Abstract
Proteins p130 and E2f4, members of the retinoblastoma protein (pRb) family/E2F transcription factor family, are the key elements in regulation of cell cycle and differentiation. The functional role of the p130/E2f4 in mesenchymal stem cells (MSC) is unclear. We demonstrate here that activation of the Wnt/β-catenin pathway in mouse MSC is associated with accumulation of active forms of the p130, E2f4, and β-catenin but does not result in inhibition of cell cycle progression. The levels and phosphorylation patterns of p130, E2f4, and β-catenin in MSC do not change during cell cycle progression. This is different from control T98G glyoblastoma cells that accumulated differently phosphorylated forms of the p130 in quiescence, and under active proliferation. In MSC, synchronized at G0/G1 and S cell cycle phases, the p130 and β-catenin physically interact each other, whereas Gsk3β was associated and co-precipitated with both p130 and β-catenin. Our results indicate that Wnt/β-catenin and pRb signal pathways interact with each other and form common p130/Gsk3β/β-catenin complex during MSC cycle progression. Physiological relevance of such complex may be associated with coupling of the cell cycle and differentiation in MSC, which is related to a wide differentiation potential of these stem cells.
Introduction
Coupling of the signaling pathways that regulate cell cycle progression and cell differentiation in majority of cell lines occurs at R1 point of G1 phase [1]. Exit from G1 is under the control of the p130, a member of the retinoblastoma gene product (pRb) family. P130 forms a repressor complex with transcription factor E2f4 [2,3]. E2f4 belongs to the E2F protein family, which is the core transcriptional regulator of multiple genes representing key elements of the cell cycle, replication, and mitotic machineries [4].
The p130/E2f4 repressor complex is formed in quiescence [5]. At G1/S transition the levels of p130 are sharply decreased, and in some cells this protein is undetectable until the end of mitosis [3]. Physiological importance of the decline of p130 levels in proliferating cells is in the elimination of its suppressor effect on synthesis of the Cyclin E/A-Cyclin-dependent kinase (Cdk) complexes required for cell cycle progression [6]. It was shown recently that p130 and E2f4 are included in multi-subunit protein complexes that are highly conserved in evolution and functionally associated with regulation of chromatin status and activity of cell cycle genes [7–9].
The ability of p130 to interact with E2f4 is regulated by phosphorylation. Twenty-two phosphorylation sites on p130 include the specific motif with Ser and Thr amino acids phosphorylated in vivo [10]. During cell cycle progression p130 is sequentially modified by Cyclin D-Cdk4/6 and Cyclin E/A-Cdk2, whereas in quiescence—by Gsk3β [11,12].
The molecular population of the p130 is subdivided into 3 groups, containing hypophosphorylated (p1), phosphorylated (p2), and hyperphosphorylated (p3) forms possessing distinct electrophoretic mobility [13]. The p1 and p2 forms poorly include radioactive label, are stable in culture during cell cycle progression, and accumulate at G0/G1. These forms represent the entire pool of the p130 in the tissues characterized by cell quiescence and high levels of differentiation [14]. The form p3 occurs at G1/S transition [15]. All 3 forms of the protein are produced in the tissues and cell lines containing actively proliferating cells, for example, human glyoblastoma T98G, mouse myoblasts C2C12, and rat myoblasts L6. However, the synthesis of p3 is inhibited in these lines after induction of differentiation. In contrast, tissue hepatocytes in quiescence produce only p1 and p2, but begin to produce all 3 forms of the protein 6–12 h after experimental hepatoectomy [14]. The mechanism of the p1 formation is still unclear. It may be the result of p130 phosphorylation by non-Cdks or Cdks; however, the level of the Cdks at G0/G1 is very low [12]. The form p2 is produced at G0/G1 due to p130 phosphorylation by Gsk3β. The p1 and p2 are transformed to the p3 form at G1/S under treatment with CyclinD-Cdk4/6 [15].
One of the main physiological targets of Gsk3β is β-catenin—transmitter of the Wnt signals in canonical Wnt/β-catenin pathway. The Wnt signals promote inactivation of the Gsk3β and result in cytosolic accumulation of the β-catenin and its translocation into nucleus. In nucleus β-catenin interacts with transcription factors of the T-cell factor/lymphoid enhancer factor (LEF/TCF) family involved in regulation of different cell functions, including proliferation and differentiation [16]. The Gsk3β-mediated modification of p130 and β-catenin may couple the pRb and Wnt/β-catenin pathways in combined regulation of cell cycle and differentiation. The Gsk3β interaction with β-catenin is cell cycle independent in contrast to that of the p130, which is effective at G0/G1. It is still unclear whether Gsk3β modifies p130 after G1/S transition in cell lines keeping the protein in active form during cell cycle progression. Interaction of Gsk3β, β-catenin and p130 at G0/G1 suggests that these proteins may form a stable complex existing until the end of mitosis. Published results describing phosphorylation of β-catenin and p130 are obtained in cells of somatically differentiated cell lines [10,12]. It is unknown whether the Gsk3β-mediated regulation of β-catenin and p130 is active in stem cells, specifically in mesenchymal stem cells (MSC).
The goal of this study was to investigate the interaction of Gsk3β, β-catenin, and p130 in mouse MSC under normal conditions, at different phases of the cell cycle and under conditions of stimulation of the Wnt/β-catenin signaling pathway. Our first aim was to determine whether p130 and E2f4 are activated in MSC by co-culture with A-549 cells or by lithium ions, both of which induce the Wnt/β-catenin pathway [17–20]. The second aim was to determine the proliferation status of MSC in co-culture with A-549 cells activating the MSC differentiation. The third aim was to compare the levels and phosphorylation status of p130 and β-catenin in MSC with those in T98G cells. The fourth goal was to investigate whether p130 and β-catenin form complex in MSC and how this complex is modified at different cell cycle phases. The final goal was to determine whether p130 and β-catenin may form a complex including Gsk3β in MSC under normal conditions and after stimulation of the Wnt/β-catenin pathway. The results obtained indicate for the first time that Wnt/β-catenin and pRb signaling pathways interact with each other and complexes of p130/β-catenin/Gsk3β are formed during cell cycle progression.
Materials and Methods
Cell culture, synchronization, and flow cytometry analysis
The A-549 human non-small-cell-lung carcinoma cells, T98G human glyoblastoma cells, and 293 human embryonic kidney cells transformed by adenovirus were obtained from ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% of fetal bovine serum (FBS). MSC were prepared earlier [19] from bone marrow of the transgenic male mice C57BL/6-Tg(ACTbEGF)1Osb/J (GFP) purchased from the Jackson Labs.
For growth arrest, the subconfluent T98G cells were grown for 72 h in the growth medium (GM): DMEM with 0.1% FBS, re-stimulated with 10% FBS, and used in synchronization experiments in 6-h intervals after re-stimulation. For growth arrest of the MSC we used cell cultures with 40% of saturation density that were grown for 48 h in DMEM containing 0.15% FBS. Then, the medium was changed for 48 h to GM containing 5 mM thymidine (Sigma). To obtain the MSC in S phase the growth-arrested cells attached to plastic were washed twice with phosphate-buffered saline (PBS) and cultured for 5 h in normal GM. For the flow cytometry analysis, the synchronized T98G cells or MSC were washed twice with PBS and trypsinized. Then, cells were washed twice in PBS containing 1% of bovine serum albumin. For nuclear staining, cells were resuspended in appropriate volume of PBS, containing 250 μg/mL of RNAse A and 50 μg/mL of propidium iodide to make the 5×105 cells/mL concentration, and then incubated at 37°C for 30 min. To stain MSC, 200 μg/mL of saponin (Sigma) was added to the staining solution. Flow cytometry analysis was done by ATC 1000 (Brucker) instrument.
For co-culture experiments, 1×105 A-549 cells were resuspended in 1 mL of GM and placed into the upper compartment of 1 well of a 6-well plate (Costar) separated from the lower compartment by a collagen-coated Transwell permeable support insert with 0.4 μM pores (Corning). Three milliliters of GM was added to the lower compartment of the same well. Then, 1×105 MSC were placed into the lower compartment of the well containing sterile pieces of cover glasses and the plate was incubated for the following 72 h. After 72 h of incubation, the cells on cover glasses were used for immunofluorescent analysis. The cells, adherent to the bottom of the well, were used on the next day for western blotting (WB) analysis. The MSC amounts and cell cycle progression were determined every 24 h. For experiments with LiCl cell cultures were incubated for 4 h in the GM supplemented with 10 mM LiCl.
To assess the levels of the β-catein phosphorylated by Gsk3β asynchronously growing MSC and the 293 cell line (used as reference) were grown for 4 h in normal GM or in the medium containing 10 mM LiCl. Thirty minutes before harvesting cells for WB 12.5 or 25 ηM phosphatase inhibitor, calyculin (Sigma) was added to the media.
Subcellular fractionation
Preparation of nuclear and cytosolic fractions was done as described earlier [21]. The cells were washed twice with ice-cold PBS, scraped by rubber policeman, and collected by spinning for 5 min at 1,500 rpm. Cell pellets were allowed to swell for 20 min in 2 packed volumes of hypotonic buffer [10 mM HEPES (pH 7.5), 10 mM KCl, 3 mM MgCl2, 1 mM EDTA (pH 8.0), 10 mM β-glycerolphosphate, 10 mM NaF, 0.1 mM Na3VO4, 1 mM PMSF, 1 mM DTT, 1 mg/mL of aprotinin, and leupeptin]. Then, the cells were subjected to 10 slow strokes with a Dounce homogenizer. Nuclei were separated by a 500 g spin for 5 min and washed 3 times in the hypotonic buffer containing 0.1% of NP-40. Nuclear and cytosol fractions were then treated with the lysis buffers for Western blotting or immunoprecipitation (IP), followed by the protein assay analysis.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and WB
Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 8% polyacrylamide gel. For preparation of total extracts cells were removed from culture dishes with a rubber policeman, washed twice in PBS, and lysed in Western blotting buffer [25 mM Tris (pH 7.4), 250 mM NaCl, 0.25% NP-40, and protease inhibitor cocktail (Sigma) 1:100], placed in an ice bath, and shaken vigorously every 5 min for a total of 30 min. The cell debris was spun down at 4°C for 15 min, total protein concentration was determined by Bradford assay for each sample, and the lysates were normalized to equivalent total protein levels. After the SDS-PAGE, the gel was blotted using semidry transfer technique to a sheet of PVDF membrane (Millipore). After 1 h of blotting, proteins were observed using standard enhanced chemiluminescence (ECL) approach.
Immunoprecipitation
Twice-washed whole cells or their nuclear and cytosol fractions were lysed for 30 min on ice in the precipitation buffer solution [50 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween 20, protease, and phosphatase inhibitor cocktails (Sigma) in dilution 1:100]. The cells placed on ice were treated on Cole Parmer 130-Watt Ultrasonic Processor using the following parameters: amplitude 60%, 3 pulses for each 10 s. One microgram of primary rabbit antibody was added to 500 μL of the cell extract containing 500 μg of the total protein, and the mixture was rocked for 2 h at 4°C. Then, 10 μg of goat anti-rabbit antibody was added for 1.5 h, the lysates were kept rocking, and 15 μL of the protein A/G–sepharose was added to the lysates with primary antibodies for 1 h. The pellets were spun down, and washed 5 times in the IP buffer and 2 times in the same buffer without Tween 20.
Immunofluorescent staining
Cells were cultured in GM in an initial concentration of 2×105 per 60 mm Petri dish containing sterile pieces of cover glasses, and allowed to adhere overnight. The next day, the cover glasses with adherent cells were transferred to 35 mm culture dishes, washed once with PBS, fixed in 4% paraformaldehyde in PBS (pH 7.4) for 15 min and 70° distilled ethanol over night at 4°C, washed once with PBS, treated with 0.2% Triton X-100 for 10 min, and then with the blocking solution containing 3% of bovine serum albumin and 0.1% of Tween 20 in PBS. Combined treatment with paraformaldehyde and distilled ethanol did not interfere with native green fluorescence but allowed to decrease background after treatment with antibodies. All primary antibodies in blocking solution were applied overnight at 4°C in dilution 1:50, and then slides were washed twice with PBS, treated with different secondary antibodies in dilution 1:200 for 1 h at room temperature, washed twice with PBS, treated with 4′,6-diamidino-2-phenylindole (DAPI) dye (Sigma) in concentration of 0.1 μg/mL for 10 min at room temperature, washed twice with PBS, transferred to microscopic slides, and covered with cover glasses in Anti-Fade reagent (BioRad). Immunofluorescence was performed on laser scanning confocal microscope Leica TCS SP5 with objective HCX PL APO 40×/1.25Oil, lasers 405, 488, 543, and 633 nm.
We used the following antibodies: rabbit polyclonal anti-p130 (C-20) sc-317, rabbit polyclonal anti-E2f4 (C-20) sc-866, mouse monoclonal anti-E2f4 (D3) sc-6851, mouse monoclonal anti-Gsk3β (H-76) sc-9166, and mouse monoclonal anti-β-catenin (E5) sc-7963 were purchased from Santa Cruz Biotechnology Co.; rabbit polyclonal anti-β-catenin (ab2982) was purchased from Abcam; rabbit polyclonal anti -β-catenin (C2206), monoclonal anti-β-actin, clone AC-15, goat anti-rabbit IgG (R0881) were purchased from Sigma; anti-phospho-β-catenin antibody (#9561) recognizing Ser33/37/Thr41 residues specifically phosphorylated by Gsk3β was purchased from Cell Signaling Technology, Inc.; peroxidase-conjugated affinity purified goat anti-mouse IgG (H+L) (170–6516) from BioRad; rabbit anti-Gsk3β (AB8687) from Millipore; and Alexa Fluor® 633 Fab fragment of goat anti-rabbit IgG (A21072) and Alexa Fluor® 543 Fab fragment of rabbit anti-mouse IgG from Invitrogen.
Results
Treatment of MSC with lithium ions or co-culture with A-549 cells increases the levels and activates p130 and E2f4
Bone marrow-derived MSC from GFP mouse at passages 12–25 were used as described by us earlier [19]. MSC were characterized earlier by phenotype, proliferative capacity, differentiation potential, and clonogenic, adhesive, and tumorigenic activity.
Immunofluorescence analysis demonstrated that under normal conditions in culture MSC expressed little amounts of the E2f4, which was distributed equally in the cytosol and in the nucleus, whereas p130 showed preferentially nuclear localization (Fig. 1A). Treatment with lithium chloride or co-culture with A-549 cells induced nuclear translocation of β-catenin. The co-culture induced translocation of β-catenin more effectively than the LiCl treatment; however, under LiCl treatment conditions, we observed an increase in the level of the membrane-bound β-catenin (Fig. 1, arrows). The levels of p130 and E2f4 in cells treated with lithium chloride were also increased, and these proteins translocated to the nuclear compartments. Under the conditions of co-culture with A-549 cells, the total levels of p130 and E2f4 were more increased as compared to lithium chloride treatment, as well as their translocation to nucleus (Fig. 1A).
FIG. 1.
Induction of the Wnt/β-catenin signaling in MSC leads to activation of the cell cycle regulators p130 and E2f4, which was not associated with cell cycle arrest. (A) Immunofluorescent analysis of p130, β-catenin, and E2f4 expression in MSCs co-cultured with A-549 cells. 1, DAPI staining; 2, specific antibody staining; 3, native green fluorescence; 4, combined image. (B) WB evaluation of p130 and E2f4 activation in MSC after induction of Wnt/β-catenin signaling. MSC were co-cultured for 72 h with A-549. LiCl was added to the growth medium in 10 mM concentration for 4 h. (C) Proliferative activity of MSC in co-culture with A-549 cells. Data are shown as mean±standard deviation. (D) Flow cytometry of asynchronously growing and synchronized at G0/G1 and S phases MSC. Number of experiments (N) for this and all following figures = 3–5. MSC, mesenchymal stem cell; WB, western blotting. Arrows indicate membrane-associates β-catenin. Color images available online at www.liebertonline.com/scd
WB results in which the levels and phosphorylation patterns of p130, E2f4, and β-catenin in MSC were determined corroborated the above-described immunofluorescence data. The levels of β-catenin were increased and electrophoretically fast hypophoshorylated forms of β-catenin also appeared. To investigate how the electrophoretic mobility of distinct forms of β-catenin corresponds to its phosphorylation levels, we used the anti-phospho-β-catenin antibody recognizing Ser33/37/Thr41 phosphorylated by Gsk3β. The 293 human cell line was used as a reference of phospho-β-catenin production. The cells were treated with phosphate inhibitor calyculin to prevent dephosphorylation of β-catenin and promote its detection by the phospho-specific antibody. The results showed that both asynchronous 293 cells and MSC produce small amounts of the phosphorylated β-catenin, which revealed the same electrophoretic mobility as the major part of total β-catenin (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). The level of phosphorylated β-catenin strongly increased after treatment with calyculin. On the other hand, molecular population of total β-catenin included fast-migrating hypophosphorylated form of β-catenin, not detected with the phosphospecific antibody. Treatment the MSC with lithium decreased the amounts of phosphorylated β-catenin to the undetectable level (not shown).
Co-culture conditions resulted in higher levels of the β-catenin compared with the LiCl treatment. The levels of p130 in MSC under co-culture conditions were also strongly increased, and p130 became hypophosphorylated and migrated faster than under normal conditions. Surprisingly, MSC with induced Wnt/β-catenin signaling did not show production of hyperphosphorylated form of the protein (Fig. 1B). The E2f4 levels in MSC under normal conditions were low and its electrophoretic pattern was composed mostly of hypophosphorylated forms. After LiCl treatment the E2f4 levels were increased, which was associated with appearance of its hyperphosphorylated form composing the larger part of the electrophoretic band (Fig. 1B).
These results suggest that activation of the Wnt/β-catenin signaling in MSC induced by growth in the medium containing LiCl or by co-culture with A-549 cells induce an increase in the levels of p130, E2f4, and β-catenin, promoting their nuclear translocation and appearance of active forms. It is known that activated p130 and β-catenin become hypophosphorylated in contrast to the E2f4, which activation results in its hyperphosphorylation [11,13,14].
Evaluation of cell cycle parameters of the MSC co-cultured with A-549 cells
Since the induction of Wnt/β-catenin pathway resulted in activation of p130 and E2f4 in MSC, our next aim was to determine whether these proteins form suppressor complex similar to that in somatic differentiated cells like T98G. To answer this question we first determined the proliferation rate of the MSC co-cultured with A-549 cells. Surprisingly, in the co-culture with A549, MSC increased their proliferation rate instead of decreasing it as we might suggest according to the active status of p130 and E2f4 in these cells (Fig. 1C). Absence of the inhibition of the cell cycle progression in MSC with activated p130 and E2f4 proteins might be associated with specific status of the p130/E2f4 repressor complex in these cells. To determine the activity of repressor complex in different phases of cell cycle in MSC, we first synchronized MSC at G0/G1 and S phases. Growth of MSC under serum deprivation conditions led to accumulation of 75% of the cells at G0/G1 but was associated with death of approximately 10% of the cell population. Because re-stimulation of the quiescent MSC with FBS was accompanied with increase of the MSC death, we used another approach for synchronization—treatment of the cells with thymidine. This allowed us to enrich S phase population of MSC up to 55% (Fig. 1D). In contrast to MSC, the control T98G cells were much more sensitive to synchronization under serum deprivation and restimulation with FBS, and the amounts of the dead T98G cells did not exceed 3% of total cell population.
Characterization of the levels and phosphorylation patterns of p130, E2f4, and β-catenin in cycling MSC
The comparison of the levels and phosphorylation patterns of p130 in asynchronously growing, or synchronized at G0/G1 and S phase MSC did not reveal significant differences. All cell populations contained p1, p2, and p3 forms of p130, whereas mouse hepatocytes, used as reference cells, demonstrated presence of p1 and p2, but not p3 form of the protein (Fig. 2A). In T98G cells (control) p130 was hypophosphorylated at G0/G1 and contained p1 and p2 forms. The p3 form of p130 appeared at G1/S that was associated with decrease in the p130 levels at the following S and G2/M phases (Fig. 2B). MSC produced 4 distinct molecular forms of E2f4 in quiescence, which phosphorylation did not change during cell cycle progression, whereas its levels were elevated in S-phase cells (compared to asynchronously growing and quiescent cells, Fig. 2A). On the other hand, control hepatocytes and T98G cells in quiescence produced high levels of E2f4 with hyperphosphorylated forms. After G1/S transition the level of E2f4 was sharply decreased, and it did not contain hyperphosphorylated forms until the end of mitosis (Fig. 2B).
FIG. 2.
WB and IP analysis of the levels, phosphorylation patterns, and physical interaction of p130 and E2f4 in MSC. (A) WB characteristics of p130 and E2f4 in asynchronously growing and synchronized at G0/G1 and S phases MSC. (B) WB evaluation of p130 and E2f4 in asynchronously growing and synchronized at G0/G1 and S cell cycle phases T98G cells. (C) IP analysis of physical interaction between p130 and E2f4 in MSC. E2f4 was precipitated from the extracts of MSC by specific antibody; p130 and β-catenin were detected in the precipitates by WB. (D) Evaluation of p130 and E2f4 interaction in T98G cells by IP. The extracts of T98G cells synchronized at various cell cycle phases were treated with antibody to E2f4, followed by detection the E2f4 and p130 in the precipitates by WB. IP, immunoprecipitation.
The level of β-catenin increased in MSC at G0/G1 and S phases, as compared to the asynchronously growing cells, though its phosphorylation pattern did not change. Hepatocytes produced more hypophosphorylated and electrophoretically fast β-catenin than MSC (Fig. 2A).
p130 and E2f4 form stable complex included β-catenin in MSC at G0/G1 and S phases
Antibody to E2f4 precipitated all 4 molecular forms of the protein from extracts of MSC in each phase of cell cycle and co-precipitated p1 and p3 forms of p130, but did not precipitate p2 form (Fig. 2C). In control mouse hepatocytes and in synchronized T98G cells antibody to E2f4 co-precipitated form p1 of p130 from quiescent cells (results not shown). This antibody also co-precipitated p1 and p3 forms of the p130 from the G1/S phase cells and small amount of the hyperphosphorylated form p3 from S and G2/M phase cells (Fig. 2D).
The p130/E2f4 complex in MSC was associated with β-catenin, which was co-precipitated from the extracts of asynchronously growing or synchronized at G0/G1 and S phases cells (Fig. 2C). Amounts of the co-precipitated β-catenin increased at G0/G1 and S phases compared to asynchronously growing cells.
Complex p130/E2f4/β-catenin in MSC includes Gsk3β
Antibody to p130 precipitated the hypophosphorylated forms p1 and p2 of p130 from extracts of normal and stimulated with LiCl MSC, but did not precipitate the hyperphosphorylated form p3. Form p3 was recognized by the same antibody in WB (Fig. 3A) and was co-precipitated by antibody to E2f4 (Fig. 2C). Although treatment with LiCl led to accumulation of p130 in MSC detected by WB (Fig. 3A, lines 1 and 2), it was not associated with the increase of this p130 form in the precipitates (Fig. 3A, lines 1 and 2). Precipitates were composed preferentially of form p2, similar to those prepared from unstimulated with LiCl MSC (Fig. 3A, lines 3 and 4). Antibody to p130 co-precipitated β-catenin from normal and stimulated with LiCl MSC (Fig. 3A). The increase in the total amounts of β-catenin after treatment with LiCl (Fig. 3A, lines 1 and 2) did not result in the increase of co-precipitated protein (Fig. 3A, lines 3 and 4). This difference was presumably associated with different affinity of the anti-p130 antibody to various molecular forms of p130. In WB the antibody recognized increasing amounts of p1 and p3 forms in the MSC treated with LiCl (Fig. 3A, lines 2 and 1), whereas in IP the antibody mostly precipitated form 2, which was not induced by this treatment (Fig. 3A, lines 4 and 3).
FIG. 3.
p130, Gsk3β, and β-catenin form complexes in MSC. (A) Antibody to p130 precipitates β-catenin from extracts of MSC. The cell extracts prepared from MSC grown under normal conditions or in the medium with LiCl were treated with antibody to p130. The p130 and E2f4 were detected in the precipitates by WB with specific antibody. (B) Antibody to β-catenin precipitates p130 from extracts of MSC. The same MSC extracts were treated with antibody to β-catenin; the p130 and β-catenin were detected in the precipitates by WB with specific antibody. (C) Antibody to Gsk3β precipitates β-catenin and p130 from extracts of MSC. The same MSC extracts were treated with antibody to Gsk3β; the Gsk3β, p130, and β-catenin were detected in the precipitates by WB with specific antibody. (D) Evaluation of physical interaction between Gsk3β, p130, and β-catenin in MSC co-cultured with A-549 cells. MSC were co-cultured with A-549 cells in the Transwell plates for 72 h to obtain the cell extracts. The extracts were treated with antibody to Gsk3β for IP, and Gsk3β, p130, and β-catenin were detected in the precipitates by WB with specific antibody.
Antibody to β-catenin precipitated larger amounts of this protein from the MSC stimulated with LiCl, as compared to unstimulated cells (Fig. 3B lines 3 and 4), as well as to precipitates prepared with antibody to p130 (Fig. 3A, lines 3 and 4). Antibody to β-catenin also co-precipitated larger amounts of the p130 from MSC stimulated by LiCl as compared to unstimulated cells. These precipitates contained preferentially form p2 of the p130 (Fig. 3B, lines 7 and 8). Antibody to Gsk3β co-precipitated same amounts of β-catenin from extracts of the MSC stimulated and unstimulated with LiCl (Fig. 3C, lines 7 and 8). The p130 was also physically associated with Gsk3β and detected in these precipitates. The antibody to Gsk3β co-precipitated all 3 forms of the p130 and amounts of the hyperphosphorylated form p3 were increased in the precipitates prepared from MSC extracts treated with LiCl (Fig. 3C, lines 11 and 12).
MSC co-cultured with A-549 cells demonstrated activation of the Wnt/β-catenin signaling as revealed by the increased levels of β-catenin (Fig. 3D). The antibody against Gsk3β co-precipitated both β-catenin and p130 composed of all 3 distinctly phosphorylated forms. The amount of the hyperphosphorylated form p3 co-precipitated with Gsk3β and β-catenin was increased in the extracts of the co-cultured cells (Fig. 3D).
Next we asked whether the p130/Gsk3β/β-catenin complex accumulates in nuclear compartment in proximity to the p130 and β-catenin potential targets? To access the complex compartmentalization, the untreated or those treated with LiCl MSC were lysed in hypotonic buffer. Efficacy of the compartmentalization procedure was evaluated in WB by antibody to actin, which did not detect this protein in the nuclear compartment (Supplementary Fig. S2A, lines 3 and 4). Total amounts of β-catenin were larger in the nucleus than in cytosol (Supplementary Fig. S2A, lines 3 and 1). Treatment with LiCl led to clear increase in the level of isoforms of hyper- and hypophosphorylated β-catenin in the nucleus, but not in cytosol (Supplementary Fig. S2A, lines 3 and 4 compare to 1 and 2). Total amounts of p130 were also larger in the nucleus than in cytosol (Supplementary Fig. S2A, lines 3 and 1). Notably, in the nucleus p130 was composed of all 3 phosphorylated forms, whereas in cytosol, only forms p2 and p3 were observed (Supplementary Fig. S2B, 1 compare to Fig. 2C, 1). Treatment with LiCl increased amounts of p1 and p3 p130 in the nucleus (Supplementary Fig. S2B, lines 2 and 1), but not in cytosol (Supplementary Fig. S2C, lines 2 and 1). Antibody to Gsk3β co-immunoprecipitated more β-catenin from nuclear fraction as compared to cytosol fraction in cells treated or untreated with LiCl (Supplementary Fig. S2B, lines 3 and 4, compare to Supplementary Fig. S2C, lines 3 and 4). Interestingly, the p130 co-precipitated with β-catenin from nuclear compartment was composed of all 3 phosphorylated forms in contrast to the cytosol precipitates, which included only p2 and p3 (Supplementary Fig. S2B, lines 3 and 4 compare to Supplementary Fig. S2C, lines 3 and 4). Treatment with LiCl increased the amounts of p1 and p3 forms co-precipitated with Gsk3β and β-catenin from the nucleus but not from the cytosol (Supplementary Fig. S2C, 3 and 4 compare to Fig. 2B, 3 and 4).
Double immunofluorescence revealed accumulation of active forms of β-catenin, p130, and E2f4 in the MSC treated with LiCl
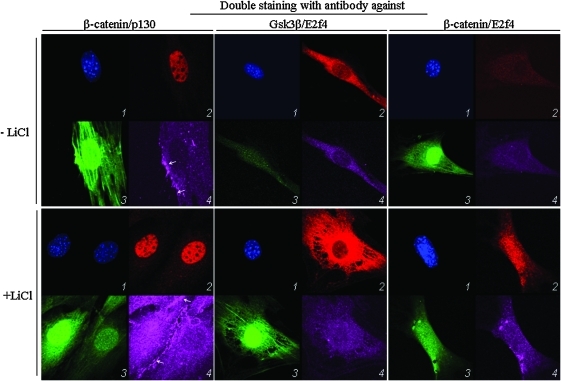
Formation of the p130/E2f4/β-catenin complex in MSC suggests that the levels of these proteins increase in the same cell compartment under induction of the Wnt/β-catenin signaling. To test this hypothesis we did double immunofluorescence staining of MSC treated with LiCl using various combinations of antibodies to β-catenin, p130, Gsk3β, and E2f4. Double immunofluorescence staining confirmed our results. Beta-catenin and E2f4 were observed in cytoplasm and nucleus; p130 was mostly located in the nucleus, whereas Gsk3β showed preferentially cytoplasmic accumulation (Fig. 4). Treatment with LiCl induced the increase in the total levels of p130, β-catenin, and E2f4 in the nucleus, whereas the level and distribution of the Gsk3β was unchanged.
FIG. 4.
Increased activity of β-catenin, p130, and E2f4 in MSC with activated Wnt/β-cateinin signaling. Asynchronously growing MSC were cultured for 4 h in the growth medium with 10 mM LiCl and double stained for p130/β-catenin, Gsk3β/E2f4, or β-catenin/E2f4. 1, DAPI staining; 2 (left), p130; 2 (middle), Gsk3/3, 2 (right), E2f4 staining, 3, native green fluorescence; 4 (left), β-catenin; 4 (middle), E2f4, 4 (right), β-catenin. Red fluorescence shows secondary antibody coupled to Alexa Fluor® 633; magenta fluorescence, secondary antibody coupled to Alexa Fluor® 543. Arrows indicate membrane-associated β-catenin. Color images available online at www.liebertonline.com/scd
Discussion
Here we demonstrate that induction of the Wnt/β-catenin pathway in MSC growing in the medium containing LiCl or co-cultured with A-549 cells was associated with activation of the cell cycle regulator p130 and transcription factor E2f4. In stimulated MSC the levels of p130 and E2f4 increased, their phosphorylation pattern changed, and the proteins were activated, translocated into nucleus, and formed a complex. However, the p130/E2f4 complex did not induce cell cycle arrest of the MSC co-cultured with A-549 cells; on the contrary, these cells increased their proliferation rate. Synchronization of MSC at G0/G1 or S phases did no result in change of the p130 and E2f4 phosphorylation pattern in contrast to mouse hepatocytes and T98G cells. The p130/E2f4 complex formation took place in asynchronously growing, synchronized at G0/G1 or S phases MSC in contrast to T98G cells. In T98G cells the p130/E2f4 complex was formed in quiescence, but was not observed at S phase. The p130/E2f4 complex also combined with β-catenin in asynchronously growing and quiescent MSC. The phosphorylation pattern of the p130 precipitated with specific antibody or co-precipitated with β-catenin was similar in untreated and treated with LiCl MSC, and was composed mostly by the form p2. In contrast, Gsk3β was co-precipitated with all 3 forms of p130 and amounts of the co-precipitated form p1 and p3 was increased in the MSC with activated Wnt/β-catenin pathway.
Thus, we demonstrate for the first time that cell cycle regulator p130 and transcription factor E2f4 are activated in MSC with induced Wnt/β-catenin pathway and form complex in quiescent and proliferating cells; p130, β-catenin, and Gsk3β form stable complex in quiescent and synchronized at G0/G1 and S phase MSC; and p130/Gsk3β/β-catenin complexes in MSC may include distinctly phosphorylated forms of the p130.
Activation of the p130 and E2f4 in response to induction of the Wnt/β-catenin signaling (Fig. 1A, B) was an unexpected finding. Recent studies show that canonical Wnt signaling keeps somatic stem cells from various tissues in a self-renewing and undifferentiated state associated with active cell proliferation [16]. On the other hand, Wnt/β-catenin pathway cross-interacts with pRb signaling. C-Myc and Cyclin D1 overexpression induced by β-catenin leads to phosphorylation of the pRb family members and drives the cells into cell cycle [22,23].
Our results showed that both the control 293 cell line and MSC produce a small amount of the β-catenin phosphorylated on Ser33/37/Thr41 residues in comparison with the total level of the protein under normal conditions. The level of phosphorylated β-catenin strongly increased after treatment with phosphatase inhibitor calyculin (Supplementary Fig. S1). These data demonstrated that the phosphorylated β-catenin migrated with same electrophoretic mobility as the larger part of the β-catenin (revealed by the antibody). These data suggest that using phospho-specific β-catenin antibody would benefit analysis of β-catenin to discriminate between active and inactive protein populations. However, the levels of phospho-β-catenin induced by Gsk3β are very low and undetectable in MSC after induction of Wnt/β-catenin signaling. On the other hand, the phosphorylated on Ser33/37/Thr41 β-catenin possesses the same electrophoretic mobility as the major fraction of the protein, which is presumably phosphorylated on other phospho-specific sites by various kinases. In contrast to the phosphorylated β-catenin, the level of the hypophosphorylated protein isoform possessing fast electrophoretic mobility is evidently increased after induction of Wnt/β-catenin signaling. This allows us to consider the increase in the level of fast migrated β-catenin as an evidence of activation of the Wnt/β-catenin pathway.
Physiological relevance of the p130/Gsk3β/β-catenin complex may be associated with the β-catenin and p130 ability to bind the LEF/TCF or E2f proteins and regulate transcription of their targets. Our results give the evidence that p130/Gsk3β/β-catenin complex accumulates in the nuclear compartment in proximity to the β-catenin and E2f4 transcription targets, but not in the cytosol. In the nucleus of cycling MSC with activated Wnt/β-catenin pathway Gsk3β forms the p130/Gsk3β/β-catenin complex in inactive form. This complex includes p1 and p3 of the p130, whereas its p2 form phosphorylated by Gsk3β is accumulated in the cytosol (Supplementary Fig. S2B, C lines 4). In this way, repressor complex p130/E2f4 in MSC may acquire new abilities. In contrast to differentiated cells like T98G in which the p130/E2f4 represses in quiescence the genes regulating cell cycle progression [24–26], in MSC its formation may be compatible with cell cycle progression due to contribution of β-catenin and Gsk3β. Induction in MSC the Wnt/β-catenin signaling increases the amounts of membrane-bound β-catenin (Figs. 1A and 4). These results are in agreement with the concept that Wnt/β-catenin pathway and cadherin-mediated cell-to-cell interaction depends on the same pool of β-catenin [27]. The increase in the level of intracellular β-catenin resulted from inhibition of Gsk3β by LiCl may lead to relocation of β-catenin from cytoplasm to plasma membrane.
Recent studies suggest that repressor p130/E2f4 might represent a small core unit for building of multisubunit protein complex highly conserved in evolution. In Drosophila this complex is called Drosophila melanogaster, RBF, E2F and MYB (dREAM); in Caenorhabditis elegans, the synthetic multivulva class B (synMuvB); and in mammalian; DP, RB-like, E2F and MuvB (DREAM) [7,8,28]. These complexes may also include multiple proteins functionally associated with differentiation and cell cycle genes. Our results show that induction of Wnt/β-catenin pathway in mouse MSC results in activation of p130 and E2f4, which form complexes in quiescent and proliferating cells. Functional role of these complexes in MSC might be associated with specific mechanism regulating cell cycle and differentiation in stem cells.
Supplementary Material
Acknowledgments
This work was supported by RFBR grant 09–04-00595 to B.P., CHRCO Jordan Family Fund to V.S., and St. Petersburg's Government Grant to N.P.
Author Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 2.Tommasi S. Pfeifer GP. In vivo structure of the human cdc2 promoter: release of a p130-E2F-4 complex from sequences immediately upstream of the transcription initiation site coincides with induction of cdc2 expression. Mol Cell Biol. 1995;15:6901–6913. doi: 10.1128/mcb.15.12.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith ES. Leone G. DeGregorio G. Jakoi L. Nevins JR. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 5.Vairo G. Livingston DM. Ginsberg D. Functional interaction between E2F4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 6.Chevalier S. Couturier A. Chartrain I. Le Guellec R. Beckhelling C. Le Guellec K. Philippe M. Ford CC. Xenopus cyclin E, a nuclear phosphoprotein, accumulates when oocytes gain the ability to initiate DNA replication. J Cell Sci. 1996;109:1173–1184. doi: 10.1242/jcs.109.6.1173. [DOI] [PubMed] [Google Scholar]
- 7.Korenjak M. Taylor-Harding B. Binné UK. Satterlee JS. Stevaux O. Aasland R. White-Cooper H. Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell. 2004;119:181–193. doi: 10.1016/j.cell.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Litovchick L. Sadasivam S. Florens L. Zhu X. Swanson SK. Velmurugan S. Chen R. Washburn MP. Liu XS. DeCaprio JA. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Longworth MS. Dyson NJ. pRb, a local chromatin organizer with global possibilities. Chromosoma. 2010;119:1–11. doi: 10.1007/s00412-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen K. Farkas T. Lukas J. Holm K. Ronnstrand L. Bartek J. Phosphorylation-dependent and–independent functions of p130 cooperate to evoke a sustained G1 block. EMBO J. 2001;20:422–432. doi: 10.1093/emboj/20.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayol X. Garriga J. Grana X. Cell cycle-dependent phosphorylation of the retinoblastoma related protein p130. Oncogene. 1995;11:801–808. [PubMed] [Google Scholar]
- 12.Litovchick L. Chestukhin A. DeCaprio JA. Glycogen synthase kinase 3 phosphorylates RBL2/p130 during quiescence. Mol Cell Biol. 2004;24:8970–8980. doi: 10.1128/MCB.24.20.8970-8980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayol X. Grana X. The p130 pocket protein: keeping order at cell cycle exit/re-entrance transitions. Front Biosci. 1998;3:11–24. doi: 10.2741/a263. [DOI] [PubMed] [Google Scholar]
- 14.Garriga J. Limón A. Mayol X. Rane SG. Albrecht JH. Reddy EP. Andrés V. Graña X. Differential regulation of the retinoblastoma family of proteins during cell proliferation and differentiation. Biochem J. 1998;333:645–654. doi: 10.1042/bj3330645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tedesco D. Lukas J. Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reya T. Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 17.Stambolic V. Ruel L. Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 18.Etheridge SL. Spencer GJ. Heath DJ. Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 19.Popov BV. Serikov VB. Petrov NS. Izusova TV. Gupta N. Matthay A. Lung epithelial cells A549 induce epithelial differentiation in mouse mesenchymal BM stem cells by paracrine mechanism. Tissue Eng. 2007;13:2441–2450. doi: 10.1089/ten.2007.0001. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y. Sun Z. Qiu X. Li Y. Qin J. Han X. Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2009;390:1309–1314. doi: 10.1016/j.bbrc.2009.10.143. [DOI] [PubMed] [Google Scholar]
- 21.Popov B. Chang L-S. Serikov V. Cell cycle-related transformation of the E2F4-p130 repressor complex. Biochem Biophys Res Commun. 2005;336:762–769. doi: 10.1016/j.bbrc.2005.08.163. [DOI] [PubMed] [Google Scholar]
- 22.Tetsu O. McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Hughes TA. Brady HJ. E2F1 up-regulates the expression of the tumour suppressor axin2 both by activation of transcription and by mRNA stabilisation. Biochem Biophys Res Commun. 2005;329:1267–1274. doi: 10.1016/j.bbrc.2005.02.102. [DOI] [PubMed] [Google Scholar]
- 24.Chestukhin A. Litovchick L. Rudich K. DeCaprio JA. Nucleocytoplasmic shuttling of p130/RBL2: novel regulatory mechanism. Mol Cell Biol. 2002;22:453–468. doi: 10.1128/MCB.22.2.453-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun A. Bagella L. Tutton S. Romano G. Giordano A. From G0 to S phase: a view of the roles played by the retinoblastoma (Rb) family members in the Rb-E2F pathway. J Cell Biochem. 2007;102:1400–1404. doi: 10.1002/jcb.21609. [DOI] [PubMed] [Google Scholar]
- 26.Ren B. Cam Y. Takahashi Y. Volkert T. Terragni J. Young RA. Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heuberger J. Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:1–24. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korenjak M. Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005;15:520–527. doi: 10.1016/j.gde.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.