Abstract
The impacts of the M9.0 Tohoku Earthquake on deep-sea environment were investigated 36 and 98 days after the event. The light transmission anomaly in the deep-sea water after 36 days became atypically greater (∼35%) and more extensive (thickness ∼1500 m) near the trench axis owing to the turbulent diffusion of fresh seafloor sediment, coordinated with potential seafloor displacement. In addition to the chemical influx associated with sediment diffusion, an influx of 13C-enriched methane from the deep sub-seafloor reservoirs was estimated. This isotopically unusual methane influx was possibly triggered by the earthquake and its aftershocks that subsequently induced changes in the sub-seafloor hydrogeologic structures. The whole prokaryotic biomass and the development of specific phylotypes in the deep-sea microbial communities could rise and fall at 36 and 98 days, respectively, after the event. We may capture the snap shots of post-earthquake disturbance in deep-sea chemistry and microbial community responses.
Tectonic events such as earthquakes often trigger the typical environmental disturbances that include numerous topographic failures and landslides on the land and coast, and sudden outflows as well as cessations and changes in the chemical composition of groundwater and hot springs1,2,3. In past, disturbance in deep-sea environment after a M5 level earthquake swarm was detected as the turbulent diffusion of sediments and a manganese concentration anomaly in a seafloor observatory4,5. However, the earthquake swarm was still on very local scale. These M5 earthquakes are not entirely comparable with the millennial M9 earthquake such as the Tohoku Earthquake occurring on March 11, 20116,7,8,9. To determine the immediate and existing conditions in the deep ocean following the M9.0 earthquake and its aftershocks is a top-priority objective of multidisciplinary science and a basis for the socially necessary restoration of the marine ecosystem and fisheries in Japan. To this end, the initial chemical and microbiological observations10,11,12,13,14,15,16 in the deep-sea water environments were made on April 15 and June 16, 2011 at the Tohoku Earthquake epicentral region, the Japan Trench.
Results
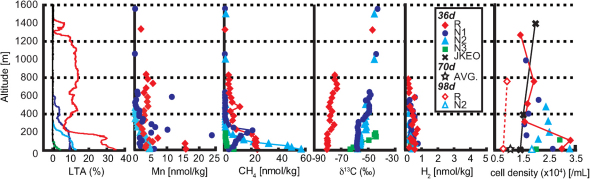
At all the stations (Fig. 1) at the time of 36 days after the M9 event, an intense light transmission anomaly (LTA) was found in the deep-sea bottom water (Fig. 2). The intensity and extent of LTA were greater and thicker, respectively, at the stations near the trench axis. The maximum LTA intensity and thickness were ∼35% and ∼1500 m, respectively. These values were very unusual in deep-sea environments, even for hydrothermally active regions17. Seismological investigations indicated that the largest vertical seafloor displacement occurred around the stations R and N17,8. Indeed, the actual seafloor displacement (horizontally 50 m and vertically 7 m) was observed in the trench slope between the large normal fault (Fig. 1) and the trench axis9. However, turbidity in the deep-sea bottommost water was still observed at all the stations investigated (except for Stn. JKEO) even 98 days after the M9 event (data not shown), although the turbidity anomaly pattern was generally similar in both the 36- and 98-days-after samples. Thus, it seems very likely that the highly turbid and sizeable deep-sea water mass was produced by the turbulent diffusion of sediments during the seafloor ruptures and landslides, while it was hardly concluded whether the diffusion of sediments was caused by the M9 earthquake or by the frequently continuing aftershocks.
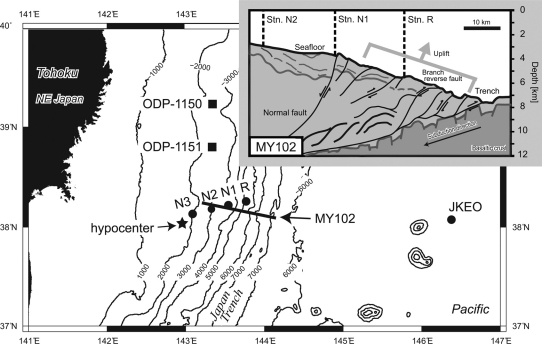
Figure 1. [Main body] Map of study area.
Sampling stations in this study (filled circles), past drilling sites (filled squares), a multi-channel seismic reflection survey line (bold line), and a hypocenter of the 3.11 M9.0 Tohoku Earthquake (filled star) are shown. [Insert] Potential sub-seafloor structure and tectonic properties of the landward slope of the Japan Trench around the epicentral region of the 3.11 Tohoku Earthquake (MY102). The sub-seafloor structure and tectonic properties are based on a multi-channel seismic reflection image7. Locations of CTD-hydrocast stations (vertical dash lines) are shown.
Figure 2. Vertical profiles of light transmission anomaly, manganese and methane concentrations, stable carbon isotopic composition of methane, H2 concentration and microbial cell density in the 36-days-after deep-sea bottom water of all the stations.
The altitude of sampling point above the seafloor is used as a vertical axis. LTA in the 36-days-after deep-sea water of each five station (R, N1, N2, N3, and JKEO) are distinguished by colors (see legend). An open star in the cell density profile represents the average microbial cell density (1.1 ± 0.12×104 cells/ml) in the deep-sea bottom water samples of the Japan Trench approximately 70 days after the M9 event (see SI for details). Open diamonds and triangle respectively represent samples taken at Stns. R and N2 98 days after the M9 event.
As previously observed as a post-earthquake chemical disturbance in seawater associated with the diffusion of sediments4,5, anomalous increase in dissolved manganese concentration relative to the background level (<1 nmol/kg) was found in the 36-days-after deep-sea water samples showing significant LTA (Fig. 2). Although most of the dissolved manganese concentrations seemed to be relevant to the LTA values, several water samples showed highly variable dissolved manganese concentrations (Fig. 2).
Methane is a frequently-used, effective chemical tracer for the sub-seafloor fluid inputs18,19,20 whereas the post-earthquake methane influx from sub-seafloor reservoirs into deep-sea water has rarely been discussed18. In fact, the methane concentrations in the 36-days-after deep-sea bottom water were significantly higher than the usual background level in deep-sea water (∼0.5 nmol/kg) (Fig. 2). Because of the entirely oxic conditions indicated by dissolved oxygen (DO) concentration data (Fig. S1), it is very unlikely that the methane anomaly was induced by in situ microbial production (methanogenesis).
The stable carbon isotope composition (δ13C) of deep-sea water methane was nearly constant at each station but differed among the stations in the 36-days-later samples (Fig. 2). Although it was known that microbial methanotrophic function increased the δ13C values of deep-sea water methane21,22, a Keeling plot analysis23 demonstrated that most of the δ13C values of the deep-sea water methane could be interpreted as consequences of the simple dilution between each of two different types of endmember methane and ambient seawater methane (Fig. S2). One of the estimated endmember methane sources was highly 13C-depleted and was obtained from Stn. R (−80.3‰). The others were relatively 13C-enriched and were obtained from Stns. N1 (−58.6‰), N2 (−56.6‰) and N3 (−65.5‰). The δ13C values of deep-sea water methane at the shallowest station of N3 demonstrated a considerably different mixing pattern from the other stations (Fig. S2). This result can be due to the potential mixing at this station between the sub-seafloor source of methane and the dissolved methane in the relatively shallower seawater, in which the 13C-enriched methane has been sometime found24.
At fault movements, molecular hydrogen (H2) can be mechanochemically generated25 and spread from the sub-seafloor fault interfaces26. Prior to the investigation, we had hypothesized that trace amounts of the earthquake-generated H2 might be detected in deep-sea water soon after the gigantic earthquake and its aftershocks. However, the deep-sea water H2 was not abundant for detection (Fig. 2). In addition, we did not find deep-sea water samples containing detectable sulfide (data not shown). Since it has been known that the H2 and sulfide inputs in the deep seawater rapidly disappear through chemical and microbial processes27,28, our data collected 36 days after the M9 earthquake do not necessarily decline the possible earthquake-induced influxes of these substances from the sub-seafloor environments.
The possible responses of deep-sea planktonic microbial communities to the earthquake-induced environmental disturbances were also investigated at two periods of time after the M9 event. The microbial cell densities at several depths of the 36-days-after deep-sea water increased toward the seafloor were more than 2.0×104 cells/ml (Fig. 2 and Table S1). The cell densities in the deep-sea bottom water samples (water depths of 5720 m and 5000 m at Stn. R and 2940 m at Stn. N2) collected 98 days after the M9 event were 8.3×103, 9.9×103 and 1.9×104 cells/ml, respectively (Fig. 2 and Table S1). The cell densities in the 36-days-after deep-sea bottom water samples were higher than those in the 98-days-after deep-sea bottom water samples at the similar depths (Fig. 2). The spatial difference in the microbial cell densities was also found in the comparison between the 36-days-after deep-sea bottom water samples at the trench regions (Stns. R, N1, N2 and N3) and at the abyssal plain (Stn. JKEO) (Fig. 2 and Table S1). The 16S rRNA gene numbers in the whole microbial DNA assemblages showed quite similar patterns in spatial and temporal variation of microbial populations in the deep-sea bottom water environments as observed in the microbial cell densities (Table S1). In addition, when the proportion of archaeal 16S rRNA gene number in the whole prokaryotic 16S rRNA gene number was compared among the deep-sea bottom water samples, the bottommost water samples at Stn. R at the times of 36 and 98 days after the M9 event exclusively showed the significantly lower proportion of archaeal 16S rRNA gene number in the whole prokaryotic 16S rRNA gene number (33.7 and 40.2%, respectively) than any other deep-sea water samples (73.4% on an average) (Table S1).
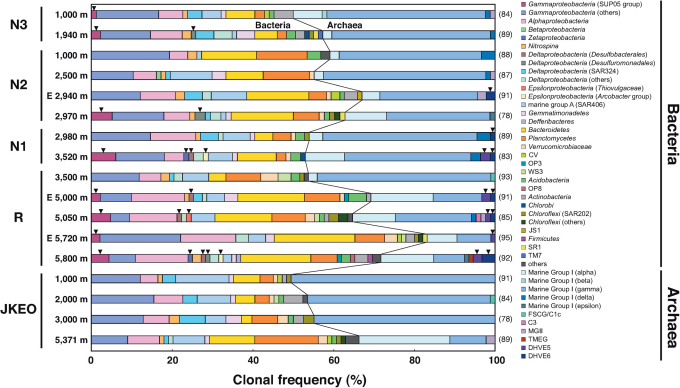
As other responses of microbial community in the deep-sea water, spatial and temporal variability in the 16S rRNA gene phylotype composition was also examined in the deep-sea bottom water (Fig. 3). The whole prokaryotic phylotype compositions were broadly similar among the stations including Stn. JKEO, the various depths and the different sampling periods (Fig. 3). However, several signature phylogenetic groups were identified only in the deep-sea bottom water samples of the stations in the Japan Trench landward slope that were placed in the potentially largest seafloor displacement region: SUP05 phylogroup34,35 within Gammaproteobacteria (Stns. N1, N2, N3 and R); zeta-proteobacterial phylogroup36,37 (Stns. N1 and R); Desulfobacterales phylogroup38 within Deltaproteobacteria (Stns. N1 and R), Desulfuromonadales phylogroup39 within Deltaproteobacteria (Stns. R, N2 and N3), Arcobacter phylogroup40,41 within Epsilonproteobacteria (Stns. N1 and R), DHVE5/Rice cluster V phylogroup42 (Stns. N1 and R) and DHVE6 phylogroup42 of the Archaea (Stns. R, N1 and N2). In addition, as demonstrated by the quantitative PCR estimation, the whole prokaryotic phylotype compositions in the bottommost deep-sea water samples (both 36- and 98-days-after samples) at Stn. R revealed the lower abundance of archaeal 16S rRNA gene phylotype populations than the other samples (Fig. 3).
Figure 3. Whole prokaryotic 16S rRNA gene phylotype compositions in the deep-sea water samples at all stations.
The number in parenthesis shown in the right of the bar chart represents the number of 16S rRNA gene clones sequenced. Black reverse triangles indicate signature phylogenetic groups in the post-earthquake deep-sea water (see SI). Most of the names and abbreviations of the phylogenetic groups indicated at the right follow the Hugenholtz database43. The classification of Marine Group I crenarchaeota subgroups and other archaeal phylotypes follow a reference44.
To understand the differences in the phylogenetic context of the post-earthquake deep-sea microbial communities, a statistical analysis (UniFrac)16 was conducted with the 16S rRNA gene phylotype compositions in the deep-sea water samples at the time of 36 and 98 days after the M9 earthquake (Fig. S3). In the 36-days-after deep-sea water samples, the microbial phylotype compositions were broadly classified into 6 types: the bottommost water types of Stn. JKEO, Stn. R, Stn. N1 and Stn. N2, respectively, about 5000 m deep water type of Stn. R, and the overlying deep-sea water type at all the stations including the bottommost water sample of Stn. N3 (Fig. S3). When the microbial phylotype compositions were characterized in several 98-days-after deep-sea water samples at the similar depth locations, the principal coordinates analysis (PCoA) revealed the significant time-course changes in the microbial phylotype composition (Fig. S3). The most drastic change was found in the phylotype composition at the bottommost water of Stn. R and the phylotype composition became much closer to the composition in the bottommost water at Stn. JKEO, a reference composition (Fig. S3).
Discussion
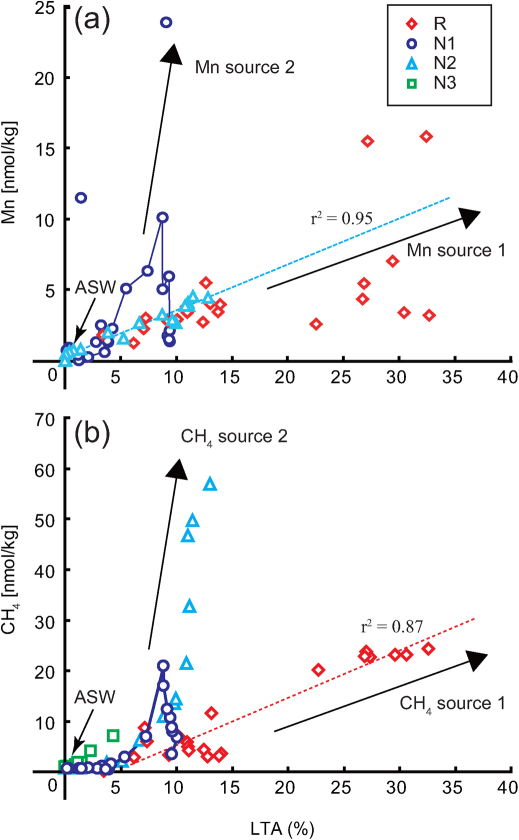
Potential chemical signs of the post-earthquake environmental disturbances were identified in the LTA anomaly, the concentrations of manganese and methane and the stable carbon isotope composition of methane in the deep-sea water environments 36 days after the M9 event. The relationships between LTA intensity and manganese or methane concentration in deep-sea water were examined to estimate the input sources of manganese and methane29,30. The turbulent diffusion of sediments should accompany diffusive input of sediment pore-water chemistry into the overlying deep-sea water. Therefore, the manganese and methane concentrations would be linearly correlated with the LTA intensity caused by the turbulent diffusion of shallow sediments. A reliable linear correlation of the LTA intensity with the manganese concentration was found in the deep-sea water samples at Stn. N2 and Stn. R when the extremely high-LTA (>20%) samples were excluded (Fig. 4a). The highly variable manganese concentrations in the high-LTA deep-sea water samples at Stn. R were probably due to the occurrence of complex leaching and scavenging processes of manganese with numerous numbers of suspended particles under the oxic condition. A similar linear correlation was identified only between the LTA intensity and the methane concentration in the deep-sea water samples at Stn. R (Fig. 4b). The correlations strongly suggested that the manganese and methane in the deep-sea water samples at these stations would originate from the turbulent diffusion of shallow sediments. In contrast, the methane (Stns. N1 and N2) and manganese concentrations (Stn. N1) in the deep-(Fig. 4). These results indicated a possible input of these components from the sources other than the shallow sediments.
Figure 4. Relationship between (a) manganese and (b) methane concentrations and LTA value.
ASW means ambient seawater. The blue and red broken lines for each panel are least-squares fitted lines for data from Stns. N2 and R, respectively.
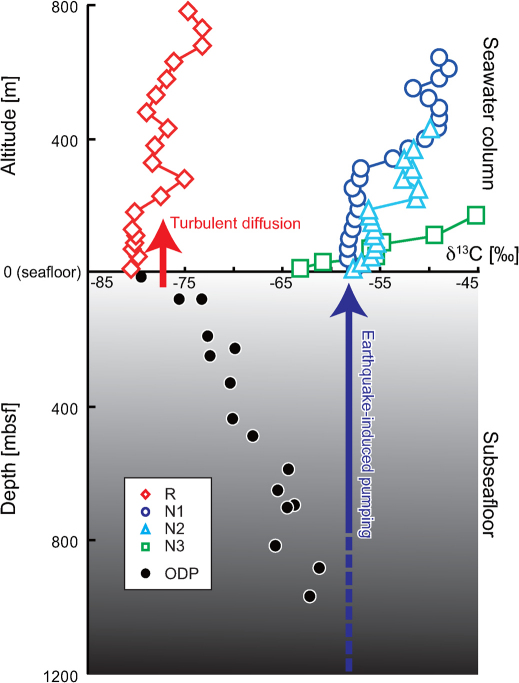
Depth profile of stable carbon isotope composition of reserved methane in the sub-seafloor sediments was characterized at the sites of the Japan Trench landward slope near the epicenter of M9 earthquake during Expedition 186 of the Ocean Drilling Project (ODP)31 (Fig. 1). The profile indicated the highly 13C-depleted methane (∼−80‰) in shallow sediments and the increases of the δ13C values of subseafloor methane with depth31 (Fig. 5). Similar vertical trends of the δ13C(CH4) values were known also in other subseafloor methane field32,33. The relatively 13C-enriched methane, with a value of ∼−60‰, were found only in the sediments at depths of >800 m below the seafloor. Thus, potentially two types of endmember methane inferred from the δ13C values would be derived from the different sedimentary reservoirs at different depths (Fig. 5). One source is the methane sorbed in the shallow fresh sediments, and is predominant in the deep-sea water at Stn. R. The other is the deep sub-seafloor methane introduced into the deep-sea water at Stns. N1, N2 and N3. Although it is not yet known how such deep sub-seafloor methane migrate to the deep-–N3 are located in a more landward area of the Japan Trench than Stn. R, where the sub-seafloor environment is characterized by the tectonic extension and many normal faults7 (Fig. 1). The massive earthquakes possibly induced hydrological changes by rapidly changing the pore-fluid pressure and local rock permeability, thereby altering the sub-seafloor fluid flows1,2. Thus, it seems likely that the deep subseafloor-derived methane would seep into the deep-sea water through earthquake-induced fluid pumping (Fig. 5).
Figure 5. Vertical profiles of carbon isotope composition of methane in deep-sea bottom water in this study (altitude in meter [m]) and in sub-seafloor reservoir obtained from the expedition 186 of the Ocean Drilling Project31 (depth in meter below seafloor [mbsf]).
As discussed in the literature, we can recognize distinct possible methane sources of turbulent diffusion of surface fresh sediment at Stn. R (a red arrow) and earthquake-induced pumping of deep sub-seafloor components at Stns. N1, N2, and N3 (a blue arrow).
Both the microbial cell densities and the 16S rRNA gene numbers in the deep-sea bottom water at Stns. R, N1, N2 and N3 were evidently increased at the time of 36 days after the M9 event but decreased 98 days later to the levels that were comparable to the data at the reference station of JKEO (Fig. 2 and Table S1). These results strongly suggest that the increased microbial populations in the bottom water of the Japan Trench landward slope represent a response of the microbial community to the earthquake-induced environmental disturbances. Although both the 36- and 98-days-after deep-sea bottom water samples were highly turbid and were likely influenced by the diffusion of sediments, the response of microbial biomass was found only in the 36-days-after deep-sea water environments. This may imply that the earthquake-induced chemical impact in the deep-sea water such as discharges of subseafloor reduced chemical substances (e.g., CH4, H2, sulfide, ferrous iron and organics) could remain significant by the time of 36 days after the gigantic earthquake.
The post-earthquake, spatial and temporal variation of the planktonic microbial communities in the deep-sea bottom water was also delineated by the 16S rRNA gene phylotype analysis (Fig. 3 and Fig. S3). Although the whole prokaryotic phylotype compositions were broadly similar among the various depths and the different sampling periods of all the stations, several signature phylogenetic groups were identified only in the deep-sea bottom water samples of the stations in the Japan Trench landward slope (Fig. 3). In addition, most of these signature phylogenetic groups were detected in the 36-days-after deep-sea bottom water but became the undetected components at the similar deep-sea water environments 98 days after the M9 event (Fig. 3). Based on the phylogenetic affiliation, the signature phylogroups of the Bacteria and Archaea were related with potential chemolithoautotrophic or chemolithotrophic populations that thrive in the planktonic and sedimentary oxic-anoxic interface and the reducing habitats of the deep sea (see Supplementary Information). Thus, these signature phylotypes are probably derived from two sources: (i) indigenous benthic microbial components that inhabited the shallow sediments before the earthquakes and spread into the deep-sea water through earthquake-induced sediment diffusion and (ii) fresh planktonic components that dominated the microbial communities by responding to the earthquake-induced influxes of reduced energy sources in the deep-sea water.
The post-earthquake spatial variation of deep-sea planktonic microbial community was illustrated in the comparison between the 36-days-after deep-sea bottommost water samples of all the stations (Fig. S3). Although Stns. R, N1, N2 and N3 were geographically located close together on the landward slope of the Japan Trench, the microbial phylotype compositions were considerably different among these stations, and the composition at the bottommost water of Stn. R (5800 m deep) was the most distantly related with those in the bottommost water of Stn. JKEO and in any other deep-sea water samples of the stations (Fig. S3). The distinctive microbial phylotype composition at the 36-days-after bottommost water of Stn. R is probably derived not only from the high frequency of emerging signature phylogenetic groups and but also from the relative enrichment of bacterial phylotype populations in the phylotype composition (Table S1, Fig. 3 and Fig. S3). The microbial community in the bottommost water at Stn. JKEO is considered to represent a reference microbial community for the abyssal plain of the Pacific plate and to show little influence from the earthquake-induced environmental disturbances. Thus, the difference in the deep-sea microbial community between Stn. JKEO and the other stations may represent the earthquake-induced environmental impact in the deep-sea bottom water habitats between the on-trench and off-trench locations, and even among the on-trench locations. The post-earthquake, temporal variation of deep-sea planktonic microbial community was inferred from the comparison between the 36- and 98-days-after deep-sea bottom water samples of Stns. R and N2 (Fig. S3). The phylotype compositions at the 98-days-after bottom water of these stations shifted closer to the composition of the 36-days-after bottommost water of JKEO (Fig. S3). The temporal shifts suggest that the microbial phylotype compositions in the 36-days-after deep-sea bottom water environments represent certain short-lived responses specifically to the chemical disturbance of the habitats induced by the gigantic earthquake. In addition, as observed in the variation of the microbial biomass and the signature phylogenetic components, the deep-sea bottom planktonic microbial communities may retrace the ordinal states relatively soon after the mitigation of post-earthquake environmental disturbance.
Our results illuminated the transient and present natures of deep-sea chemical environments and microbiological communities after the 3.11 Tohoku Earthquake. The impacts of the M9.0 Tohoku Earthquake on deep-sea environment we detected were less severe than expected from the enormous energy release. However, the evidence of impact was spatially and temporally extensive. This evidence served to identify the earthquake-induced chemical influxes in the deep-sea water resulting from sub-seafloor fluid migration. A few oceanographic reconnaissances cannot reveal the detailed processes involved in earthquake-induced chemical disturbances and the corresponding transition in the microbial ecosystem. Ongoing oceanographic expeditions and further multidisciplinary investigations will clarify the full impact of the massive earthquakes initiated from the March 2011 Tohoku Earthquake on the marine environments and ecosystems of northeastern Japan in the future.
Methods
The Japan Trench is located on the northwestern margin of the Pacific Plate that is subducting beneath the northeastern Japan Arc (Supplementary Fig. S1). Multi-channel seismic reflection data at the epicentral region were acquired by the R/V Kairei (Japan Agency for Marine-Earth Science and Technology: JAMSTEC) in 1999 (Line MY102 of KR99-08, analyzed in the reference7) (Fig. 1). Vertical hydrocasts of the CTD-CMS (Conductivity Temperature Depth profiler with Carousel Multiple Sampling system) were conducted 36 days after the M9.0 event during the MR11-03 cruise of R/V Mirai (JAMSTEC) at the epicentral region (Fig. 1). Stations N1 (38°10.6′N, 143°33.0′E, depth: 3502 m), N2 (38°08.7′N, 143°19.0′E, depth: 2954 m), and N3 (38°06.8′N, 143°05.0′E depth: 1948 m) are located on the western direction of the landward slope of a ridge associated with displacement along an outstanding normal fault that was considered to be the potentially largest slip of the Tohoku Earthquake7. Station R (38°12.5′N, 143°47.2′E, depth: 5715 m) is above a branch reverse fault (Fig. 1). The stations N2 and R were revisited during the YK11-E04 cruise of R/V Yokosuka (JAMSTEC) with another CTD-CMS. The station JKEO (38°00′N, 146°30′E, depth: 5381 m) is the site of JAMSTEC Kuroshio Extension Observatory located in the abyssal plain of the Pacific plate. The Ocean Drilling Project (ODP) Sites 1150 (39°11′N, 143°20′E) and 1151 (38°45′N, 143°20′E) were the drilled sites during the expedition conducted in 1998 and the pore-water chemistry was already reported31.
The wired CTD-CMS system of the MR11-03 cruise consisted of a CTD (SBE9 Plus, Sea-Bird Electronics), a CMS (SBE32, Sea-Bird Electronics), 36 Niskin-X bottles (12-liter type, General Oceanics), a dissolved oxygen sensor (RINKO-III, JFE Advantech), and a light transmissometer (C-star 25-cm light-path type, WET Lab). The Light Transmission Anomaly (LTA), calculated from the difference between the in-situ light transmission value (Tr: %) and the value of the transparent layer at intermediate depth for each hydrocast, is used to describe deep-sea water turbidity. The CTD-CMS system of the YK11-E04 cruise consisted of a CTD (SBE11 Plus, Sea-Bird Electronics), a CMS (SBE32, Sea-Bird Electronics), and 12 Niskin-(12-liter type, General Oceanics).
The seawater samples taken by the Niskin bottles were immediately subsampled into several optimized bottles for various geochemical and microbiological analyses10,11,12. For the analysis of manganese concentration10, the subsampled seawater in an acid-washed plastic bottle was filtered using a 0.22-µm pore-size PTFE filter and acidified with nitric acid (TAMA Chemical) and analyzed by the luminol-H2O2 chemiluminescence detection method. For the analysis of methane11, sample seawater was subsampled into a 120 ml glass vial capped by a butyl-rubber septum after the addition of 0.5 ml HgCl2-saturated solution for poisoning. The methane concentration and carbon isotope ratio were simultaneously determined with a combination of purge and trap techniques and continuous-flow isotope ratio mass spectrometry. The stable carbon isotope ratio is presented in general delta notation on a permillage scale with respect to the Vienna PDB. For the analysis of molecular hydrogen concentration12, the subsampling was conducted in a manner similar to that used for methane, except that a Teflon-coated butyl-gum septum was used rather than the untreated butyl-gum septum used for methane. The molecular hydrogen concentration was analyzed onboard using the headspace method with a gas chromatograph equipped with a trace-reduced gas detector (TRD-1: Round Science Inc., Japan)12 within six hours after the subsampling to avoid sample alteration during storage. If a hydrogen sulfide-like smell was detected in a sample, the seawater was subsampled and placed in a Nalgene bottle to quantify the H2S concentration using methylene blue colorimetry.
Microbial cell counts were determined by a DAPI-staining direct count (Supporting Information). For extraction of microbial DNA, a portion (3 L) of deep-sea water was filtered with a 0.22-µm-pore-size cellulose acetate filter (Advantec, Tokyo, Japan) and was preserved onboard at −80°C. DNA was extracted by using the Ultra Clean Mega Soil DNA Isolation kit (MO Bio Laboratory, Solana Beach, CA, USA). Quantitative PCR of archaeal and entire prokaryotic 16S rRNA genes was performed using 7500 Real Time PCR System13,14. Prokaryotic 16S rRNA gene clone analysis was conducted with another PCR experiment15 (also see SI for details). To assess difference in phylogenetic context of the post-earthquake deep-sea microbial communities in the bottommost deep-sea water, an online tool, UniFrac16, was used for the principal coordinates analysis (PCoA).
Author Contributions
S.K. M.C.H., W.L., H.K., and K.T. designed the study; S.K., Y.Y-T, T.No., M.C.H., H.U., T.Nu., J.M. and K.T. conducted the onboard observations; S.K., Y.Y-T, T.No., H.I., F.N., U.T., K.O., Y.T., T.Nu, and M.H. performed the experiments and analyzed the data; and S.K. and K.T. wrote the manuscript.
Supplementary Material
supplementary info
Acknowledgments
We are grateful to the captains, crews, and technical staff (GODI, MWJ, and NME) of the R/V Mirai MR11-03 and YK11-E04 cruises for their technical expertise. We thank T. Hirose for comments and discussions. This work was supported in part by the Trans-crustal Advection and In-situ reaction of Global sub-seafloor Aquifer (TAIGA) project, the Super-Deep KAIKO Drilling (KANAME) project, and a Grant-in-Aid for Young Scientists (No. 22710024).
References
- Elkhoury J. E., Brodsky E. E. & Agnew D. C. Seismic waves increase permeability. Nature 441, 1135–1138 (2006). [DOI] [PubMed] [Google Scholar]
- Roeloffs E. A. Fault stability changes induced beneath a reservoir with cyclic variations in water level. J Geophys Res-Solid 93, 2107–2124 (1988). [Google Scholar]
- Tsunogai U. & Wakita H. Precursory chemical changes in ground water: Kobe earthquake, Japan. Science 269, 61–63 (1995). [DOI] [PubMed] [Google Scholar]
- Gamo T., Okamura K., Mitsuzawa K. & Asakawa K. Tectonic pumping: earthquake-induced chemical flux detected in situ by a submarine cable experiment in Sagami Bay, Japan. P Jpn Acad B-Phys 83, 199–204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasaya T. et al. Trial of multidisciplinary observation at an expandable sub-marine cabled station “off-Hatsushima Island Observatory” in Sagami Bay, Japan. Sensors-Basel 9, 9241–9254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. et al. Displacement above the hypocenter of the 2011 Tohoku-oki earthquake, Science. 332, 1395 (2011). [DOI] [PubMed]
- Tsuji T. et al. Potential tsunamigenic faults of the 2011 Tohoku earthquake. Earth Plants and Space 63, 831–834 (2011). [Google Scholar]
- Lay T., Ammon C. J., Kanamori H., Kim M. J. & Xue L. Possible large near-trench slip during the great 2011 Tohoku (Mw 9.0) earthquake. Earth Plants and Space 63, 687–692 (2011). [Google Scholar]
- Fujiwara T., Kodaira S., No T., Kaiho Y., Takahashi N. & Kaneda Y. (2011) The 2011 Tohoku-Oki Earthquake: displacement reaching the trench axis. .Science 334, 1240 (2011). [DOI] [PubMed] [Google Scholar]
- Nakayama E., Isshiki K., Sohrin Y. & Karatani H. Automated-determination of manganese in seawater by electrolytic concentration and chemi-luminescence detection. Anal Chem 61, 1392–1396 (1989). [Google Scholar]
- Hirota A., Tsunogai U., Komatsu D. D. & Nakagawa F. Simultaneous determination of δ15N and δ18O of N2O and δ13C of CH4 in nanomolar quantities from a single water sample. Rapid Commun. Mass Spectrom. 24, 1085–1092 (2010). [DOI] [PubMed] [Google Scholar]
- Kawagucci S. et al. Gas geochemical characteristics of hydrothermal plumes at the HAKUREI and JADE vent sites, the Izena Cauldron, Okinawa Trough. Geochem J 44, 507–518 (2010). [Google Scholar]
- Nunoura T. et al., Quantification of mcrA by fluorescent PCR in methanogenic and methanotrophic microbial communities. FEMS Microbiol Ecol 64, 240–247 (2008). [DOI] [PubMed] [Google Scholar]
- Takai K. & Horikoshi K. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66, 5066–5072 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K., Moser D. P., DeFlaun M., Onstott T. C. & Fredrickson J. K. Archaeal diversity in waters from deep South African gold mines. Appl Environ Microbiol 67, 5750–5760 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C. & Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71, 8228–8235 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murton B. J., Baker E. T., Sands C. M. & German C. R. Detection of an unusually large hydrothermal event plume above the slow-spreading Carlsberg Ridge: NW Indian Ocean. Geophys Res Lett 33, 10.1029/2006GL026048 (2006). [Google Scholar]
- Tsunogai U., Ishibashi J., Wakita H. & Gamo T. Methane-rich plumes in the Suruga Trough (Japan) and their carbon isotopic characterization. Earth Planet Sc Lett 160, 97–105 (1998). [Google Scholar]
- German C. R. et al. Diverse styles of submarine venting on the ultraslow spreading Mid-Cayman Rise. Proc Natl Acad Sci USA 107, 14020–14025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine D. L. et al. Propane respiration jump-starts microbial response to a deep oil spill. Science 330, 208–211 (2010). [DOI] [PubMed] [Google Scholar]
- Coleman D. D., Risatti J. B. & Schoell M. Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria. Geochim Cosmochim Ac 45, 1033–1037 (1981). [Google Scholar]
- Gamo T. et al. Microbial carbon isotope fractionation to produce extraordinarily heavy methane in aging hydrothermal plumes over the southwestern Okinawa Trough. Geochem J 44, 477–487 (2010). [Google Scholar]
- Keeling C. D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Ac 13, 322–334 (1958). [Google Scholar]
- Sansone F. J. Popp B. J., Gasc A., Graham A. W. & Rust T. M. Highly elevated methane in the eastern tropical North Pacific and associated isotopically enriched fluxes to the atomosphere. Geophys Res Lett 28, 4567–4570 (2001). [Google Scholar]
- Hirose T., Kawagucci S. & Suzuki K. Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophys Res Lett 38, L17303 (2011). [Google Scholar]
- Wakita H., Nakamura Y., Kita I., Fujii N. & Notsu K. Hydrogen release - new indicator of fault activity. Science 210, 188–190 (1980). [DOI] [PubMed] [Google Scholar]
- Kadko D. C., Rosenberg N. D., Lupton J. E., Collier R. W. & Lilley M. D. Chemical reaction rates and entrainment within the Endeavour Ridge hydrothermal plume. Earth Planet Sci Lett 99, 315–335 (1990). [Google Scholar]
- Punshon S., Moore R. M. & Xie H. Net loss rates and distribution of molecular hydrogen (H2) in mid-latitude coastal waters. Mar Chem 105, 129–139 (2007). [Google Scholar]
- Kawagucci S. et al. Methane, manganese, and helium-3 in newly discovered hydrothermal plumes over the Central Indian Ridge, 18°–20°S, .Geochem Geophys Geosyst 9, Q10002, doi:10.1029/2008GC002082 (2008). [Google Scholar]
- Mottl M. J. et al. Manganese and methane in hydrothermal plumes along the East Pacific Rise, 8°40′ to 11°50′N. Geochim Cosmochim Ac 59, 4147–4165 (1995). [Google Scholar]
- Ijiri A. et al. Enrichment of adsorbed methane in authigenic carbonate concretions of the Japan Trench. Geo-Mar Lett 29, 301–308 (2009). [Google Scholar]
- Hinrichs K. et al. Biological formation of ethane and propane in hte deep marine subsurface. Proc Natl Acad Sci USA 103, 14684–14689 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer V. B. et al. The stable carbon isotope biogeochemistry of acetate and other dissolved carbon species in deep subseafloor sediments at the northern Cascadia Margin. .Geochim Cosmochim Ac 73, 3323–3336 (2009). [Google Scholar]
- Sunamura M., Higasi Y., Miyako C., Ishibashi J. & Maruyama A. Two bacteria phylotypes are predominant in the Suiyo seamount hydrothermal plume. Appl Environ Microbiol 70, 1190–1198 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A. et al. Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326, 578–582 (2009). [DOI] [PubMed] [Google Scholar]
- Emerson D. et al. A novel lineage of Proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE 2, e667 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D., Emerson D., Fleming E. J. & McBeth J. M. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol 64, 561–583 (2010). [DOI] [PubMed] [Google Scholar]
- Kuever J. Rainey F. A. & Widdel F. In Bergey's manual of systematic bacteriology, second edition, volume two, Brenner D. J., , Krieg N. R., , and Staley J. T. (eds.). New York: Springer, 961 (2005). [Google Scholar]
- Kuever J. Rainey F. A. & Widdel F. In Bergey's manual of systematic bacteriology, second edition, volume two, Brenner D. J., , Krieg N. R., , and Staley J. T. (eds.). New York: Springer, 1007 (2005). [Google Scholar]
- Vandamme P. et al., In Bergey's manual of systematic bacteriology, second edition, volume two, New York: Springer, 1161 (2005). [Google Scholar]
- Wirsen C. O. et al. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl Environ Microbiol 68, 316–325 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K. & Horikoshi K. Genetic diversity of archaea in deep-sea hydrothermal vent environments. Genetics 152, 1285–1297 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P. Exploring prokaryotic diversity in the genomic era. Genome Biol 3, reviews0003.1–0003.8 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoura T. & Takai K. Comparison of microbial communities associated with phase-separation-induced hydrothermal fluids at the Yonaguni Knoll IV hydrothermal field, the Southern Okinawa Trough. FEMS Microbiol Ecol 67, 351–370 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary info