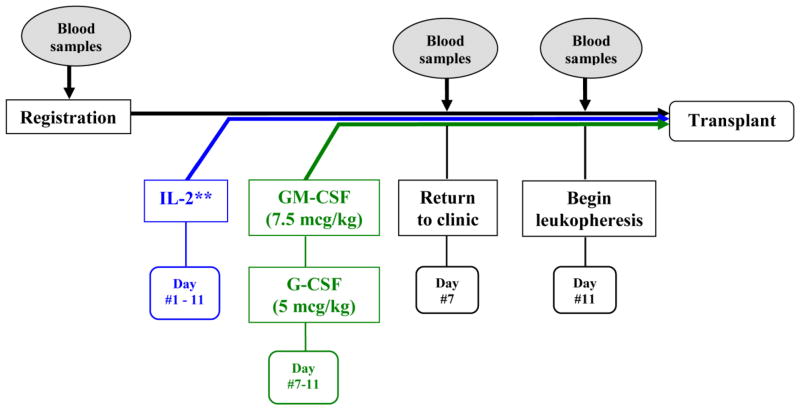
After signing an informed consent, patients were treated as detailed in Figure 1. PBPC were mobilized using IL-2 (Chiron, Emeryville, CA, USA). Treatment with IL-2 began at dose level 1 (6 × 105 IU/m2/day) administered as a daily subcutaneous injection for 11 days. On day 7 of mobilization treatment, G-CSF (5 mcg/kg/day; Amgen, Thousand Oakes, CA, USA) and GM-CSF (7.5 mcg/kg/day; Bayer Pharmaceuticals, Pittsburgh, PA, USA) were started with a daily subcutaneous dose of each medication and continued until completion of leukapheresis. On day 11 of therapy, leukapheresis was initiated if the peripheral blood CD34+ cell number was ≥5 CD34+ cells/μL. Daily leukaphereses of approximately 15–20 L whole blood (approximately 3.5–4.5 total blood volumes over the course of 300 min) were performed and continued until 3 × 106 CD34+ cells/kg had been collected. Each patient was followed for engraftment and toxicity post-transplant.
Figure 1.
Treatment schema. IL-2 was administered on day 1 and continued until completion of leukapheresis. GM-CSF and G-CSF were started on day 7 and continued until completion of leukapheresis.
IL-2 therapy: dose escalation and dose adjustments
The trial was designed to establish the maximum tolerated dose of IL-2 in patients conditioned with this regimen. IL-2 was administered as a single daily subcutaneous injection each evening until completion of leukapheresis (Table I). Patients were started on level 1 (6 × 105 IU/m2/day). If a patient developed grade 2 cardiac or Central Nervous System (CNS) signs/symptoms, or any other ≥ grade 3 toxicity during treatment with IL-2, the IL-2 was held until toxicities recovered to a ≤ grade 1 level. The treatment was then resumed at a 50% dose. If toxicities re-occurred, no further IL-2 was given. IL-2 was discontinued if any dose-limiting toxicity failed to resolve within 72 h. If toxicities occurred whilst the patient was taking IL-2 and growth factors, the IL-2 was held while continuing the G-CSF and GM-CSF.
Table I.
IL-2 dose escalation.
| Level 0 | 3 × 105 IU/m2/day for 11 days | |
| Level 1 | 6 × 105 IU/m2/day for 11 days | Starting dose level |
| Level 2 | 1 × 106 IU/m2/day for 11 days | |
| Level 3 | 1.5 × 106 IU/m2/day for 11 days | |
| Level 4 | 2 × 106 IU/m2/day for 11 days |
PBPC harvest, collection and storage
On day 11 of mobilization, a central venous catheter was placed if peripheral veins could not be used. The collection, concentration and storage procedures of PBPC were standard for each patient. Leukaphereses were performed daily using a COBE Spectra cell separator (CaridianBCT, Lakewood, CO, USA). Collected cells were concentrated to 50–100 mL in a refrigerated centrifuge. PBPC concentrates were cryopreserved in a medium consisting of Normosol-R (Abbott Laboratories, North Chicago, IL, USA), pH 7.4, 10% final concentration dimethyl sulfoxide (DMSO; Cryoserv Research Industries, Salt Lake City, UT, USA) and autologous plasma. Cells were cryopreserved in Cryocyte freezing bags (Nexell Therapeutics Inc., Irvine, CA, USA) in a CryoMed Freezer (Thermo Electron Corporation, Waltham, MA, USA). At the conclusion of freezing, the cells were transferred to the vapor phase of a monitored liquid nitrogen freezer (CryoPlus III; Forma Scientific, Marietta, OH) and stored at a temperature of −120°C or below until infusion.
The cryopreserved PBPC were thawed and then re-infused on day 0 following the induction regimen. Prior to infusion of stem cells, diphenhydramine (50 mg) and acetaminophen (650 mg) were administered.
Monitoring toxicities
Toxicities were monitored and graded using National Cancer Institute (NCI) common toxicity criteria version 3.0. CTCAEv3.0 8-9-2006 (NCI: Bethesda, MD) www.cancer.gov. Once the IL-2 was started, a designated data manager telephoned the patient every other day and evaluated the patient for any potential signs or symptoms of toxicity. Patients returned to the clinic 7 days after starting the IL-2. During this visit, toxicity was evaluated by the treating physician and the designated data manager. In addition, blood tests were obtained, including a complete blood count, liver and kidney tests. The designated data manager also evaluated each patient while receiving leukaphereses. Following transplantation, toxicity was monitored until day 30 or until discharge from the hospital, whichever occurred sooner.
Post-transplantation monitoring
Patients were seen and evaluated daily during their hospital course by a transplant physician using a physical examination and blood work, including a complete blood count, electrolytes, kidney and liver function tests. Routine supportive care was used, including the infusion of packed red blood cells (PRBC) or single donor platelet (SDP) transfusions, and management of infections, as required. PRBC or SDP transfusions were administered if patients were symptomatic or if the hemoglobin level fell below 8 gm/dL, or the platelets dropped below 5 × 109/mm3, respectively. The number of platelet or red blood cell units administered was recorded from the day of admission until discharge.
Engraftment was defined as an absolute neutrophil count (ANC) of ≥500/mm3 for 3 days and a platelet count of ≥20 000/mm3 for 3 days (non-transfused). The first of the 3 days was defined as the engraftment day. All clinical data were placed into a bone marrow transplant-specific database (Stem Soft Company, Vancouver, British Columbia, Canada) and each patient was given a unique patient number.
Laboratory analysis
Phenotypic analyzes of peripheral blood mononuclear cells and leukapheresis products
Blood samples were obtained from each patient at baseline (pre-mobilization) and on day 7. Leukapheresis products were used for the day 11 analyzes (date of collection). Cells were analyzed using standard multicolor flow cytometry (FACscan; Becton-Dickinson, San Jose, CA, USA) with a standard lymphocyte gate and enumerating a common number of events for each cell subset. Following Ficoll–Hypaque separation, mono-nuclear cells from each sample were incubated with antibodies for 60 min at 4°C. After washing three times with cold phosphate-buffered saline (PBS), cells were fixed with 0.5% paraformaldehyde (Sigma, St. Louis, MO). Monoclonal antibodies were used directed against CD3, CD4, CD8, CD56, CD28 and CD25 conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or phycoerythrin-Texas Red (ECD) (Beckman Coulter, Fullerton, CA, USA). Cells were analyzed using standard multicolor flow cytometry (FACscan; Becton-Dickinson).
Phenotypic analyzes were performed using peripheral blood mononuclear cells (PBMNC) from day 0 (baseline) and day 7, while mobilized leukapheresis mononuclear cells were used for the day 11 evaluations. For comparisons, samples from the study patients were compared with samples from control cancer patients. Control patients were cancer patients who were mobilized with G-CSF alone. Appropriate figure legends describe whether controls were used.
Tumor cell lines
Two human myeloma cell lines, RPMI-8226 and U266, were obtained from American Type Culture Collection (Rockville, MD, USA). Cell lines were cultured in RPMI-1640 (Cellgro, Herndon, VA, USA) containing 10% fetal bovine serum (FBS; Life Technologies, Rockville, MD, USA), 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen Corp., Carlsbad, CA, USA).
Cytotoxicity of mobilized blood mononuclear cells
The cytotoxicity of the patients’ PBMNC on the day of collection was evaluated prior to starting therapy (day 0) and day 7 of mobilization. Mobilized leukapheresis mononuclear cells were used for the day 11 evaluations. Cryopreserved PBMNC were thawed rapidly in a 37°C water bath and washed twice with AIM-V medium (Life Technologies, Rockville, MD). Following determination of viability using trypan blue, 1 × 106/mL cells were placed in T-75 cm2 tissue culture flasks (Costar, Cambridge, MA, USA) in serum-free AIM-V medium, supplemented with 1000 IU/mL IL-2 (Chiron) and incubated in 5% CO2 at 37°C for 4 h. Mononuclear cells were harvested, washed twice with AIM-V medium, and used as the effector cells in a standard 4-h51 Cr-release assay. A human myeloma cell line, RPMI-8226, was used for the target cells. Each experiment was performed using triplicate samples. The percentage lysis was calculated using a standard formula.
Statistical analyzes
The trial was designed to establish the maximum tolerated dose of IL-2 in patients conditioned with this regimen. Cohorts of three or six patients were to be treated at each dose level, beginning with dose level 1. The Maximum Tolerable Dose (MTD) was defined as the dose level at which fewer than 33% of patients experienced dose-limiting toxicity (DLT). Beginning with dose level 1, three patients were to be treated at each dose level. If no grade 2 cardiac or CNS, or ≥ grade 3 toxicity, was observed, subsequent patients would be entered into higher dose levels. If DLT was observed in one of these three patients, the cohort would be expanded to a total of six patients treated at that dose level. Dose escalation would occur (i.e. a new cohort of three patients would be treated at the next higher dose level) if DLT was observed in 0 of three or one of six patients. Otherwise, if, at any time, DLT was observed in at least two patients in a given cohort, accrual of patients to that cohort would stop. The MTD would be declared as the next lower dose level and the trial would stop. When analyzing the flow cytometry and cytotoxicity data using patient samples, the differences were compared using a Student’s t-test.
Results
Patient demographics and disease characteristics
Thirteen patients were accrued (multiple myeloma n = 11, NHL n = 2). Despite demonstrating chemotherapy-sensitive disease, one patient with lymphoma progressed during mobilization and was removed from the protocol. Six patients were female, and the median age was 61 years (range 45–69 years). The number of pre-transplant treatment regimens was one (median; range 1–2).
Mobilization process and stem cell collection
There were no difficulties with the collection of autologous cells and each patient mobilized enough cells for the transplant (Table II). Patients required 2.5 leukaphereses (median; range 1–3). The median number of CD34+ cells/kg collected was 3.2 × 106 CD34+ cells/kg (mean; range 1.9–6.6 × 106 CD34+ cells/kg). The median number of mononuclear cells collected was 17 × 108/kg (median; range 3.8–21 × 108/kg).
Table II.
IL-2 dose levels, cell collections and toxicities.
| Unique patient numbers | Diagnosis IL-2 dose level | Required number of leukaphereses | Total cells collected
|
Toxicities (≥grade 2) | ||
|---|---|---|---|---|---|---|
| CD34+ cells/kg | MNC cells/kg | |||||
| UPN 001 | MM | Level 1 | 2 | 2.3 × 106 | 6.4 × 108 | Back pain, gr 2 |
| UPN 002 | NHL | Level 1 | NA | NA | NA | |
| UPN 003 | MM | Level 1 | 2 | 3.1 × 106 | 5.8 × 108 | None |
| UPN 004 | MM | Level 1 | 3 | 1.9 × 106 | 12 × 108 | None |
| UPN 005 | MM | Level 1 | 2 | 2.6 × 106 | 8.7 × 108 | None |
| UPN 006 | MM | Level 1 | 3 | 3.5 × 106 | 21 × 108 | None |
| UPN 007 | MM | Level 2 | 2 | 3.7 × 106 | 8.9 × 108 | Fever, gr 2 |
| UPN 008 | MM | Level 2 | 1 | 3.2 × 106 | 3.8 × 108 | Nausea, gr 2; back pain, gr 2 |
| UPN 009 | MM | Level 2 | 2 | 4.4 × 106 | 9 × 108 | None |
| UPN 010 | MM | Level 3 | 1 | 6.6 × 106 | 4.3 × 108 | None |
| UPN 011 | MM | Level 3 | 3 | 2.1 × 106 | 6.9 × 108 | Fatigue, gr 2; fluid retention/vascular leak, gr 2 |
| UPN 012 | MM | Level 3 | 2 | 4.4 × 106 | 19 × 108 | Fever, gr 2; chills, gr 2 |
| UPN 013 | NHL | Level 3 | 3 | 1.9 × 106 | 14 × 108 | Anorexia, gr 3; diarrhea, gr 3 |
| Median | 2.5 | 3.2 × 106 | 17 × 108 | |||
All toxicities ≥ grade 2 are listed; gr, grade; NA, not applicable; MNC, Mono Nuclear Cells; MM, Multiple Myeloma.
UPN 002 was removed from the trial during mobilization. UPN 011 and UPN 013 experienced dose-limiting toxicities and IL-2 was discontinued.
Toxicities and defining the maximum tolerated dose of IL-2
There were no treatment-related mortalities. One patient with lymphoma was removed from treatment because of disease progression during mobilization (UPN 002). Of the remaining 12 patients, 10 completed the full course of IL-2 and growth factors. Toxicities (≥ grade 2) observed during the mobilization course were minimal and are listed in Table II. Treatment with IL-2 began at dose level 1 (6 × 105 IU/m2/day). As one of the first three patients was removed because of disease progression, six patients were treated at dose level 1. Two patients at dose level 3 of IL-2 (1.5 × 106 IU/m2/day) experienced IL-2 toxicities on the day of collection, including fluid overload/capillary leak (UPN 011) and diarrhea with anorexia (UPN 013). Although these toxicities did not meet the criteria for discontinuing IL-2 therapy, enough cells were collected for transplant (although not meeting the trial criteria of 3 × 106 CD34+ cells/kg) and the treating physician believed the toxicities were clinically significant and caused by the IL-2. As a result, the maximum tolerated dose of IL-2 was defined as 1 × 106 IU/m2/day (level 2).
Engraftment, blood product support and length of stay
Following transplantation, all patients engrafted without delay. The number of days required for the ANC to reach 500 cells/mm3 was 12.3 (median; range 9–14). The number of days required for the platelet count to reach 20 000/mm3 was 10.5 days (median; range 0–17 days). The median number of red blood cell units transfused to each patient was 2 (range 0–4 units). The median number of platelet transfusions required per patient was 1 (range 0–3 units). The length of hospital stay was 14.8 days (median).
Recovery of T-cell subsets in patients’ mobilized progenitor cells
Prior to combining the data, the results from each of the three IL-2 dose levels were analyzed. As there were no statistically significant differences among all the dose levels, the data from the patient samples were grouped together and the results are presented in aggregate.
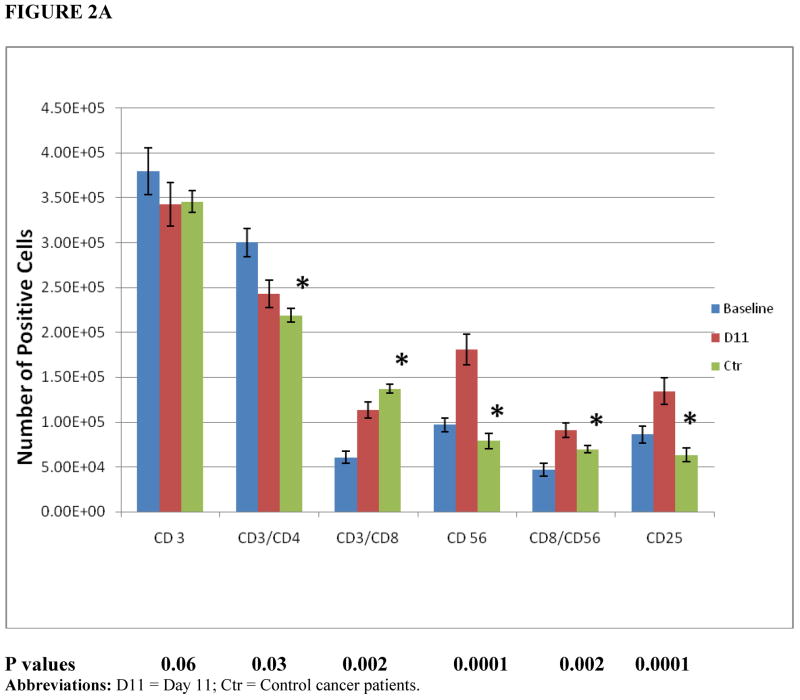
PBMNC were collected from patients prior to initiating mobilization and at day 7, after 7 days of IL-2 therapy and before starting G-CSF and GM-CSF. Leukapheresis products were analyzed on day 11, the day of collection. Figure 2A demonstrates the phenotypic changes of effector cells within the leukapheresis products after 11 days of mobilization, compared with baseline and control cancer patients. Immune mobilization resulted in an increased number of CD3+ CD4+ T cells (P = 0.03), CD56+ NK cells (P = 0.0001) and CD8+ CD56+ T cells (P = 0.002) within progenitor cells on collection (day 11), compared with control cancer patients. The expression of CD25, the IL-2 receptor, markedly increased on day 11 (P = 0.0001). There was no difference in the number of CD3+ T cells between the control cancer group and the patients on trial (P = 0.06).
Figure 2.
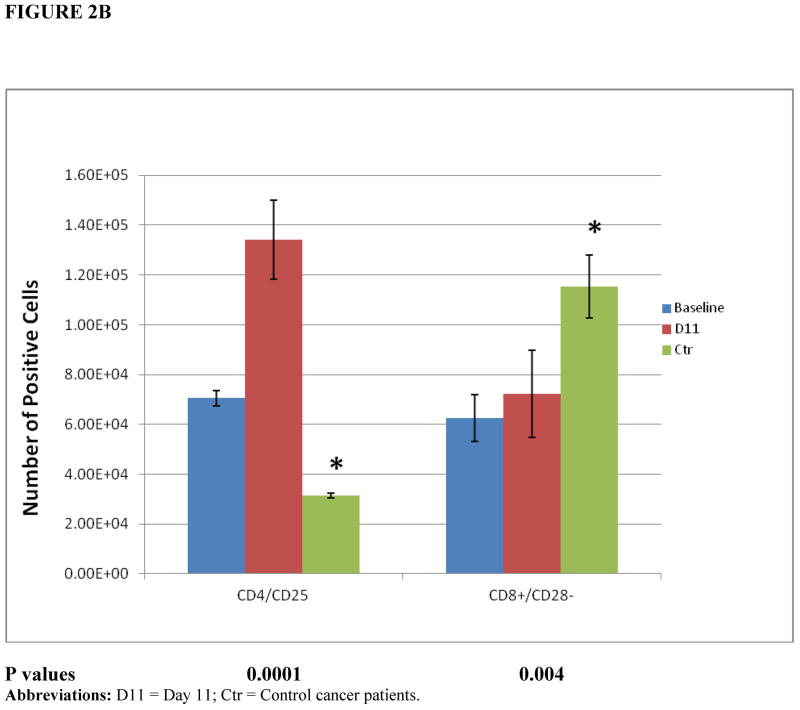
(A) Cell populations within patients’ mobilized progenitor cells. Patients’ PBMNC (baseline, day 0) or leukapheresis products (day 11 of therapy) were analyzed using multicolor FACscan analyzes with a standard lymphocyte gate and enumerating a common number of events for each cell subset (n = 8 patients, n = 3 controls). Control results are depicted for day 11 only. (B) Activated T cells (CD4+ CD25+) and T-suppressor cell populations (CD8+ CD28−). Patients’ PBMNC (day 0) or leukapheresis products (day 11 of therapy) were analyzed using multicolor FACscan analyzes with a standard lymphocyte gate and enumerating a common number of events for each cell subset (n = 8 patients, n = 3 controls). Control results are depicted for day 11 only. The effector:target (E:T) ratio was 20:1. Control group: cancer patients who were mobilized with G-CSF alone. *Statistical significance comparing day 11 results from the control cancer group with the treatment group. D11 = Day 11; Ctr = Control cancer patients.
The number of activated T cells, defined as CD4+ CD25+ T cells, increased markedly compared with control patients (P = 0.0001). CD8+ CD28− T-suppressor cell numbers were much higher in control patients compared with the trial patients (P = 0.004) (Figure 2B).
Cytotoxicity of PBMNC
The regimen mobilized an increased number of effector cells that demonstrated marked increased lysis of human myeloma cells (Figure 3). When testing against a human myeloma cell line, there was a marked increase in lysis of RPMI-8226 myeloma cells after 7 days of IL-2 (P = 0.03), and this increase in lysis was sustained at day 11, the day of collection (P = 0.02).
Figure 3.
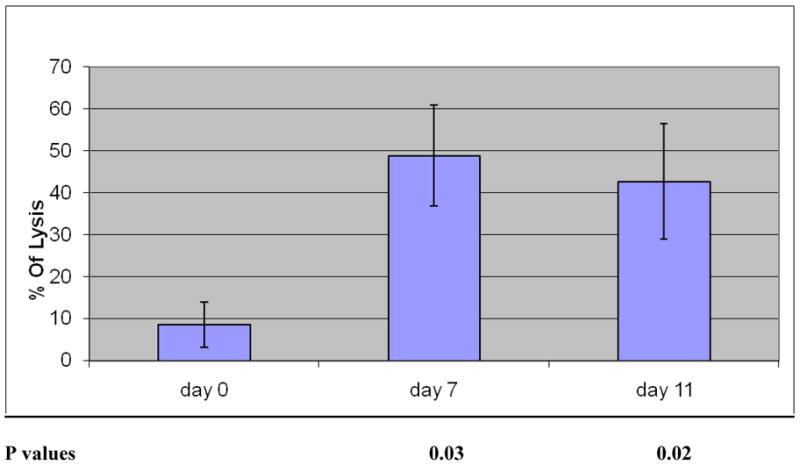
Cytotoxic function of the immune mobilized progenitor cells. Myeloma patients received IL-2 for 11 days and GM-CSF and G-CSF for the last 5 days. Leukapheresis occurred on day 11. Patients’ mobilized mononuclear cells served as effector cells. Targets were RPMI-8226, a human myeloma cell line. P-values compared cytotoxicity at baseline (day 0) with other days. E:T = 20:1; n = 6 patients; P < 0.03 (day 7), 0.02 (day 11).
NKG2D+ CD8+ T cells
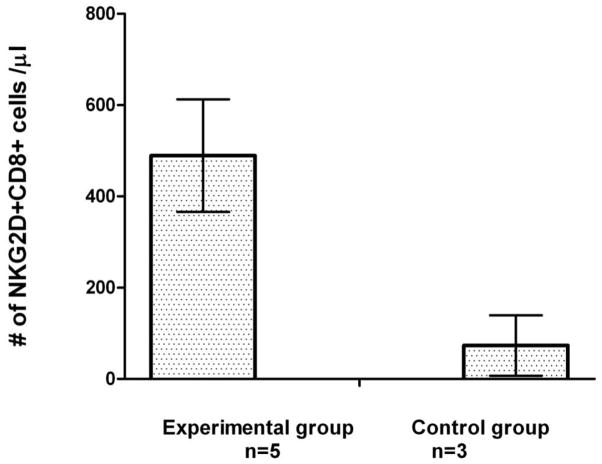
Our ex vivo expansion methods of mononuclear cells had previously identified a population of CD8+T cells that lysed myeloma cells in a major histocompatibility complex (MHC) and T-cell receptor (TCR)-independent manner (18–22). These CD8+T cells acquired NKG2D during ex vivo expansion. NKG2D+ CD8+T cells are quite aggressive against autologous myeloma cells and kill patients’ myeloma cells. Compared with cancer patients receiving G-CSF mobilization alone (control patients), patients receiving immune mobilization demonstrated an increased number of NKG2D+ CD8+T cells at the time of collection, demonstrating that this regimen increased the number of NKG2D+ CD8+T cells in vivo (Figure 4).
Figure 4.
Immune mobilization: increase in circulating NKG2D+ CD8+ T cells. The number of NKG2D+ CD8+ T cells collected from patients receiving immune mobilization with IL-2, GM-CSF and G-CSF (n = 5 patients) was compared with patients receiving mobilization with G-CSF alone (control group; n = 3 patients). Control group: cancer patients who were mobilized with G-CSF alone. Immune mobilization markedly increased the number of NKG2D+ CD8+ T cells mobilized compared with control patients (P < 0.002).
Discussion
Myeloma patients experience an improved survival if they receive an increased number of lymphocytes within transplanted cells or demonstrate an elevated lymphocyte recovery within 2 weeks of transplant (10–17). We designed a mobilization regimen to increase the number and function of lymphocytes within the collected cells that would be used for transplant. We postulated that IL-2 and growth factors would mobilize populations of cytotoxic effector cells with an efficient number of CD34+ hematopoietic progenitor cells. We proposed that these ‘effector cells’, in particular T cells and NK cells, would markedly enhance the immune function of the graft. The results demonstrate that immune mobilization is well tolerated. Ten of 12 patients completed the full course of therapy. The pre-determined definition of the maximum tolerated dose was based on the clinical tolerability of IL-2 by each patient at each dose level, not on laboratory results. Based on clinical toxicities, 1 × 106 IU/m 2/day IL-2 (dose level 2) was defined as the maximum tolerated dose.
This immune mobilization regimen increases the number of CD3+ CD8+ T cells, CD8+ CD56+ T cells and CD56+ NK cells within the collected cells. As expected, expression of CD25, the IL-2 receptor, markedly increased on day 11 in the treatment cohort, as exogenously administered IL-2 up-regulates IL-2 receptor expression (CD25). It was surprising that there was no difference in the number of CD3+ T cells between the control group and the patients on trial, as we anticipated the T-cell number to increase as a result of IL-2. The number of cells expressing CD4+ CD25+, probably reactive T cells, increased markedly compared with control patients, while CD8+ CD28− T-suppressor cell numbers were lower in the study patients. There was no detrimental effect on subsequent marrow engraftment following transplant. However, our results indicated that a log more CD34+ cells/kg were collected on day 11 in the control patients, compared with the study patients, suggesting that IL-2 may be suppressing mobilization of hematopoietic cells.
This regimen also mobilized an increased number of cytotoxic effector cells with increased killing of myeloma cells. Following transplant, the recovery of T and NK cells results partly from peripheral expansion of the infused cells. As a result, the increased number and function of these effector cells within the autologous graft may offer a potential benefit, by aggressively eradicating residual malignant cells in vivo (16,17).
The mobilization regimen determines the number of CD34+ cells collected. Within the past few years, research has shown that a larger number of lymphocytes within the infused cells is associated with an improved survival (11,14,15). Hence, if a mobilization regimen increases the number of lymphocyte subsets within the collected cell product, patient survival may improve. Within the transplant setting, clinicians are optimizing high-dose therapy options and also focusing on post-transplant maintenance therapies, especially in the setting of myeloma. Our results suggest an additional focus should be the pre-transplant mobilization regimen, as disparate mobilization regimens will produce different cellular subsets, which may affect immune reconstitution and, perhaps, survival.
The selection of a mobilization regimen is based on the potential cell yield, risk of tumor cell contamination and costs. The mobilization regimen not only affects the number and cell populations collected, but also determines subsequent engraftment and clinical course (1,17). For example, using a mobilization trial with a large number of patients, Gertz et al. (23) showed that the addition of cyclophosphamide to growth factors yielded an increased CD34+ cell number but resulted in longer times to engraftment of both platelets and neutrophils, compared with growth factors alone (P = 0.001). In addition, patients receiving cyclophosphamide experienced a higher incidence of post-transplant bacteremia (23).
The use of IL-2 for mobilization is relatively novel. To date, three small clinical trials have evaluated the use of IL-2 in the mobilization of autologous progenitor cells. While these trials were unique at the time they were performed, they were small and often did not compare laboratory results with clinical endpoints (24–26). Although both G-CSF and GM-CSF can be used for mobilization, GM-CSF was added to our regimen because only GM-CSF possesses potent immune effects (27–29). For example, GM-CSF will (a) enhance the tumor-directed lytic ability of PBMNC; (b) activate macrophages and (c) mediate the proliferation, maturation and migration of dendritic cells. In addition, GM-CSF mobilization may enhance innate and acquired immunity after transplant, because it decreases the number of type 2 dendritic cells (DC2) within the mobilized cells (30,31). These dendritic cells may inhibit cellular immune responses.
Although a relatively small clinical trial, the current study satisfied its pre-determined goal of identifying a maximum tolerated dose of IL-2 (in combination with growth factors) that would mobilize an efficient number of CD34+ cells. The correlative laboratory results of the trial support the initial hypothesis that immune mobilization increases the number of functionally active effector cells that recognize and kill tumor cells. Although the trial was designed for patients with any hematologic malignancy, 11 of 13 patients accrued had myeloma. As a result, the conclusions need to be interpreted cautiously because they may be unique to myeloma patients.
We identified a few interesting clinical findings during the trial. For example, we discovered that neither the peripheral blood counts nor the lymphocyte numbers during mobilization were predictive of the timing of progenitor cell collection. In addition, despite the use of IL-2 for 7 days, there was no corresponding increase in peripheral blood lymphocytes, probably because of the low dose of IL-2 utilized. Although the regimen was well tolerated, toxicities generally occurred after the addition of growth factors to the IL-2. For example, UPN 11 and UPN 13 were removed at the time of apheresis, after receiving 11 days of IL-2 and 5 days of G-CSF and GM-CSF. Finally, the low MTD dose of IL-2 needs to be addressed. Based on the past experiences of the investigators, the dose of IL-2 tolerated by patients undergoing autologous stem cell transplant for a hematologic malignancy is much lower than the IL-2 dose tolerated by patients with renal carcinoma. This was true in this trial, as, once again, a low dose of IL-2 (1 × 106 IU/m2/day) was defined as the biologically active dose.
In summary, we present a unique immune mobilization trial using a combination of IL-2 and growth factors. This trial demonstrates that the mobilization regimen is a critical part of the treatment, as the therapy used to mobilize cells determines the cellular subsets and activity of the effector cells collected and used for transplant. The regimen mobilizes CD34+ progenitor cells and improves the immune properties of the mobilized stem cells by increasing the number and function of activated T cells and NK cells. These activated cells may enhance immune reconstitution following transplantation. Ongoing laboratory studies and clinical trials at our institution are examining ways to optimize pre-transplant immune mobilization as part of the transplant process. Our current laboratory analyzes are examining methods to improve tumor cell killing by these immune mobilized effector cells, by examining novel receptor–ligand interactions between the mobilized effector cells and malignant cells (18,19).
Acknowledgments
This study was sponsored in part by a grant from the Hitchcock Foundation, Dartmouth Medical School and the Dartmouth-Hitchcock Medical Center (KRM), Leukemia and Lymphoma Society Translational Research Grant 6061-06 (KRM), Dartmouth College NIH COBRE Award (KRM), R21 CA112761 (KRM, MSE) and R01CA095648 (MSE). The research was also funded through an unrestricted research grant from Bayer Pharmaceuticals.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–95. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 2.Cesana C, Carlo-Stella C, Regazzi E, Garau D, Sammarelli G, Caramatti C, et al. CD34+ cells mobilized by cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) are functionally different from CD34+ cells mobilized by G-CSF. Bone Marrow Transplant. 1998;21:561–8. doi: 10.1038/sj.bmt.1701133. [DOI] [PubMed] [Google Scholar]
- 3.Webb IJ, Eickhoff CE, Elias AD, Ayash LJ, Wheeler CA, Schwartz GN, et al. Kinetics of peripheral blood mononuclear cell mobilization with chemotherapy and/or granulocyte-colony-stimulating factor: implications for yield of hematopoietic progenitor cell collections. Transfusion. 1996;36:160–7. doi: 10.1046/j.1537-2995.1996.36296181930.x. [DOI] [PubMed] [Google Scholar]
- 4.Demirer T, Buckner CD, Storer B, Lilleby K, Rowley S, Clift R, et al. Effect of different chemotherapy regimens on peripheral blood stem cell collections in patients with breast cancer receiving granulocyte colony-stimulating factor. J Clin Oncol. 1997;15:684–90. doi: 10.1200/JCO.1997.15.2.684. [DOI] [PubMed] [Google Scholar]
- 5.Talmadge JE, Reed EC, Kessinger A, Kuszynski CA, Perry GA, Gordy CL, et al. Immunologic attributes of cytokine mobilized peripheral blood stem cells and recovery following transplantation. Bone Marrow Transplant. 1996;17:101–9. [PubMed] [Google Scholar]
- 6.Verma UN, Mazumder A, Meehan KR. Interleukin-2 in combination with cyclophosphamide for peripheral blood stem cell mobilization. Cancer Res. 1997;38:3268a. [Google Scholar]
- 7.Gajewski J, Rondon G, Mehra R, et al. Preliminary results of a randomized trial comparing intensive chemotherapy with growth factor for peripheral blood progenitor cell mobilization to growth factor alone for hematopoietic rescue after high dose chemotherapy. Blood. 1998;92:271a. [Google Scholar]
- 8.Ojeifo JO, Wu AG, Miao Y, Herscowtiz HB, Meehan KR. Docetaxel-induced mobilization of hematopoietic stem cells in a murine model: kinetics, dose titration, and toxicity. Exp Hematol. 2000;28:451–9. doi: 10.1016/s0301-472x(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–85. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Sohn HJ, Kim S, Lee JS, Kim WK, Suh C. Early lymphocyte recovery predicts longer survival after autologous peripheral blood stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2006;37:1037–42. doi: 10.1038/sj.bmt.1705373. [DOI] [PubMed] [Google Scholar]
- 11.Porrata LF, Litzow MR, Inwards DJ, Gastineau DA, Moore SB, Pineda AA, et al. Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33:291–8. doi: 10.1038/sj.bmt.1704355. [DOI] [PubMed] [Google Scholar]
- 12.Porrata LF, Litzow MR, Tefferi A, Letendre L, Kumar S, Geyer SM, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–8. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 13.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–6. [PubMed] [Google Scholar]
- 14.Hiwase DK, Hiwase S, Bailey M, Bollard G, Schwarer AP. Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14:116–24. doi: 10.1016/j.bbmt.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Porrata LF, Gertz MA, Geyer SM, Litzow MR, Gastineau DA, Moore SB, et al. The dose of infused lymphocytes in the autograft directly correlates with clinical outcome after autologous peripheral blood hematopoietic stem cell transplantation in multiple myeloma. Leukemia. 2004;18:1085–92. doi: 10.1038/sj.leu.2403341. [DOI] [PubMed] [Google Scholar]
- 16.Condomines M, Quittet P, Lu ZY, Nadal L, Latry P, Lopez E, et al. Functional regulatory T cells are collected in stem cell autografts by mobilization with high-dose cyclophosphamide and granulocyte colony-stimulating factor. J Immunol. 2006;176:6631–9. doi: 10.4049/jimmunol.176.11.6631. [DOI] [PubMed] [Google Scholar]
- 17.Gazitt Y. Immunologic profiles of effector cells and peripheral blood stem cells mobilized with different hematopoietic growth factors. Stem Cells. 2000;18:390–8. doi: 10.1634/stemcells.18-6-390. [DOI] [PubMed] [Google Scholar]
- 18.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–8. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 19.Wu JY, Ernstoff MS, Hill JM, Cole B, Meehan KR. Ex vivo expansion of non-MHC-restricted cytotoxic effector cells as adoptive immunotherapy for myeloma. Cytotherapy. 2006;8:141–8. doi: 10.1080/14653240600620218. [DOI] [PubMed] [Google Scholar]
- 20.Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as a novel immunotherapy for multiple myeloma. J Exp Heme. 2008;36:1318–28. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J, Wu J, Webber S, Ernstoff MS, Szczepiorkowski Z, Sentman C, Meehan KR. Immune mobilization with direct in-vivo effector response: favorable implications for post-transplant outcome. Blood. 2007;10:361a. [Google Scholar]
- 22.Meehan KR, Wu J, Ernstoff MS, Szczepiorkowski Z, Barber A, Sentman C. Development of a laboratory model for ex vivo expansion of multiple populations of effector T cells. Cytotherapy. 2008;10:30–7. doi: 10.1080/14653240701762398. [DOI] [PubMed] [Google Scholar]
- 23.Gertz MA, Kumar SK, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43:619–25. doi: 10.1038/bmt.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Gast GC, Vyth-Dreese FA, Nooijen W, van den Bogaard CJ, Sein J, Holtkamp MM, et al. Reinfusion of autologous lymphocytes with granulocyte–macrophage colony-stimulating factor induces rapid recovery of CD4+ and CD8+ T cells after high-dose chemotherapy for metastatic breast cancer. J Clin Oncol. 2002;20:58–64. doi: 10.1200/JCO.2002.20.1.58. [DOI] [PubMed] [Google Scholar]
- 25.Schiller GJ, Vescio R, Berenson J. Cytomegalovirus infection following transplantation of autologous CD34-selected progenitor cells. Blood. 2000;96:1194–5. [PubMed] [Google Scholar]
- 26.Sosman JA, Stiff P, Moss SM, Sorokin P, Martone B, Bayer R, et al. Pilot trial of interleukin-2 with granulocyte colony-stimulating factor for the mobilization of progenitor cells in advanced breast cancer patients undergoing high-dose chemotherapy: expansion of immune effectors within the stem-cell graft and post-stem-cell infusion. J Clin Oncol. 2001;19:634–44. doi: 10.1200/JCO.2001.19.3.634. [DOI] [PubMed] [Google Scholar]
- 27.Grabstein KH, Urdal DL, Tushinski RJ, Mochizuki DY, Price VL, Cantrell MA, et al. Induction of macrophage tumoricidal activity by granulocyte–macrophage colony-stimulating factor. Science. 1986;232:506–8. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 28.Joshi SS, Lynch JC, Pavletic SZ, Tarantolo SR, Pirruccello SJ, Kessinger A, et al. Decreased immune functions of blood cells following mobilization with granulocyte colony-stimulating factor: association with donor characteristics. Blood. 2001;98:1963–70. doi: 10.1182/blood.v98.6.1963. [DOI] [PubMed] [Google Scholar]
- 29.Wing EJ, Magee DM, Whiteside TL, Kaplan SS, Shadduck RK. Recombinant human granulocyte/macrophage colony-stimulating factor enhances monocyte cytotoxicity and secretion of tumor necrosis factor alpha and interferon in cancer patients. Blood. 1989;73:643–6. [PubMed] [Google Scholar]
- 30.Hock BD, Haring LF, Ebbett AM, Patton WN, McKenzie JL. Differential effects of G-CSF mobilization on dendritic cell subsets in normal allogeneic donors and patients undergoing autologous transplantation. Bone Marrow Transplant. 2002;30:733–40. doi: 10.1038/sj.bmt.1703734. [DOI] [PubMed] [Google Scholar]
- 31.Lonial S, Hicks M, Rosenthal H, Langston A, Redei I, Torre C, et al. A randomized trial comparing the combination of granulocyte–macrophage colony-stimulating factor plus granulocyte colony-stimulating factor versus granulocyte colony-stimulating factor for mobilization of dendritic cell subsets in hematopoietic progenitor cell products. Biol Blood Marrow Transplant. 2004;10:848–57. doi: 10.1016/j.bbmt.2004.07.008. [DOI] [PubMed] [Google Scholar]