Abstract
Embryonic stem (ES) cells differentiating as aggregates self-organize dependent on Wnt signaling that is initially localized to discrete sites in the aggregate. As differentiation proceeds, Wnt signaling expands to most of the aggregates, thus resulting in widespread differentiation of mesendodermal progenitors. This process resembles primitive streak formation, but the lack of organized positional information makes the differentiating aggregates develop in a disorganized fashion. Here, we report that exogenous, cellular signaling sources can control the site where differentiation initiates in ES cell aggregates. Fibroblasts engineered to express cadherins are assembled with ES cells to form composite aggregates where the fibroblasts are positioned as a discrete pole. When engineered to express secreted Wnt agonists or antagonists, this pole functions to localize signaling in a way that polarizes the differentiating aggregates. The use of cell adhesion molecules to control morphology of developing stem cell aggregates should be widely applicable in tissue engineering.
Introduction
Embryonic stem ((ES) cells are capable of limitless self-renewal in vitro and can differentiate into cells constituting all 3 primitive germ layers: endoderm, mesoderm, and ectoderm, as well as germ cells. ES cells are typically differentiated by removal of factors that sustain pluripotency, in either adherent culture on tissue culture plastic coated with extracellular matrix components, in coculture with stromal cells, or in suspension culture where the ES cells form aggregates known as embryoid bodies (EBs), typically composed of 500–2,000 cells [1,2]. ES cells differentiating as EBs self-organize dependent on Wnt signaling that is initially localized to discrete sites in the aggregate [3]. As differentiation proceeds, Wnt signaling expands to most of the aggregates, thus resulting in widespread differentiation of mesendodermal progenitors. This process resembles primitive streak formation, but the lack of organized positional information makes the differentiating aggregates develop in a disorganized fashion. One can guide the differentiation along specific developmental lineages by varying the EB size, by addition of growth factors to the culture medium, but endogenous, intra-EB signaling cannot be readily controlled [1,4]. Conceivably, an exogenous, cellular source of secreted agonists (or antagonists), activating (or repressing) specific signaling pathways could be used to control the spatial extent of differentiation in EBs, if the geometry of the composite aggregate can be controlled.
Here, we show that EBs containing exogenous, cellular signaling sources can be assembled and that the signaling source can control the site where differentiation initiates in ES cell aggregates. We use fibroblasts engineered to express cadherins together with undifferentiated ES cells to assemble composite aggregates where the fibroblasts are positioned as a discrete pole. When engineered to additionally express secreted Wnt agonists or antagonists, this pole functions to localize signaling in a way that polarizes the differentiating aggregate.
Materials and Methods
Cloning
Mouse Cdh1 and Cdh2 (encoding E- and N-cadherin, respectively) cDNA was amplified from E7 whole mouse embryo cDNA by PCR. PCR products were cloned into pENTR/D-TOPO (Invitrogen). After sequencing, E-cadherin cDNA was recombined into the pCGIG destination vector and N-cadherin cDNA into the pCGImycT destination vector by way of Gateway® cloning. The pCGIG destination vector [5] was a kind gift from Dr. Anne Grapin-Botton. The resulting constructs encoded E-cadherin and GFPNLS (pCGIG-E-cadherin) or N-cadherin and a myc-tagged dTomato (pCGImycT-N-cadherin). Wnt3a-HA was amplified by PCR from Wnt3a-HA-pUSEamp (Upstate Biotechnology). PCR products were cloned into pENTR/D-TOPO (Invitrogen). After sequencing, Wnt3a-HA was recombined into pEFDEST51 (Invitrogen) by Gateway cloning. Mouse Dkk1 was PCR amplified and cloned into pCR Blunt TOPO (Invitrogen). The Dkk1 cDNA was excised by NheI-BspE1 digestion and cloned into pIRES-Puro3 (Clontech).
Cell lines and culture
Ltk- cells, a thymidine kinase deficient subclone of L cells [6], were chosen as inducer cells, as L cells do not express classic cadherin molecules at appreciable levels [7]. Ltk- cells were cultured in DMEM, 4,500 mg/L glucose supplemented with Glutamax®, 10% fetal calf serum, 50 U/mL penicillin, 50 μg/mL streptomycin, and 1 mM sodiumpyrovate (all from Invitrogen). Ltk- cells were transfected with pCGIG-E-cadherin or pCGImycT-N-cadherin by using Lipofectamine 2000 (Invitrogen). The cells were cotransfected with a plasmid containing the herpes simplex virus thymidine kinase gene (pPGK1-HSV-TK) conferring resistance to selection in HAT medium (Invitrogen). A cell line expressing GFPNLS but not E-cadherin was also generated by using the original pCGIG vector. As expected, the HAT resistant cells became sensitive to gancliclovir and GFP- or Tomato-positive (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertonline.com/scd). Expression of E- and N-cadherin was demonstrated by western blotting and immunofluorescent (IF) analysis (Supplementary Fig. S1C–F), and the functional activity was tested by cell mixing experiments as described [7], which showed the expected preference for homotypic adhesion (Supplementary Fig. S1G). For further culture of transfected cells, the medium was supplemented with 1xHAT (Invitrogen). L-Ecad-GFP and L-Ncad-Tomato cells were transfected with the expression constructs pDEST51-Wnt3a-HA and pmDkk-1 IRES Puro3, respectively, by using Lipofectamine 2000. Transfected cells were selected in the presence of 10 ng/mL blasticidin or 5 μg/mL puromycin (both from Invivogen) for ∼14 days, after which resistant colonies were picked and expanded. Generation of Rosa26R26–60-TOP-TA-CFP ES cells will be described in detail elsewhere. Briefly, we first replaced the luciferase reporter in pSuperTOP-Flash [8] with Cerulean fluorescent protein (CFP) [9], and the entire SuperTOP-TA-CFP cassette was via a shuttle vector targeted to a modified, transcriptional inactive Rosa26 locus via recombinase-mediated cassette exchange in Rosa26LCA ES cells [10]. E14 and Rosa26R26–60-TOP-TA-CFP ES cells were essentially cultured under serum-free conditions in 2i/lif medium as previously described [11–13]. For manipulation of spontaneous EB differentiation, the medium was supplemented with the following compounds: 1,500 U/mL LIF (Chemicon), 1 μM SB431542 (Calbiochem), 50 ng/mL Noggin (R&D Systems), and 10 μM dorsomorphine (Tocris Bioscience).
Aggregation of L and ES cells in hanging drops
ES cells were dissociated by using nonenzymatic cell dissociation solution (Sigma) and diluted in standard serum containing medium without LIF to the relevant concentration for the desired EB size and transferred to 50 mL polystyrene trays (Corning). Approximately, a hundred 20 μL drops were applied to the lid of a 14 cm cell culture dish (Nunc). EB size ranged from 125 to 1,000 cells. L-cells were added to the drops as single cells, either mixed in suspension with the ES cells (for L-Ncad-Tomato) or the next day after EB formation had occurred (for L-Ecad-GFP; see Fig. 1). The next day, EBs were washed down with HBSS and allowed to sediment in 15 mL centrifuge tubes for 1–2 min. HBSS was removed by suction, and EBs were transferred to standard serum-free medium (supplemented with N2B27) in bacterial dishes for suspension culture for a variable number of days. Tracking of individual EBs was done in hanging drops or after transfer to individual bacterial dishes.
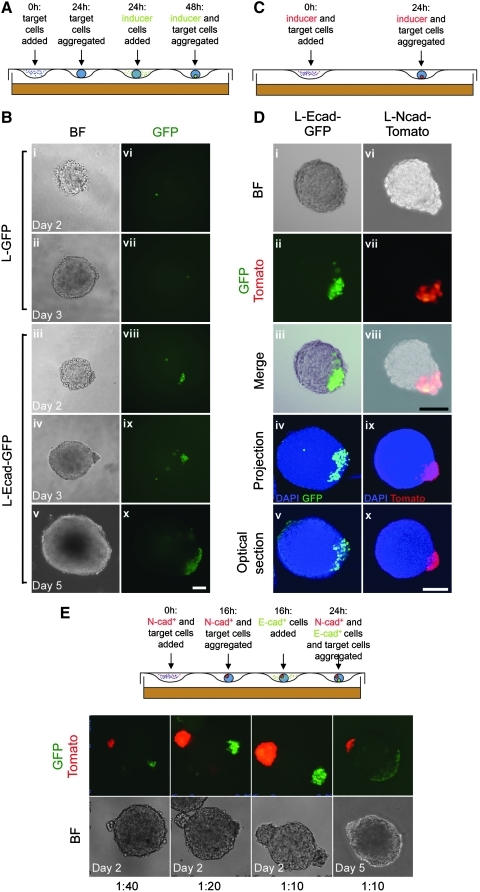
FIG. 1.
Assembly of composite EBs. (A) Schematic protocol for assembly of composite EBs composed of ES cells and L-Ecad-GFP cells. (B) 250 ES cells and 25 L-GFP (i, ii, vi, vii) or L-Ecad-GFP cells (iii–v and viii–x) were assembled as shown in (A) and subsequently transferred to a bacterial Petri dish. Images were taken on day 2, immediately after transfer, and on days 3 and 5. Note that L-Ecad-GFP cells integrate into a composite EB, whereas L-GFP cells do not. (C) Schematic protocol for assembly of composite EBs composed of ES cells and L-Ncad-Tomato cells. (D) Aggregates consisting of 250 ES cells and 25 L-Ecad-GFP (i–v) or L-Ncad-Tomato cells (vi–x) were assembled as shown in (A) and (C), respectively, before being transferred to bacterial Petri dishes. Images were taken on day 3. Confocal images are of composite EBs whole-mount stained with antibodies against GFP (iv, v) and the myc-tag on Tomato (ix, x), respectively. Counterstaining with DAPI was used to visualize DNA. (E) Upper part shows a schematic protocol for assembly of composite EBs composed of ES cells, L-Ecad-GFP cells, and L-Ncad-Tomato cells. Lower part shows composite EBs generated from 500 ES cells and 12 (1:40), 25 (1:20) or 50 (1:10) L-Ecad-GFP and L-Ncad-Tomato cells. Note that composite EBs with 2 distinct poles are stable for up to 5 days. Scale bars are 100 μm. EB, embryoid body; ES, embryonic stem.
Western blotting
L-Ecad-GFP cells were harvested in 1× cell extraction buffer (Invitrogen) supplemented with Complete Protease Inhibitor Cocktail (Roche). Proteins were separated on NuPage 4%–12% Bis-Tris polyacrylamide gels (Invitrogen) and transferred to a nitrocellulose membrane (Invitrogen). Cadherins were detected by using rat-anti-E-cadherin (Zymed) and rat-anti-mouse N-cadherin (DSHB), and both were labeled with a horseradish-peroxidase-conjugated goat anti-rat secondary antibody (Santa Cruz). α-tubulin and β-actin were used as loading control. Primary antibodies: Mouse anti-bovine α-tubulin (Molecular Probes) and mouse-anti-β-actin (Sigma). Secondary antibodies: horseradish-perioxidase-conjugated goat anti-mouse (Santa Cruz) or donkey anti-mouse (Jackson). Proteins were visualized on HyperFilm ECL (Amersham Bioscience) by using SuperSignal Substrate (Pierce).
Whole-mount immunocytochemistry
EBs were fixed for 30 min in Lilly's fixative (Bie and Berntsen) and transferred to phosphate-buffered saline (PBS). Immunocytochemistry (ICC) was performed by using a protocol for whole-mount immunohistochemistry (IHC) of embryos [14]. Briefly, EBs were incubated in methanol (MeOH) for 1 h, then in MeOH:DMSO:H2O2, 4:1:1 for 1 h at room temperature. EBs were washed in PBS and blocked in 0.5% TNB buffer for 1 h (Perkin-Elmer). The primary antibody was diluted in PBS+0.3% Triton-X+0.25% bovine serum albumin (BSA) and incubated overnight at 4°C with gentle rocking. EBs were washed in PBS (3×10 min), and secondary antibodies were diluted in PBS+0.3% Triton-X+0.25% BSA and applied overnight at 4°C with gentle rocking. EBs were washed in PBS (3×10 min) and transferred to 100% MeOH. MeOH was changed thrice with 10 min interval to ensure that PBS was completely washed out. EBs can be stored at −20°C for months in 100% methanol without loss of fluorescence intensity. Primary antibodies used were as follows: Alexa 488 conjugated rabbit anti-GFP to (Molecular Probes/Invitrogen); goat anti-brachyury (R&D Systems); mouse anti-GFP (Nordic Biosite); rabbit anti-Myc (Santa Cruz). Secondary antibodies used were Cy2-donkey-anti-rabbit, Cy3-donkey-anti-goat, Cy2-donkey-anti-mouse, Cy3-donkey-anti-rabbit, and Cy5-donkey-anti-goat, all from Jackson ImmunoResearch Laboratories. DAPI was used at 3 μg/mL.
Confocal microscopy
EBs stored in 100% methanol were cleared in BABB (1:2 mixture of benzyl alcohol to benzyl benzoate) and transferred to petridishes with covered glass bottoms (FluoroDish™; World Precision Instruments). Images were obtained on a Zeiss AxioVert 200M META microscope connected to a LSM 510 lazer module by using the Achroplan 20x/0.5 W Ph2 objective or the Plan-Apochromat 20x/0.75 objective. Z sections were recorded at 5 or 10 μm intervals and projected to 3D by using Zeiss LSM software.
Statistics
Statistical analyses were performed by using Fisher's exact test.
Results
We first explored whether step-wise assembly of composite aggregates could be achieved by forming EBs in hanging drops and, subsequently, adding a small number of L-cells expressing a nuclear localized EGFP (L-GFP cells) and allowing these to sediment in close vicinity of the preformed EB (Fig. 1A). When using L-GFP cells, a few cells were occasionally found to attach loosely to the surface of the EBs at 24 h, but these were invariably lost when transferring the EBs to suspension culture in bacterial dishes and after 48 h, we could no longer find L-GFP cells in contact with the EB (Fig. 1B). We suspected that the low endogenous cadherin activity in L-cells [7] prevented them from stably adhering to the E-cadherin expressing ES cells in the EBs, and, therefore, repeated the experiment after stably introducing recombinant Cdh1 into L-GFP cells (L-Ecad-GFP cells; Supplementary Fig. S1). Addition of 50–100 L-Ecad-GFP cells to a preformed EB results in the creation of a composite EB with the L-Ecad-GFP cells forming a discrete cap on more than 50% of the EBs (n>300), which was stable for up to 5 days after transfer to bacterial dishes (Fig. 1B). The requirement for E-cadherin expression in L-cells in order for these to adhere efficiently to preformed EBs led us to speculate that the ability of classical cadherins to organize tissue morphogenesis [15] could be further exploited to control the geometry of composite aggregates in a single-step assembly process. We, therefore, generated L-cells expressing N-cadherin and a myc-tagged dTomato (L-Ncad-Tomato cells; Supplementary Fig. S1) and attempted to generate composite aggregates between these and ES cells. We mixed dissociated L-Ncad-Tomato cells with dissociated E14 ES cells at a 1:10 ratio and cultured them overnight in hanging drops (Fig. 1C). After 24 h, the cells had segregated according to cadherin expression with the ES cells forming the bulk of the composite EB and the L-Ncad-Tomato cells forming one (∼35%) or more (∼50%) discrete poles (n>300; Fig. 1D), which remained stable for up to 5 days after transfer to suspension culture (not shown). Whole-mount IF analysis revealed that L-Ecad-GFP and L-Ncad-Tomato cells were mostly attached to the surface of the EB with little contribution to the interior (Fig. 1D).
Signaling in vivo is often regulated by a localized source of an agonist combined with a distant sink, acting either via enzymatic degradation of the agonist or by secretion of protein that prevents activity by sequestering the agonist. We, therefore, tested whether we could assemble composite aggregates between ES cells and 2 separate and distinguishable clusters of engineered L-cells. We found that this was readily achieved by combining the self-organizing properties of ES cell/L-Ncad-Tomato cells in a first step, followed by addition of L-Ecad-GFP cells, which were allowed to sediment in hanging drops containing the preformed ES cell/L-Ncad-Tomato aggregates (Fig. 1E). Up to 5% of the aggregates (n>300) display a geometry shown in Fig. 1E with the 2 poles positioned on opposite sides of the ES cells, whereas a much larger fraction (∼50%) displayed 2 or more distinct poles located more closely to each other. Additionally, the size of the poles and, thus, the amount of putative growth factor secreted from these can be controlled by varying the number of engineered L-cells in the assembly process (Fig. 1E).
To test whether a growth factor expressed in L-Ecad-GFP cells could activate signaling in the ES cell component of our composite aggregates, we introduced a Wnt3a expression construct into the cells, generating L-Ecad-GFP-Wnt3a cells and generated composite EBs with ES cells carrying a novel fluorescent reporter of canonical Wnt signaling; Rosa26R26–60-TOP-TA-CFP (see Materials and Methods). We first mapped the time and extent of spontaneous, endogenous Wnt signaling in normal EBs formed by Rosa26R26–60-TOP-TA-CFP ES cells in the absence of L-Ecad-GFP-Wnt3a cells in serum-free medium. After 72 h of differentiation, there was no sign of endogenous activation of the Wnt reporter, but 24 h later, most of the EBs had activated the reporter (n>100; Supplementary Fig. S2A). This activation could be blocked by treatment with Dkk1 or the ALK4/5/7 inhibitor SB431542, which blocks canonical Wnt and nodal/activin signaling, respectively (n>100; Supplementary Fig. S2B). In contrast to a previous report [3], blocking BMP signaling with Noggin (Supplementary Fig. S2B) or dorsomorphin (not shown) had no effect on spontaneous Wnt reporter activation (n>100). Whole-mount IF analysis revealed induction of Brachyury (Bry, encoded by the T gene) expression in CFP+ cells at day 4 (Supplementary Fig. S2C), and qRT-PCR confirmed that T expression peaked at day 4 (Supplementary Fig. S2D).
We then assayed reporter activation after aggregation with L-Ecad-GFP-Wnt3a cells at 72 h, before endogenous activity could mask any induced activation. More than 75% of the of Rosa26R26–60-TOP-TA-CFP ES cell aggregates activated Cerulean expression in close vicinity to the L-Ecad-GFP-Wnt3a cells, whereas no activation was seen when control L-Ecad-GFP cells were added (n>100; Fig. 2A). Moreover, IF analysis revealed that Brachyury expression was also activated close to the L-Ecad-GFP-Wnt3a cells, thus demonstrating that localized differentiation could be induced by these (Supplementary Figs. 2B and S3A). However, the onset of differentiation raises a possible caveat when interpreting the Wnt reporter activation. As ES cells differentiate, they activate expression of Wnt proteins [3,16] capable of activating a Wnt reporter in ES cells [3]. We would, therefore, expect that our Cerulean reporter should respond to ES cell-derived Wnt-family members as well as to Wnt3a produced by the L-Ecad-GFP-Wnt3a cells, making it difficult to discriminate between the 2 sources of ligand. To prevent induction of Wnt protein expression in the ES cells, we repeated the experiment in the presence of LIF for the first 48 h, which prevents spontaneous differentiation of the aggregated ES cells (Supplementary Fig. S3B–D). In contrast, activation of the CFP reporter by L-Ecad-Wnt3a cells was not affected by LIF (n>100; Supplementary Fig. S3B), even though activation of Brachyury expression in Rosa26R26–60-TOP-TA-CFP ES cells by L-Ecad-GFP-Wnt3a cells was blocked (Supplementary Fig. S3C). Together, these data indicate that Wnt3a derived from the L-Ecad-GFP-Wnt3a cells is indeed responsible for activation of the CFP reporter in our ES cells.
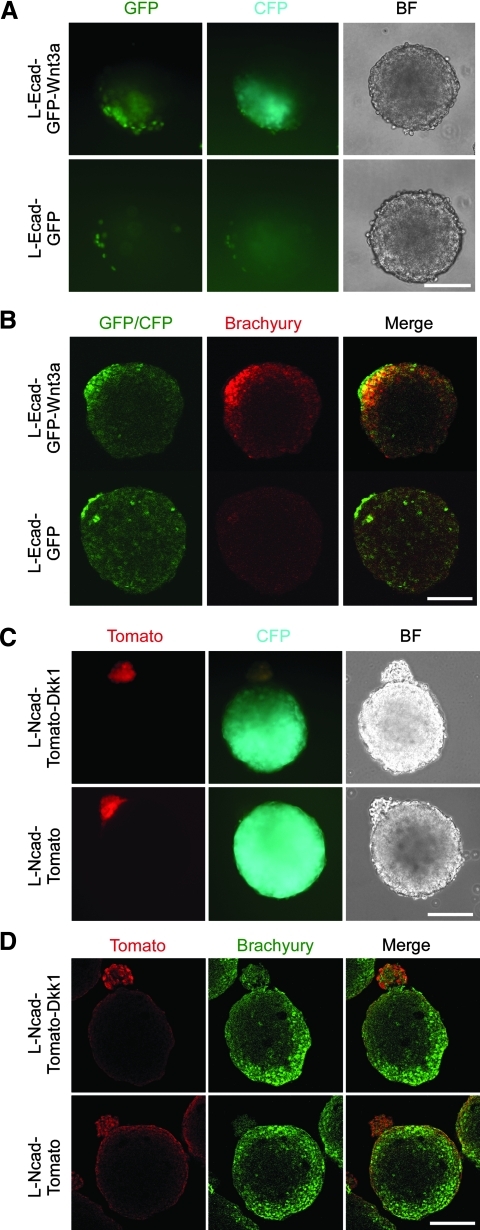
FIG. 2.
Polarization of ES cell differentiation in composite EBs. (A) BF and fluorescent micrographs of day 3 composite EBs generated from 250 Rosa26(-60)-TA-TOP-CFP ES cells aggregated with 25 L-Ecad-GFP-Wnt3a cells or L-Ecad-GFP cells as indicated. Note activation of the CFP reporter in the vicinity of the L-Ecad-GFP-Wnt3a cells, and lack of activation close to control L-Ecad-GFP cells. (B) Confocal, optical sections of day 3 composite EBs generated as in A, whole-mount stained with anti-GFP/CFP and anti-brachyury antibodies. Note expression of Bry in the vicinity of L-Ecad-GFP-Wnt3a cells, but not in control EBs made with L-Ecad-GFP cells. (C) BF and fluorescent micrographs of day 4 composite EBs generated from 250 Rosa26(-60)-TA-TOP-CFP ES cells aggregated with 25 L-Ncad-Tomato-Dkk1 cells or L-Ncad-Tomato cells as indicated. Note repression of the CFP reporter in the vicinity of the L-Ncad-Tomato-Dkk1 cells, and lack of repression close to control L-Ncad-Tomato cells. (D) Confocal, optical sections of day 4 composite EBs generated as in (C), whole-mount stained with anti-myc and anti-brachyury antibodies. Note that expression of Bry is suppressed in the vicinity of L-Ncad-Tomato-Dkk1 cells, but not in control EBs made with L-Ncad-Tomato cells. Scale bars are 100 μm. BF, bright field; CFP, Cerulean fluorescent protein.
Finally, we set out to test whether we could inhibit endogenous Wnt signaling locally by assembling composite EBs from Rosa26R26–60-TOP-TA-CFP ES cells and Dkk1 transfected L-Ncad-Tomato cells and assayed CFP reporter activation at day 4. Twelve out of fifteen EBs containing a single, well-formed pole of L-Ncad-Tomato-Dkk1 cells showed suppressed activation of the CFP reporter in the vicinity of the L-Ncad-Tomato-Dkk1 cells, compared with zero out of 8 EBs containing a single, well-formed pole of L-Ncad-Tomato cells (P<0.0005; Fig. 2C). Likewise, IF analysis revealed that Bry expression was suppressed close to the L-Ncad-Tomato-Dkk1 cells, whereas Bry expression encompasses the entire periphery in control aggregates (Fig. 2D), similar to the situation found in regular EBs (Supplementary Fig. S2C). These results demonstrate that canonical Wnt signaling as well as the associated differentiation can be locally inhibited by Dkk1-expressing cells.
Discussion
Here, we report a novel way of controlling the timing and location of ES cell differentiation by allowing ES cells to form composite aggregates with fibroblasts engineered to express appropriate cell adhesion molecules and growth factors. ES cells hold great promise as a source of differentiated cells that can be used to treat degenerative diseases. The key to generating desired cell types is the development of differentiation protocols that allow efficient and controlled formation of the particular cell type. Differentiation of ES cells is often induced by culturing the cells as “EBs,” but the investigator has little control over the process. Differentiation in EBs is self-organized via endogenous Wnt signaling [3]. To obtain a degree of control, one can add soluble growth factors and inhibitors to the medium, but this approach has its limitation, as a soluble factor will, when added to the medium, affect all cells in the periphery of the aggregate but have little or no effect on cells located within the aggregate [4]. The method described here, designed to spatially and temporally control delivery of growth factors and/or inhibitors, overcomes some of the limitations of current EB-based differentiation protocols and gives the investigator the possibility to more precisely control the signaling and differentiation of ES cell aggregates.
The method presented here makes it possible to instruct ES cells in the vicinity of the engineered fibroblasts to engage a differentiation program earlier than ES cells located further away from the source of growth factor. Further, by varying (1) the number of stem cells versus growth factor-producing cells, (2) the cell adhesion molecules used, and (3) the number of different inducer cells used to set up the composite aggregates, the investigator can design conditions that suit a particular differentiation strategy. We believe that the general principle of using adhesion molecules to guide the 3D organization of composite cell aggregates could, in theory, be expanded to encompass more families of adhesion molecules other than classical cadherins and, thus, potentially be used to guide the development of cells and tissues in a number of tissue-engineering settings.
We show here that step-wise assembly of composite aggregates between stem cells and engineered inducer cells can be achieved with predictable geometries if one has knowledge of and/or control over the expression of cell adhesion molecules expressed in stem cells and inducer cells. We use the system to provide a localized source of Wnt3a (or the Wnt antagonist Dkk1), thus resulting in local activation (or inhibition) of Wnt signaling in the composite EBs and demonstrate that differentiation is reproducibly induced (or inhibited) in the vicinity of the inducing cells.
During our characterization of spontaneous EB differentiation, we made some observations that differed from a previous study. Spontaneous, Wnt-mediated differentiation of EBs has been reported to start after 72 h [3], but in the current study, we consistently found that our aggregates showed no signs of differentiation at this time. Instead, we saw widespread differentiation at 96 h, suggesting that initiation of differentiation commenced somewhere between 72 and 96 h. This discrepancy is most likely due to different cell lines and possibly also by differences in aggregate size and culture medium. Another discrepancy between our data and those of Nusse and coworkers [3] is the dependency on BMP signaling observed by that group. We failed to unravel a dependency on BMP signaling, as treatment with the soluble BMP antagonist Noggin or a small molecule inhibitor of the type 1 BMP receptors ALK2/3/6 dorsomorphin [17] had no effect on Wnt reporter activation. Instead, we found that inhibition of nodal/activin signaling reduced Wnt reporter activation. We have no explanation for the lack of BMP dependency but speculate that different ES cell lines may differ in their dependency for additional signaling events. The requirement for nodal/activin signaling, however, is consistent with a previous study which found that nodal/activin signaling was required for Wnt3a to inhibit neuroectoderm differentiation [18].
The results presented here provide a stepping stone for more detailed studies aimed at exploiting the predictable geometry of such composite aggregates. Controlled aggregation of stem cells with growth-factor-producing cell lines, as demonstrated here, could potentially be used to examine mechanisms of growth factor signaling, particularly the formation and regulation of morphogen gradients. Much work remains before the full potential of this novel way of controlling the 3D organization of cellular aggregates can be realized, but the results presented here clearly demonstrate that differentiation of stem cells can be directed spatially and temporally by using adhesion molecules to assemble composite stem cell/inducer cell aggregates.
Supplementary Material
Acknowledgments
The authors are grateful to Randall T. Moon for the SuperTOP-Flash reporter and to Ragna Jørgensen for expert technical assistance. This work was made possible due to support from the NIDDK (DK072473) to M.A.M., K.S.Z., and P.S., and the Danish Stem Cell Doctoral School (DASCDOC) to P.S.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Bratt-Leal AM. Carpenedo RL. McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans T. Embryonic stem cells as a model for cardiac development and disease. Drug Discov Today Dis Models. 2008;5:147–155. doi: 10.1016/j.ddmod.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ten Berge D. Koole W. Fuerer C. Fish M. Eroglu E. Nusse R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3:508–518. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachlos E. Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29:4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Megason SG. McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 6.Earle WR. Production of malignancy in vitro. IV. The mouse fibroblast cultures and changes seen in living cells. J Nat Cancer Inst. 1943;4:213–227. [Google Scholar]
- 7.Nose A. Nagafuchi A. Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 8.Veeman MT. Slusarski DC. Kaykas A. Louie SH. Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo MA. Springer GH. Granada B. Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol. 2004;22:445–449. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 10.Chen SX. Osipovich AB. Ustione A. Potter LA. Hipkens S. Gangula R. Yuan W. Piston DW. Magnuson MA. Quantification of factors influencing fluorescent protein expression using RMCE to generate an allelic series in the ROSA26 locus in mice. Dis Model Mech. 2011;14:537–547. doi: 10.1242/dmm.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols J. Silva J. Roode M. Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva J. Barrandon O. Nichols J. Kawaguchi J. Theunissen TW. Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo G. Yang J. Nichols J. Hall JS. Eyres I. Mansfield W. Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahnfelt-Ronne J. Jorgensen MC. Hald J. Madsen OD. Serup P. Hecksher-Sorensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 2007;55:925–930. doi: 10.1369/jhc.7A7226.2007. [DOI] [PubMed] [Google Scholar]
- 15.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 16.Hansson M. Olesen DR. Peterslund JM. Engberg N. Kahn M. Winzi M. Klein T. Maddox-Hyttel P. Serup P. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol. 2009;330:286–304. doi: 10.1016/j.ydbio.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu PB. Hong CC. Sachidanandan C. Babitt JL. Deng DY. Hoyng SA. Lin HY. Bloch KD. Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engberg N. Kahn M. Petersen DR. Hansson M. Serup P. Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells. 2010;28:1498–1509. doi: 10.1002/stem.479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.