Abstract
Human mesenchymal stem cells (hMSCs) are an attractive choice for a variety of cellular therapies. hMSCs can be isolated from many different tissues and possess unique mitochondrial properties that can be used to determine their differentiation potential. Mitochondrial properties may possibly be used as a quality measure of hMSC-based products. Accordingly, the present work focuses on the mitochondrial function of hMSCs from umbilical cord blood (UCBMSC) cells and bone marrow cells from donors younger than 18 years of age (BMMSC <18) and those more than 50 years of age (BMMSC >50). Changes of ultrastructure and energy metabolism during osteogenic differentiation in all hMSC types were studied in detail. Results show that despite similar surface antigen characteristics, the UCBMSCs had smaller cell surface area and possessed more abundant rough endoplasmic reticulum than BMMSC >50. BMMSC <18 were morphologically more UCBMSC-like. UCBMSC showed dramatically higher mitochondrial-to-cytoplasm area ratio and elevated superoxide and manganese superoxide dismutase (MnSOD) levels as compared with BMMSC >50 and BMMSC <18. All hMSCs types showed changes indicative of mitochondrial activation after 2 weeks of osteogenic differentiation, and the increase in mitochondrial-to-cytoplasm area ratio appears to be one of the first steps in the differentiation process. However, BMMSC >50 showed a lower level of mitochondrial maturation and differentiation capacity. UCBMSCs and BMMSCs also showed a different pattern of exocytosed proteins and glycoproteoglycansins. These results indicate that hMSCs with similar cell surface antigen expression have different mitochondrial and functional properties, suggesting different maturation levels and other significant biological variations of the hMSCs. Therefore, it appears that mitochondrial analysis presents useful characterization criteria for hMSCs intended for clinical use.
Introduction
Human mesenchymal stem cells (hMSCs) hold great promises for cellular therapies, as they are relatively easy to obtain and expand in vitro, they posses 3-lineage differentiation potential, extensive self renewal capacity and there is evidence for their potential use in clinical trials [1–4]. hMSCs are traditionally derived from adult tissues, such as adipose and bone marrow, but cells from these sources seem to lose their potential during aging; and, therefore, alternative sources are sought from postnatal tissues, such as umbilical cord blood [1,5–9]. Umbilical cord blood-derived MSCs (UCBMSC) are now considered a potential substitute for bone marrow-derived mesenchymal stem cell (BMMSC) to overcome the aging problem, as they are derived from an in vivo microenvironment more similar to bone marrow with characteristic properties for hMSCs [5,10–15].
Several comparative studies on differentiation potency, proliferation rate, immunophenotype, and gene expression of hMSCs from different sources have been previously conducted [16–21]. In general, cell surface antigen characteristics are similar for hMSCs derived from umbilical cord, fetal bone marrow, adult bone marrow, and adipose tissue; but inconsistencies in differentiation potential and proliferation rate have been reported. On the other hand, the characterization criteria for hMSCs remain unspecific; and different isolation protocols or different culture media are commonly used, which may explain the conflicting results of previous studies. In addition, the tendency for hMSCs to exhibit different levels of maturation poses challenges for the development of specific characterization criteria for hMSCs [22].
Mitochondrial function plays a major role in terminally differentiated cells by fulfilling the energy demand of the cells and regulating several critical functions, such as apoptosis, cell cycle, and calcium homeostasis [23–26]. Recently, more attention has been focused on mitochondria and cellular bioenergetics of stem cells, which has revealed their unique metabolic status [27–29]. It has been shown that mitochondrial activity or dormancy plays a major role in maintaining the undifferentiated state, whereas the proper activation and function of mitochondria is essential during the differentiation processes [29–31]. In addition, mitochondrial reactive oxygen species (ROS) regulate the differentiation of several stem cell types; and recently, ROS have been shown to inhibit adhesion of hMSCs to the site of injury [32–34].
The present study focuses on the comparative functional analysis of hMSCs from different donors with special emphasis on the mitochondrial properties. To do so, mitochondrial function, exocytosed molecules, and differentiation of hMSCs from bone marrow of donors of different ages and similarly also UCBMSCs were analyzed in detail. To our knowledge, this is the first study where mitochondrial and secretory properties of hMSCs from different origins have been studied and compared. We believe that differences in mitochondrial-to-cytoplasm area ratio and function could be explained by the different maturation level of hMSCs. Moreover, we also show that an increase in mitochondrial-to-cytoplasm area ratio is one of the first steps during osteogenic differentiation. Therefore, we argue that mitochondrial analysis is an extremely important additional approach in characterization of hMSC-based cell products, and these different properties can have an impact on the intended therapies.
Materials and Methods
Isolation and culture of human bone marrow-derived hMSCs
BMMSCs were isolated from patients who were operated for osteoarthritis and from some younger patients operated for idiopathic scoliosis. BMMSCs were isolated from an unaffected bone site and expanded as earlier described [35]. Patients with neuromuscular scoliosis, autoimmune diseases such as rheumatoid arthritis, or genetic diseases have been excluded from this study. Samples from bone marrow were suspended in a proliferation medium containing alpha minimum essential medium (αMEM) buffered with 20 mM HEPES and containing 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 2 mM L-glutamine; and the suspension was transferred into a cell culture flask. hMSCs were allowed to attach for 48 h at 37°C in 5% CO2 and 20% O2. Nonattached cells were removed by changing fresh medium, and attached cells were cultured at the bottom of the flask until they reached 70%–80% confluence. The medium was changed twice a week, and cells in passages 3–5 were used in experiments.
Isolation and propagation of umbilical cord blood-derived hMSCs
Cord blood collections were performed at the Helsinki University Central Hospital, Department of Obstetrics and Gynecology, and Helsinki Maternity Hospital. UCBMSCs were isolated and expanded as earlier described [36]. Mononuclear cells were isolated by using Ficoll-Hypaque (Amersham Biosciences) gradient centrifugation. Tissue culture plates (Nunc) were coated with fibronectin (Sigma), and mononuclear cells were plated at a density of 1×106/cm2. The initial UCBMSC line establishment was performed under hypoxic conditions (5% CO2, 3% O2 at 37°C) in a medium containing αMEM with Glutamax (Gibco) and 10% fetal calf serum (Gibco) supplemented with 10 ng/mL epidermal growth factor (EGF; Sigma), 10 ng/mL recombinant human platelet-derived growth factor (R&D Systems), 50 nM Dexamethasone (Sigma), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). Cells were allowed to adhere overnight, and nonadherent cells were washed with medium changes. Hence, UCBMSCs were cultured in normoxic conditions (5% CO2 and 20% O2 at 37°C), and proliferation media was renewed twice a week. Established lines were passaged when almost confluent and replated at 1,000–3,000 cells/cm2. Cells in passages 3–6 were used.
Immunophenotype analysis of hMSCs by flow cytometry
hMSCs were detached from the culture flask and suspended in phosphate-buffered saline (PBS) with 0.5% bovine serum albumin (BSA). The characterization of cell surface antigens for hMSCs was performed by using the following conjugated antibodies: CD44 [fluorescein isothiocyanate (FITC); BD Biosciences], CD49e [phycoerythrin (PE); BD Biosciences], CD90 (FITC; Stem Cell Technologies), CD73 (PE; BD Biosciences), HLA-ABC (allophycocyanin; BD Biosciences), and CD105 (FITC; Abcam). Negative surface antigens for hMSCs were incubated simultaneously as a group in the same sample: HLA-DR (PE; BD Biosciences), CD34 (PE; BD Biosciences), CD45 (PE; BD Biosciences), CD14 (PE; BD Biosciences), and CD19 (PE; BD Biosciences). In addition, the following isotype controls were used: FITC Mouse IgG2a k (BD Biosciences) and PE Mouse IgG2a k (BD Biosciences). hMSCs were incubated at room temperature for 20 min and then washed with and finally suspended in PBS +0.5% BSA.
Samples were analyzed by using FACSCalibur (Becton Dickinson), equipped with dual lasers emitting at 488 and 633 nm. The fluorescence emissions were measured at 530±30 nm, 585±42 nm, and 661±16 nm; and data were compensated and analyzed with FlowJo (TreeStar Inc.). Positivity for a particular antibody was defined by negative and isotype controls.
Cell surface proteomics
UCBMSCs and BMMSCs were used for mass spectrometric analysis of cell surface proteins including extracellular matrix (ECM) proteins associated with these cells. The gel-based proteome analysis was performed once from 2 different cell lines, whereas liquid chromatography mass spectrometry (LC-MS) protein profiling was repeated >10 times from 3 different MSC lines. The cell surface proteins in subconfluent cell cultures were essentially biotinylated as earlier described [37]. Hereafter, the labeled proteins were harvested with streptavidin-coupled magnetic beads and analyzed by using either a gel-based analysis method or direct digestion in liquid. In the gel-based protocol, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) separation was carried out by using 12% polyacrylamide gel, which was first silver stained as earlier described [38], and then cut into 35 slices per lane. The gel pieces were washed and proteins were reduced, alkylated, and tryptically digested over night as previously described [39]. In the direct digestion approach, the proteins were reduced, alkylated, and digested with trypsin as previously described [40]. Mass spectrometric analyses of digested peptides were performed with LC-MS. Briefly, peptides were loaded to a reversed-phase precolumn (NanoEase Atlantis dC18, 180 μm×23.5 mm; Waters) and separated in a reversed-phase analytical column (PepMap 100, 75 μm×150 mm; Dionex Corporation) with a linear gradient of acetonitrile. Ultimate 3000 LC instrument (Dionex Corporation) was operated in a nano scale with the flow rate of 0.3 μL/min. Eluted peptides or glycans were introduced to LTQ (Linear Trap Quadrupole) Orbitrap XL mass spectrometer (Thermo Fisher Scientific Inc.) via an electrospray chip interface (NanoMate Triversa, Advion Biosciences Inc.) in positive-ion mode. Based on full MS scan, 6 MS/MS data-dependent scans were acquired on LTQ. Data files from mass spectrometry measurements were processed with Mascot Distiller (Matrix Science Ltd., version 2.2.1.0) via Mascot Daemon Client (Matrix Science Ltd., version 2.2.2). Protein identifications were performed by Mascot MS/MS ions search on Mascot Server (Matrix Science Ltd., version 2.2.04) against the human proteome in UniProtKB database (Release 2010_09) [41]. The enrichment of cell surface proteome was verified by calculating the proportion of integral membrane proteins identified in these analyses.
Osteogenic differentiation induction and detection of calcium deposition
Osteogenic differentiation of hMSCs was performed in 96-well plates for 5 weeks. Osteogenic induction of all hMSC types was performed in the same medium that contained BMMSC proliferation medium supplemented with 100 nM dexamethasone, 10 μM β-glycerophosphate, and 0.05 mM ascorbic acid-2-phosphate was used. The basic proliferation medium was used as a control. Cells were plated at a density of 1,500 cells/cm2 in 4 replicates; and during the 5 week period, the medium was changed twice a week. Calcium deposition was analyzed as described next. hMSCs were washed with PBS, and 0.6 M HCL was added and incubated overnight at room temperature. The next day, calcium content was measured with the o-cresolphthalein-complexone method according to the manufacturer (Roche Diagnostics Corporation). A Plate reader (Victor 2; Wallac Oy) was used to measure absorbance at 550 nm. Results were indicated as fold changes comparing osteoinduced cells with controls.
Transmission electron microscopic analysis of hMSCs
From each hMSCs type, one line was selected for transmission electron microscopy (TEM) analysis before and after 14-day osteoinduction. hMSCs grown in cell culture flasks were fixed for 10 min in 1% glutaraldehyde 4% formaldehyde mixture in 0.1 M phosphate buffer, then scraped off, and pelleted, followed by further fixation for 1 h. Then, the cell pellets were immersed in 2% agarose in distilled water, postfixed in 1% osmiumtetroxide, dehydrated in acetone, and embedded in Epon LX 112 (Ladd Research Industries). Thin sections were cut with Leica Ultracut UCT ultramicrotome, stained in uranyl acetate and lead citrate, and examined in a Philips CM100 transmission electron microscope. Image analysis was performed with MCID Core 7.0 software (InterFocus Imaging LTD).
Determination of mitochondrial superoxide levels
Mitochondrial superoxide levels were determined by MitoSOX (Molecular Probes, Invitrogen) and analyzed by flow cytometry. Three different hMSC lines from each group and 14-day osteogenic differentiated cells were included in the analysis. In addition, 2 lines from each group were treated with rotenone (Sigma-Aldrich) for 5 min before MitoSOX staining. Detached cells were suspended in PBS, incubated with 5 μM MitoSOX for 10 min at room temperature, and washed with PBS. Flow cytometry analysis was performed with FACSCalibur and equipped with lazers emitting at 488 and 633 nm. MitoSOX fluorescence was excited at 488 nm, emission was measured at 585±42 nm, and data were analyzed with FlowJo. MitoSOX positive cells were determined by a negative control. Cell debris was gated out from data analysis.
Western blot analysis
Three lines from each hMSC types were taken to the MnSOD analysis by western blot. In addition, 14-day osteoinduced cells were analyzed. Samples were suspended into lysis buffer consisting of 50 mM Tris, 0.9% NaCl, 0.2% NaN3, 0.1% Triton X-100, 0.1% deoxycholic acid, and protease inhibitor; and protein concentrations were determined by Bio-Rad Protein Assay (Bio-Rad Laboratories). Protein samples (10 μg of protein) were separated on 12% SDS-PAGE and blotted on nitrocellulose membrane. Unspecific binding of proteins was blocked by 5% milk powder in PBS +0.1% Tween20 (PBST; Sigma-Aldrich). The membrane was incubated with MnSOD (anti-rabbit, 1:100,000; Abcam) and β-actin (anti-mouse, 1:7,500; Sigma-Aldrich) primary antibodies in PBST over night at 4°C in a shaker. After secondary antibody (anti-rabbit IgG IR800 conjugated 1:1,000 and anti-mouse IgG IR700 conjugated 1:1,000) incubation for 1 h, the MnSOD and β-actin signals were measured by Odyssey Infrared Imager System (Li-Cor Biosciences). To exclude variations in amounts of loaded protein, MnSOD signals were normalized against β-actin.
Measurement of oxygen consumption
Oxygen consumption rate was measured from hMSCs and 14-day osteoinduced cells. Detached cells were suspended in medium containing 0.25 M sucrose, 20 mM MOPS pH 7.4, and 0.10 mg/mL digitonin (Sigma-Aldrich). Cells were incubated for 8 min at room temperature to permeabilize plasma membrane and outer mitochondrial membrane. Digitonin was washed away, and cells were suspended (550,000 cells/100 μL) in an oxygraph medium containing 75 mM mannitol, 25 mM sucrose, 100 mM KCl, 10 mM KH2PO4, 5 mM MgCl2, and 20 mM Tris-HCl pH 7.0. Samples were prewarmed in a 25°C water bath; and then, the whole suspension was transferred into a Mitocell (Strathkelvin Instruments) chamber thermostated at 25°C, and oxygen consumption rate was measured after addition of 5 mM glutamate+2.5 mM malate (complex I-mediated state 2 respiration)+2.5 μM ADP (state 3 respiration) and after the addition of 5 μM rotenone (complex I inhibitor)+10 mM succinate (complex II-mediated state 4 respiration)+2.5 μM ADP (State 3 respiration). The oxygen consumption rates after substrate and ADP additions were recorded and calculated with the 949 Oxygen System software (Strathkelvin Instruments). The initial oxygen concentration of the incubation mixture was taken as a 258 μmol/L, and zero oxygen calibration was made by addition of sodium dithionite.
Flow cytometric detection of mitochondrial inner membrane potential
Mitochondrial inner membrane potential (ΔΨm) specific dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcar-bocyanine iodide (JC-1; Sigma-Aldrich) was used as earlier described [30]. Cells were suspended in PBS and incubated with 1 μM JC-1 for 20 min at room temperature. Cells were washed with PBS and suspended in a concentration of 100,000 cells/mL. JC-1 signal was detected from FL1 and FL2 channels by flow cytometry (FACSCalibur), and data were analyzed with FlowJo. Fluorescence was excited at 488 nm, and the ratio of emissions at 585±42 and 530±30 nm were measured from 3 different UCBMSC and BMMSC lines without and with osteoinduction. As a zero-potential control, the protonophore carbonyl cyanide m-chlorophenylhydrazone was used to depolarize ΔΨm, which was seen as a clear decrease in 585 nm/530 nm emission ratio and also in contour plot as an accumulation of cells into JC-1 monomer region, which fully indicates depolarized cells.
Statistical analysis
Statistical analysis and all diagrams were performed by using the OriginPro version 8. All data are presented as mean±standard deviation (SD) of the results from 3 different hMSC lines unless otherwise indicated. The normality of the data was tested by using Kolmogorov-Smirnov–test where P values of P>0.05 were considered as normally distributed series. The significance level of normally distributed data was determined by 2-sample t-test and unequally distributed data by nonparametric Mann–Whitney test where P value P<0.05 was considered statistically significant.
Results
Morphologically UCBMSC and BMMSC >50 differed dramatically, whereas BMMSC <18 showed similarity with UCBMSC
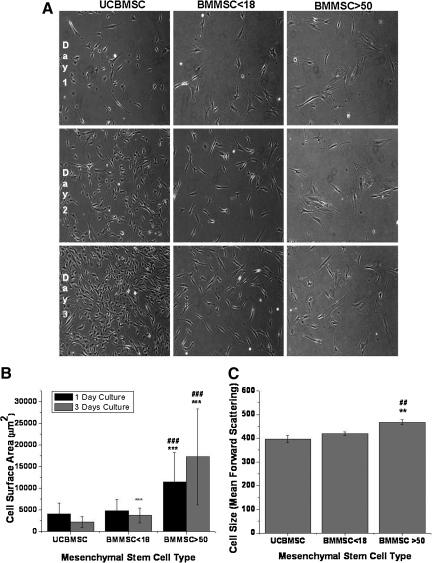
Morphologically, UCBMSCs differed from BMMSC >50, whereas BMMSC <18 were more similar to UCBMSCs (Fig. 1A). Despite the lack of detailed analysis, the proliferation rate of UCBMSCs seems to be much higher compared with BMMSC >50 (Fig. 1A). The mean cell area of 60 attached cells of each hMSC group was measured after 1 day and 3 days in culture (Fig. 1B). After 1 day culture, the cell area (mean±SD) was 4,048±2,567 μm2 in UCBMSC and 4,842±2,590 μm2 in BMMSC <18; whereas BMMSC >50 showed a dramatically greater cell surface area of 11,456±6,753 μm2, compared with UCBMSC and BMMSC <18 (P<0.001). After 3 days in culture, the cell surface area was 2,193±1,268 μm2 in UCBMSC and 3,723±1,693 μm2 in BMMSC <18; whereas in BMMSC >50, the cell surface area during 3 days in culture increased to 17,248±11,088 μm2, a value 8 times higher than that of UCBMSC and BMMSC <18 (P<0.001).
FIG. 1.
Morphological analysis of umbilical cord blood- and bone marrow-derived hMSCs. Representative images after one or 3 days in vitro culture of cells from one line of each hMSCs type. Cells were seeded at the density of 2,500 cells/cm2 in passages 3, 5 and 5 for BMMSC >50, BMMSC <18 and UCBMSC respectively. (Magnification×100) (A). Cell surface area of hMSC types after 1-day and 3-day culture. Data are mean±SD of 60 cells. ###P<0.001 of differences between BMMSC >50 and BMMSC <18 in Mann–Whitney test and P value of ***P<0.001 when compared with UCBMSC with Mann-Whitney test (B). Cell size in suspension determined by forward scattering intensity by flow cytometry (C). Results are mean±SD of 3 different cell lines. ##P<0.01 for differences between BMMSC >50 and BMMSC <18 in 2-sample t-test. **P<0.01 when compared with UCBMSC with 2-sample t-test. hMSC, human mesenchymal stem cell; UCBMSC, umbilical cord blood-derived mesenchymal stem cells; BMMSC, bone marrow-derived mesenchymal stem cells; SD, standard deviation.
The cell size was also analyzed from detached cells as forward scattering (FSC) intensity by flow cytometry. UCBMSC had a mean FSC intensity of 397±15, BMMSC <18 showed a mean FSC intensity of 420±8, and BMMSC >50 had a mean FSC of 468±10 (Fig. 1C). Although the UCBMSC and BMMSC <18 cell showed smaller cell size in suspension than BMMSC >50 (P<0.01), the difference was not as great as in attached cells. We also analyzed 3 BMMSC >50 lines from the iliac crest of patients with myocardial infarction without a known history of osteoarthritis. The origin of the cells derived from this alternative group was found to have no effect on their phenotypic characteristics, that is, cell surface antigens, osteogenic differentiation, and mitochondrial function, as compared with the BMMSCs >50 group (data not shown).
UCBMSC and BMMSC lines have different mitochondrial-to-cytoplasm area ratio and abundance of the rough endoplasmic reticulum
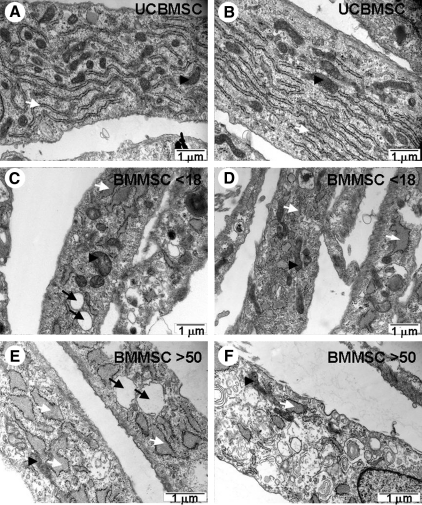
From each hMSC type, one cell line was selected for TEM analysis. Closer observation of mitochondria and rough endoplasmic reticulum (rER) revealed differences between UCBMSCs and BMMSCs as well as between BMMSC <18 and BMMSC >50 (Fig. 2A–F). UCBMSCs showed abundant stacks of rER, whereas BMMSC <18 showed fewer but more dilated rER. The most striking difference between UCBMSC and BMMSC <18 was the presence of clear vacuoles in BMMSC <18, which were almost completely missing in UCBMSC. BMMSC >50 showed more dilated rER than BMMSC <18, and also clear vacuoles were common. In all, hMSC types mitochondria were distributed evenly in the cytoplasm.
FIG. 2.
Transmission electron micrographs indicating differences between hMSC types in abundance of rER and mitochondrial-to-cytoplasm area ratio. Representative images of UCBMSC showing abundant rER stacks and mitochondria (A and B). Representative images of BMMSC <18 showing fewer rER with more dilated structure and presence of several mitochondria. Also, clear vacuoles were seen (C and D). Ultrastructure of BMMSC >50 showing dilated rER and presence of clear vacuoles but lower abundance of mitochondria compared with UCBMSC and BMMSC <18 (E and F). White arrow indicates rER, black arrowhead indicates mitochondria, and black arrow indicates vacuoles. rER, rough endoplasmic reticulum.
Mitochondrial area from 20 individual cells was normalized against total cytoplasmic area of each cell. UCBMSC showed mitochondrial-to-cytoplasm area ratio of 6.3%±2.6%, BMMSC <18 showed mitochondrial-to-cytoplasm area ratio of 5%±2.3%, whereas BMMSC >50 had mitochondrial-to-cytoplasm area ratio of 2.2%±1%, which was dramatically lower than UCBMSC and BMMSC <18 (P<0.001) (Fig. 3M). Mitochondrial morphology did not differ dramatically between hMSC types. Inner and outer mitochondrial membranes were visible, but clearly defined cristae were quite rare.
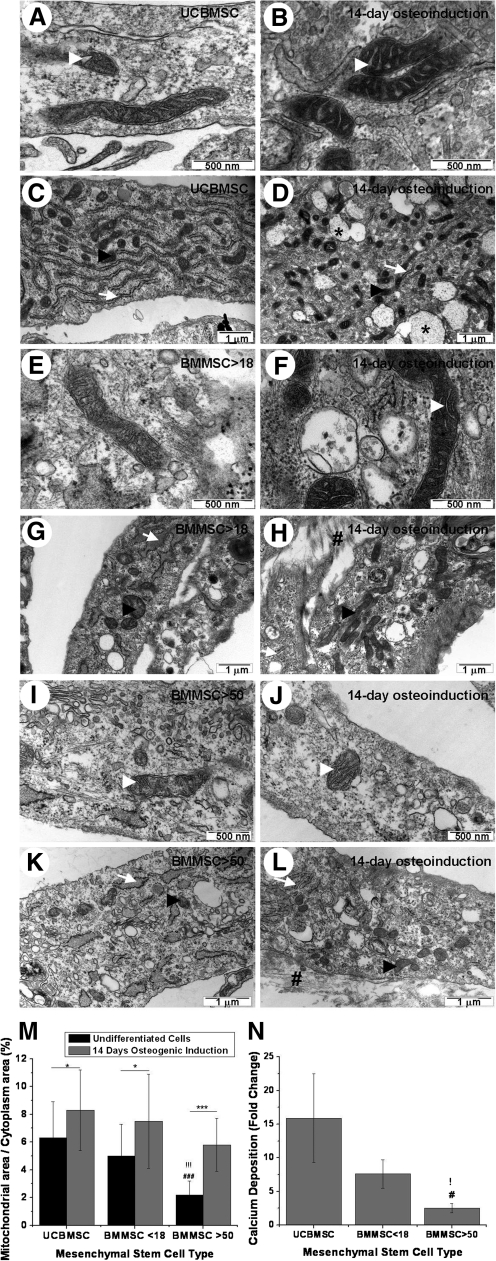
FIG. 3.
Effect of osteogenic differentiation on ultrastructure and quantity of mitochondria. Electron micrographs of undifferentiated UCBMSC, BMMSC <18, and BMMSC >50 respectively (A, E, and I, respectively) and after osteoinduction (B, F, and J). In UCBMSC and BMMSC <18, well-defined cristae were seen after 14-day osteoinduction. Also, density of mitochondria increased. Changes in mitochondrial structure were not as clear as in BMMSC >50. Representative images of ultrastructure in undifferentiated UCBMSC, BMMSC <18, and BMMSC >50 (C, G, and K) and after osteoinduction (magnification×24,500) (D, H, and L). Mitochondrial-to-cytoplasm area ratio increased in all hMSCs types after 14-day osteoinduction. Vesicles with electron-dense material were evident in UCBMSC, and secretion of extracellular components was evident in all hMSC types. The abundance of rER seemed to decrease after differentiation. Mitochondrial-to-cytoplasm area ratio was determined. Results were represented as mean±SD of 20 cells. * and !!!/###/*** indicates Mann–Whitney test P values of P<0.05, and P<0.001. ! indicates differences when compared with UCBMSC and # indicates differences between BMMSC <18 and BMMSC >50. * indicates differences between undifferentiated and osteoinduced cells (M). Osteogenic differentiation efficiency was measured by calcium content. Results were represented as mean±SD from 3 different hMSCs lines (N). !/# represent 2-sample t-test P values of P<0.05. White arrowhead indicates mitochondrial cristae, black arrowhead indicates mitochondria, white arrow indicates rER, black asterisk indicates vesicles with electron-dense material, and black number sign indicates secreted extracellular components.
Osteogenic differentiation is accompanied by calcium accumulation and increase in mitochondrial volume and maturation
After 14 days differentiation, UCBMSC showed clear vesicles that contained electron dense material, possibly ECM proteins and glycoproteins that were also present in extracellular space. Also, BMMSC <18 showed secretion of ECM proteins, which is a clear indication of osteogenic differentiation (Fig. 3A–H). BMMSC >50 cells did not show as evident production of ECM proteins as in UCBMSC and BMMSC <18 (Fig. 3I–L). The abundance of rER seemed to decrease after the 14-day osteoinduction. Cristae in mitochondria were well defined after 14-day osteogenic induction in UCBMSC and BMMSC <18. Clear cristae were not evident in BMMSC >50. However, the mitochondrial-to-cytoplasm area ratio increased during differentiation in all hMSC types (Fig. 3M). In UCBMSC, the increase was from 6.3%±2.6% to 8.3%±2.9% (P<0.05). In BMMSC <18 from 5%±2.3% to 7.5%±3.4% (P<0.05) and in BMMSC >50, the mitochondrial-to-cytoplasm area ratio increased from 2.2%±1% to 5.8%±1.9% (P<0.001).
To determine the differentiation potential of UCBMSC, BMMSC <18, and BMMSC >50, calcium content was determined after 5 weeks differentiation (Fig. 3N). As expected, UCBMSC showed the highest calcium content with 15.9±6.6-fold change, when compared with undifferentiated cells. BMMSCs <18 showed calcium content with fold change of 7.6±2.1, whereas the 2.5±0.6 (P<0.05) fold calcium content in BMMSC >50 was lower (P<0.05) than in UCBMSC and BMMSC <18.
hMSCs express a typical ECM scaffold on their surface
To study the nature of the extracellular scaffold secreted by these cells, we used a classical cell surface proteomics approach [37]. The selected approach offers clear benefits in comparison to analyzing the total secretome produced by these cells, where the suppression effect of the bulky cell culture media proteins might interfere with the analyses. In addition, for this analysis, undifferentiated cells were selected to obtain comprehensive analysis of the actively secreted extracellular proteome only. After differentiation, the abundant ECM prevented the optimal labeling and further prevented the analysis of secreted proteins. Both the 1-dimensional gel-based approach and direct LC-MS protein profiling were performed. More than 200 proteins were identified per analysis, and an average of 54% of proteins with known subcellular locations were identified as integral plasma membrane proteins (data not shown). In fact, 17 different secreted proteins were identified by using these analyses (Table 1), of which a majority were ECM proteins. Since proteoglycans are also closely associated with the formation of ECM, the 5 identified proteoglycan core proteins are also listed in Table 1. A difference between gel-based and direct protein profiling methods can be seen in these analyses, but also some clear differences can be seen between 2 MSC types derived from different origins. For example, BMMSCs showed different and more numerous collagen subtypes (Table 1).
Table 1.
Surface-Associated Proteins Identified from Mesenchymal Stem Cells Derived from Either Bone Marrow or Umbilical Cord Blood Using Both Gel-Based Protein Identification Workflow as Well as Liquid Chromatography Mass Spectrometry-Based Direct Protein Profiling
| |
|
BMMSC |
UCBMSC |
||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
Gel-based digest |
Direct digest |
Gel-based digest |
Direct digest |
||||
| Protein name | UniProt id | M Score | peptide | M Score | peptide | M Score | peptide | M Score | Peptide |
| ECM and secreted proteins | |||||||||
| Galectin-1 | LEG1_HUMAN | 1,739 | 41 | 1,154 | 33 | ||||
| Tenascin | Q4LE33_HUMAN | 1,298 | 53 | ||||||
| Fibulin-1 | FBLN1_HUMAN | 99 | 20 | 85 | 4 | 1,013 | 28 | ||
| Plasminogen activator inhibitor 1 | PAI1_HUMAN | 116 | 6 | 761 | 102 | ||||
| Collagen alpha-1(XII) chain | COCA1_HUMAN | 348 | 64 | ||||||
| Collagen alpha-3(VI) chain | CO6A3_HUMAN | 279 | 55 | 96 | 21 | ||||
| EMILIN-1 | EMIL1_HUMAN | 217 | 7 | 198 | 17 | ||||
| Fibronectin | FINC_HUMAN | 48 | 4 | 198 | 18 | ||||
| Gelsolin | GELS_HUMAN | 73 | 7 | 172 | 18 | 36 | 5 | ||
| Extracellular glycoprotein lacritin | LACRT_HUMAN | 131 | 2 | ||||||
| Vitronectin | VTNC_HUMAN | 77 | 18 | 125 | 10 | ||||
| Collagen alpha-1(I) chain | CO1A1_HUMAN | 111 | 16 | 36 | 6 | 78 | 8 | ||
| Lipocalin-1 | LCN1_HUMAN | 97 | 6 | ||||||
| Neutrophil defensin 2 | DEF1_HUMAN | 87 | 5 | ||||||
| Collagen alpha-1(VI) chain | CO6A1_HUMAN | 69 | 16 | ||||||
| Collagen alpha-2(VI) chain | CO6A2_HUMAN | 65 | 13 | ||||||
| Ceruloplasmin | CERU_HUMAN | 41 | 2 | ||||||
| Proteoglycans | |||||||||
| Chondroitin sulfate proteoglycan 4 | CSPG4_HUMAN | 452 | 27 | 2,675 | 203 | 1,860 | 103 | ||
| Versican core protein | CSPG2_HUMAN | 254 | 16 | 227 | 13 | ||||
| Glypican-1 | GPC1_HUMAN | 209 | 14 | 89 | 3 | ||||
| Syndecan-2 | SDC2_HUMAN | 89 | 5 | ||||||
| Hyaluronan and proteoglycan link protein 1 | HPLN1_HUMAN | 68 | 2 | ||||||
Secreted proteins and proteoglycan core proteins are listed with a recommended protein name and an accession id to UniProt Database. Protein identifications are listed according to descending Mascot (M) score, and peptide number in the experimental identifications is also reported.
UCBMSC, umbilical cord blood-derived mesenchymal stem cells; BMMSC, bone marrow-derived mesenchymal stem cell; ECM, extracellular matrix.
Mitochondrial superoxide and MnSOD levels are constitutively elevated in UCBMSCs
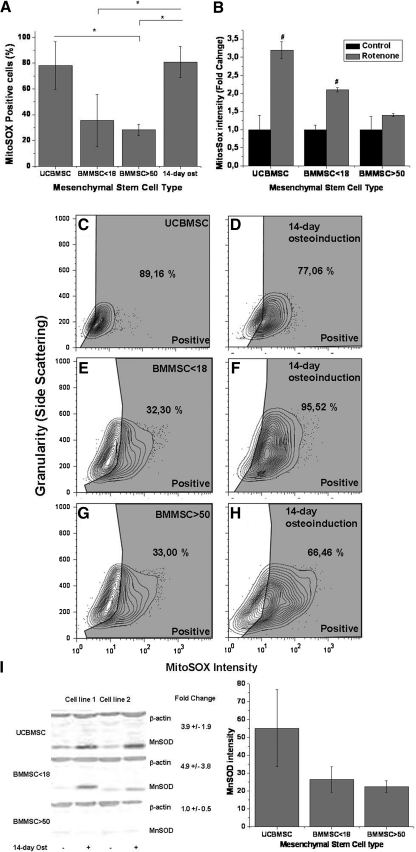
Mitochondrial superoxide levels and the amount of ROS-scavenging enzyme MnSOD were determined from undifferentiated hMSCs and 14-day osteoinduced hMSCs. The number of MitoSOX positive cells in BMMSC >50 and BMMSC <18 was 28.2%±4.4% and 35.4%±20.4%, respectively, that is, less than in UCBMSC with 78.2%±18.4% of MitoSOX positive cells (P<0.05 compared with BMMSC >50) (Fig. 4A, C, E, G). After 14-day osteoinduction, the number of MitoSOX positive cells increased in BMMSC <18 and BMMSC >50 with a fold-changes of 2.3 and 2.8, respectively (P<0.05 and P<0.05); whereas no significant increase was observed with UCBMSC with a fold change of 1.0 (Fig. 4A, C–H). Mitochondrial superoxide production increased after treatment with the complex I inhibitor rotenone. The increase was highest in UCBMSC with 3.2±0.23-fold change (P<0.05); also, BMMSC <18 showed a higher increase than BMMSC >50 with fold changes of 2.1±0.06 (P<0.05) and 1.4±0.04, respectively (Fig. 4B).
FIG. 4.
Quantification of mitochondrial superoxide and MnSOD, in UCBMSC, BMMSC <18 and BMMSC >50. Percentage of MitoSOX positive cells. Results are mean±SD of 3 different hMSC lines (A). Measurement of MitoSOX intensity after rotenone treatment. Results are represented as mean±SD of 2 different hMSCs lines (B). Representative flow cytometry contour plots of MitoSOX stainings in undifferentiated UCBMSC, BMMSC <18, and BMMSC >50 (C, E, and G) and after 14 days of osteoinduction (D, F, and H). Representative western blot of MnSOD levels from 2 different hMSC lines and comparison of MnSOD intensities from 3 different hMSC lines. Fold changes are represented as mean±SD of 3 hMSC line (I). */# represent 2-sample t-test P values P<0.05. # indicates differences between control and rotenone treatment. MitoSOX positive gate was determined by negative controls individually. Numbers inside gates indicate percent of cells positive for MitoSOX. 14-day ost refers to 14-day osteoinduction. MnSOD, manganese superoxide dismutase.
The MnSOD levels were analyzed from 3 different hMSC lines from each group. BMMSC >50 and BMMSC <18 showed lower MnSOD western blot intensities with fold changes of 0.37±0.07 and 0.36±0.08, respectively when compared with UCBMSC (Fig. 4I). UCBMSC and BMMSC <18 showed a higher increase in MnSOD concentration after 14-day osteogenic induction with fold changes of 3.9±1.9 and 4.9±3.8, respectively; whereas BMMSC >50 showed no increase in the MnSOD amount, fold-change 1.0±0.5.
Oxygen consumption increases during osteogenic differentiation in all hMSC types
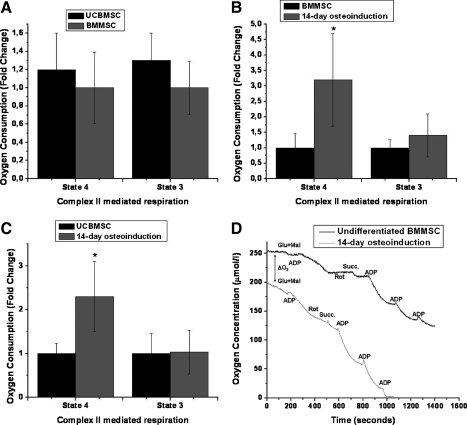
The mitochondrial activity was further studied by measuring complex I (+III+IV)—or complex II (+III+IV) mediated state 2 or 4 (addition of glutamate+malate or succinate) and state 3 (addition of ADP) oxygen consumption of hMSCs and osteoinduced cells. In complex I-mediated oxygen consumption, there was a great variability between measurements within a given hMSC line. Therefore, we decided to compare only complex II-mediated oxygen consumption, which showed more reproducible results. There were no differences in oxygen consumption rate between BMMSC <18 and BMMSC >50, but UCBMSCs showed slightly higher complex II-mediated state 4 and state 3 oxygen consumptions than BMMSCs with fold changes of 1.2±0.4 and 1.3±0.3, respectively, although the difference was not statistically significant (Fig. 5A). However, the succinate-dehydrogenase-mediated state 4 oxygen consumption rate was significantly higher after 14-day osteoinduction when compared with undifferentiated UCBMSC and BMMSC, with fold changes of 2.3±0.7 and 3.2±1.5, respectively (P<0.05). Complex II-mediated state 3 oxygen consumption showed clear increment in oxygen consumption rate, but there were no significant differences between UCBMSC or BMMSC when compared with 14-day osteoinduced cells with fold changes of 1.0±0.5 and 1.5±0.7, respectively (Fig. 5B, C). Osteoinduced cells showed more continuous and higher oxygen consumption rate, whereas undifferentiated hMSCs showed short consumption bursts after addition of substrate or ADP (Fig. 5D).
FIG. 5.
Oxygen consumption after osteoinduction of UCBMSC and BMMSC. Complex II-mediated state 4 (Succinate) and state 3 (Succinate and ADP) oxygen consumption rates of UCBMSC and BMMSC. Results are represented as a mean±SD of 3 different lines (A). The state-4-mediated oxygen consumption rate was significantly higher in 14-day osteoinduction than in BMMSC <18, whereas no significant differences between BMMSC <18 and 14-day osteoinduced cells were seen in state 3 (B). 14-day osteoinduction also showed a significant increase in state 4 but not in state 3 oxygen consumptions when compared with UCBMSC (C). Results are represented as mean of 4 parallel measurements±SD (D). Oxygen consumption rate in undifferentiated cells decreased to zero after a short period of time without inhibitors; whereas in osteoinduced cells, the oxygen consumption was more continuous. *P<0.05 in Mann–Whitney test between control and osteoinduced cells. Oxygen consumption measurements were performed with 550,000 cells in all experiments. Glu+Mal, glutamate and malate; ADP, adenosine diphosphate; Rot, rotenone; Succ, succinate, ΔO2; difference in initial oxygen concentration between cell suspensions.
Mitochondrial energy state differs in osteoinduced cells compared with hMSCs
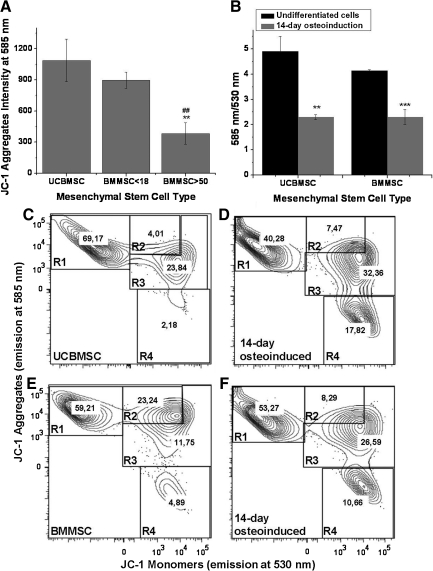
Mitochondrial energy state of hMSCs before and after 14-day osteoinduction was evaluated as ΔΨm. It was interesting to notice that the JC-1 aggregate intensities of UCBMSC and BMMSC <18 were significantly higher (P<0.01) than BMMSC >50 with intensities of 1,086±205 and 897±77, respectively; whereas BMMSC >50 had an intensity of only 384±103 (Fig. 6A). Since there were no systematic differences in 585 nm/530 nm emission ratio of JC-1 between UCBMSC, BMMSC <18, and BMMSC >50, we decided to compare undifferentiated UCBMSC and BMMSC cells with 14-day osteoinduced ones. After 14-day osteoinduction, the 585 nm/530 nm ratio decreased significantly from 4.9±0.6 to 2.3±0.2 in UCBMSC (P<0.05) and 4.1±0.03 to 2.3±0.3 in BMMSC <18 (P<0.01) (Fig. 6B). Flow cytometric analysis before and after osteoinduction demonstrates that after osteogenic differentiation, the ΔΨm is decreased in a proportion of cells, which is seen as an increment in the number of cells belonging to group R4 (Fig. 6C–F).
FIG. 6.
Mitochondrial membrane potential after short-term osteoinduction. Intensity of JC-1 aggregates (emission at 585 nm) measured by flow cytometry represented as mean±SD of 3 different hMSC lines (A). 585 nm/530 nm of JC-1 analysis represented as mean±SD of 3 parallel experiments from undifferentiated and 14-day osteoinduced cells (B). Representative flow cytometry contour plots of undifferentiated UCBMSC and BMMSC (C and E) and after 14 days of osteoinduction (D and F). Samples were stained with JC-1 and analyzed by flow cytometry. R1 means viable cells; R2 and R3, early apoptotic cells; and R4, late apoptotic cells. Numbers indicates percent of cells belonging to indicated group. **/## and *** indicates 2-sample t-test P values P<0.01 and P<0.001. In (A), * indicates differences when compared with UCBMSC and in (B), differences between undifferentiated and osteoinduced cells. # indicates differences between BMMSC <18 and BMMSC >50.
hMSCs with similar surface antigen phenotypes have differences in mitochondrial function and structure
Flow cytometric surface antigen characterization of 3 different hMSC lines from each group showed typical cell surface expression pattern for hMSCs, where cells were over 95% positive for CD73, CD90, CD105, CD44, CD49e, and HLA-ABC and under 2% positive for CD19, CD45, CD34, CD14, and HLA-DR, except for one UCBMSC line that did not satisfy the criteria. Thus, of the UCBMSCs, 78.16% of the cells were positive for CD90; and an average of 6.82% were positive for CD19, CD45, CD34, CD14, and HLA-DR. However, hMSC types showed dramatic differences in mitochondrial, morphological, and cellular properties where UCBMSCs showed already activated mitochondrial function that was more similar to osteoblasts than to BMMSC >50 (Table 2).
Table 2.
Mitochondrial and Cellular Properties Compared with Expression of Characteristics Cell Surface Antigens of Human Mesenchymal Stem Cells
| Mitochondrial and cellular properties | UCBMSC | BMMSC <18a | BMMSC >50b | 14-day osteoinduction |
|---|---|---|---|---|
| Proliferation rate | +++++ | ++++ | ++ | – |
| Mitochondrial area | ++++ | +++ | + | +++++ |
| Mitochondrial superoxide levels | +++++ | ++ | ++ | +++++ |
| Manganese superoxide dismutase levels | +++ | + | + | +++++ |
| Oxygen consumption | ++ | ++ | ++ | +++++ |
| Cell surface antigen expression | ||||
| CD90 | 78,16 (30,7) | 99,69 (0,2) | 99,77 (0,1) | – |
| CD105 | 99,52 (0,6) | 99,72 (0,4) | 99,69 (0,2) | – |
| CD73 | 99,89 (0,1) | 99,78 (0,3) | 99,42 (0,4) | – |
| CD44 | 99,70 (0,4) | 99,66 (0,2) | 99,40 (0,3) | – |
| CD49e | 99,79 (0,4) | 99,68 (0,2) | 98,95 (0,5) | – |
| HLA-ABC | 95,11 (3,3) | 99,60 (0,3) | 98,41 (0,8) | – |
| HLA-DR,CD19,CD45,CD34,CD14 | 6,82 (1,5) | 1,97 (0,1) | 0,87 (0,5) | – |
Cell surface antigen expressions are represented as mean±standard deviation from 3 different hMSC lines. Cells were stained with particular antigens and analyzed by flow cytometry. Positivity for antigens was defined by negative and isotype controls. +++++ indicates the highest value for cellular and mitochondrial properties. −indicates lack of analysis.
BMMSC <18, bone marrow mesenchymal stem cells from donors under 18 years old.
BMMSC >50, bone marrow mesenchymal stem cells from donors over 50 years old.
hMSC, human mesenchymal stem cell.
Discussion
Previous comparative studies on proliferation rate, gene expression profile, differentiation potential, and efficiency of UCBMSC, fetal BMMSC, adult BMMSC, and adipose-derived hMSCs have produced conflicting results, although all hMSC types have been demonstrated to show systematically similar cell surface antigen expression [16–21]. In this study, we focused on comparison of UCBMSCs and BMMSCs from different age groups. It is our hypothesis that the different developmental stage of these cells may reflect different initial energy metabolism levels, which could be an independent parameter in explaining the behavior of hMSC under experimental conditions.
Interestingly, morphological and ultrastructural analyses revealed clear age-dependent differences between the UCBMSCs and BMMSCs. The dramatically smaller cell surface area and higher mitochondrial-to-cytoplasm area ratio in UCBMSC and BMMSC <18 than in BMMSC >50 could indicate that these groups represent different hMSC types. In addition, UCBMSCs showed more abundant rER than BMMSCs. In previous studies, no differences have been found in mitochondrial number or structure and, to our knowledge, this is the first time that the difference in mitochondrial-to-cytoplasm area ratio has been demonstrated between these hMSC types [42]. Since the mitochondrial-to-cytoplasm area ratio also increased after 14-days osteoinduction in all hMSC types and the increase was most striking in BMMSC >50, we believe that the higher mitochondrial-to-cytoplasm area ratio in UCBMSC and BMMSC <18 compared with BMMSC >50 could indicate different overall maturation levels of these cells.
Chen et al. [29], however, have not noticed dramatic changes in mitochondrial mass during differentiation measured by nonyl acridine orange, a finding that is at variance with our results, as mitochondrial mass should increase as mitochondrial-to-cytoplasm area ratio increases. On the other hand, Chen et al. [29] used BMMSC that were expanded in presence of growth factors (fibroblast growth factor and EGF), which may drive the cells toward a more mature progenitor state with already increased mitochondrial mass. Our results suggest that the increase in mitochondrial-to-cytoplasm area ratio is one of the first observed indicators in osteogenic induction which is in line with recent work on murine cells [43]. The increase in mitochondrial-to-cytoplasm area ratio seems not to be due to mitochondrial swelling, as the area of individual mitochondria seems to stay constant. Therefore, we believe that the increase in mitochondrial-to-cytoplasm area ratio indicates an increase in abundance of mitochondria.
In addition to intracellular changes, the TEM analysis also revealed that short-term osteogenic differentiation increased secretion of electron-dense material into extracellular space. hMSC proteomics during bone differentiation has been characterized earlier in many studies, including our own previous work [35,44–46]. Here, the aim was to specifically focus on precise characterization of the exocytosed proteins that are peripherally attached to cell surface as well as glycoproteoglycansins and to compare the plasma membrane attached to BMMSC and UCBMSC secretomes, which has not been previously done. Interestingly, more tenascin-C was attached to and more numerous collagen subtypes were detected in the BMMSC than UCBMSC; whereas UCBMSCs expressed more fibulin-1 when compared with BMMSCs. Also, many other proteins were secreted differently, which might indicate different regenerative and immunomodulatory capacities of these cells. The precise role of each individual molecule remains to be elucidated further, but we believe that it is important to recognize that the origin or culture conditions also inflict a significant change in the secretome in cells, which according to standard criteria would appear to be similar.
During osteogenic differentiation, multiple changes have been shown to occur in antioxidant enzymes and in ROS production. The data also suggest that ROS could have a regulatory function during the differentiation process itself [29,32,33]. Observations in the present study showed that the UCBMSC had a higher number of MitoSOX positive cells and they expressed MnSOD constitutively at higher levels than BMMSCs. This supports the hypothesis that UCBMSCs, exposed to more numerous growth factors and, hence, differentiation signaling, may represent a more mature population of hMSCs. In addition, the amount of MnSOD clearly increased after 14 days of osteogenic induction in UCBMSC and BMMSC <18, whereas BMMSC >50 did not show any increase. This observation can be explained by a slower differentiation process due to the more native form of BMMSC >50. The explanation is supported by the finding that the initial mitochondrial-to-cytoplasm area ratio was lowest in BMMSC >50, and the proportional increment was highest in these cells after the 14 days of osteoinduction. It has been shown earlier that ROS production can decrease in spite of an increase in oxidative metabolism [29]. The present observations indicate increased superoxide production in BMMSC <18 and BMMSC >50. Again, since these cells were grown without growth factors, they may not be comparable to cells used in previous works [29]. This is supported by the finding of a slightly decreased or constant amount of MitoSOX positive cells with simultaneous increase in MnSOD amounts in UCBMCS after 14-day osteoinduction.
Measurements of succinate dehydrogenase-mediated mitochondrial respiration revealed no significant differences between BMMSC and UCBMSC. However, the state-4 oxygen consumption increased significantly in 14-day osteoinduced cells when compared with undifferentiated hMSCs, which is a clear sign of mitochondrial activation and a shift from anaerobic to aerobic metabolism and in good accordance with the work of others [29]. Interestingly, the intensity of JC-1 aggregates was higher in UCBMSC and BMMSC <18 than in BMMSC >50, which could be due to higher mitochondrial number. Whether the decrease in ΔΨm after 14 days of osteogenic differentiation may be an implication of increased mitochondrial biogenesis and oxidative metabolism remains to be elucidated. The mitochondrial superoxide production should decrease in parallel with ΔΨm [47], which was not evident in all cases in the present study. In addition, previous works done with murine MSCs have showed increased ROS-production and ΔΨm after 72 h osteoinduction [43]. In the present study, we analyzed ΔΨm after 14-day osteoinduction and also demonstrated increased calcium concentration, which may explain the findings [48,49].
As just shown, we observed several mitochondrial and cellular differences between hMSC types, which we believe are indicators of different maturation levels. To demonstrate that these differences have relevance in their function, we also showed that both UCBMSC and BMMSC <18 had higher osteogenic differentiation potential than BMMSC >50. The age-dependent decline in potency of BMMSC has been shown earlier [7–9]. In these earlier studies, the osteogenic differentiation potency of UCBMSCs was not significantly higher compared with that of BMMSCs [16–18].
The surface phenotype analysis showed that all hMSCs lines, except one UCBMSC line, fulfilled the characteristics cell surface antigens assembled by The International Society of Cellular Therapy for hMSCs, which was extended in our study [50]. Despite similar cell surface phenotype of BMMSC <18, BMMSC >50, and UCBMSC, there seem to be dramatic differences in mitochondrial and cellular properties that are not linked to the selected cell surface profile. These results clearly point out the limitations of the cell surface analysis to fully characterize the biology of hMSCs. Higher proliferation rate, higher mitochondrial-to-cytoplasm area ratio, elevated MnSOD, and superoxide levels, higher osteogenic differentiation efficiency seen in UCBMSC compared with BMMSC >50 indicate different maturation levels of hMSCs. These observations are critical when hMSCs are considered for cellular therapies, as the differences between hMSC types inevitably lead to different functional properties, which may influence, at least, the treatment of bone defects and also other cell-based therapies.
The obvious limitations in this comparative study are naturally the need to use different culture conditions for the UCBMSC when compared with BMMSCs. The underlying disease of the BMMSC donors may also have a role, whereas our preliminary data would suggest differently. BMMSCs of patients who do not have osteoarthritis showed similar characteristics to those of our standard sample of cells originating from patients with osteoarthritis (data not shown). At the age >50, osteoarthritis is very common, and we are not able to exclude the possibility that even disease-free individuals could have represented such a phenotype [51]. This depicts well the current situation, where such optimization and characterization for UCBMSC culture is still needed. Future studies should include characterization of more cell lines from each hMSC group to fully confirm the differences seen in the present study. Also, more functional testing, such as immunomodulatory and in vivo tissue regenerative capacity, should be linked to these changes in energy metabolism.
In conclusion, the present study shows that mitochondrial analysis is a valuable method for more detailed characterization of hMSCs to determine their differentiation potential and also to distinguish different maturation levels of hMSCs.
Acknowledgments
The authors want to thank laboratory technologist Minna Savilampi and Biocenter Oulu EM core technicians for their major contribution in this work. This study is funded by the Academy of Finland, grant no. 122561, and the Finnish Medical Association. We are grateful to the Finnish Red Cross, Blood Service, for the UCBMSC samples.
Author Disclosure Statement
This work does not include conflicts of interest of any kind.
References
- 1.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM. Gordon PL. Koo WK. Marx JC. Neel MD. McNall RY. Muul L. Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal stem cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitoh H. Kitakoji T. Tsuchiya H. Mitsuyama H. Nakamura H. Katoh M. Ishiguro N. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis-a preliminary result of three cases. Bone. 2004;35:892–898. doi: 10.1016/j.bone.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K. Rasmusson I. Sundberg B. Götherström C. Hassan M. Uzunel M. Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 5.Romanov YA. Svintsitskaya YA. Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord blood. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2003;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolzing A. Jones E. McGonagle D. Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Stenderup K. Justesen J. Clausen C. Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S. Greenberger JS. Epperly MW. Goff JP. Adler C. Leboff MS. Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieback K. Kern S. Kluter H. Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 11.Wang M. Yang Y. Yang D. Luo F. Liang W. Guo S. Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauta AJ. Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin HS. Bicknese AR. Chien SN. Bogucki BD. Quinn CO. Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S. Wehner R. Bornhäuser M. Wassmuth R. Bachmann M. Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 15.Meisel R. Zibert A. Lareya M. Göbel U. Däubener W. Dilloo D. Human bone marrow stromal cells inhibit allogenic T-cell responses by idoleamine-2,3-dioxygenase-mediated trypthophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 16.Kern S. Eichler H. Stoeve J. Kluter H. Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 17.Rebelatto CK. Aguiar AM. Moretao AP. Senegaglia AC. Hansen P. Barchiki F. Oliveira J. Martins J. Kuligovski C. Mansur F. Christofis A. Amaral VF. Brofman PS. Goldenberg S. Nakao LS. Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood and adipose tissue. Exp Biol Med. 2007;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 18.Guillot PV. De Bari C. Dell'Accio F. Kurata H. Polak J. Fisk NM. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem ell sources. Differentiation. 2008;76:946–957. doi: 10.1111/j.1432-0436.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 19.Panepucci RA. Siufi JL. Silva WA., Jr. Proto-Siquiera R. Neder L. Orellana M. Rocha V. Covas DT. Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 20.Miao Z. Jin J. Chen L. Zhu J. Huang W. Zhao J. Qian H. Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Hwang JH. Shim SS. Seok OS. Lee HY. Woo SK. Kim BH. Song HR. Lee JK. Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood and bone marrow. J Korean Med Sci. 2009;24:547–554. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu TM. Martina M. Hutmacher DW. Hui JH. Lee EH. Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 23.Heiskanen KM. Bhat MB. Wang HW. Ma J. Nieminen AL. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J Biol Chem. 1999;274:5654–5658. doi: 10.1074/jbc.274.9.5654. [DOI] [PubMed] [Google Scholar]
- 24.Brini M. Ca(2+) signaling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium. 2003;34:399–405. doi: 10.1016/s0143-4160(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 25.Nesti C. Pasquali L. Vaglini F. Siciliano G. Murri L. The role of mitochondria in stem cell biology. Biosci Rep. 2007;27:167–171. doi: 10.1007/s10540-007-9044-1. [DOI] [PubMed] [Google Scholar]
- 26.Holley AK. St Clair DK. Watching the watcher: regulation of p53 by mitochondria. Future Oncol. 2009;5:117–130. doi: 10.2217/14796694.5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondoh H. Lleonart ME. Nakashima Y. Yokode M. Tanaka M. Bernard D. Gil J. Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 28.Lonergan T. Brenner C. Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 29.Chen CT. Shih YV. Kuo TK. Lee OK. Wei Y. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 30.Pietilä M. Lehtonen S. Närhi M. Hassinen IE. Leskelä HV. Aranko K. Nordström K. Vepsäläinen A. Lehenkari P. Mitochondrial function determines the viability and osteogenic potency of human mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16:435–445. doi: 10.1089/ten.tec.2009.0247. [DOI] [PubMed] [Google Scholar]
- 31.Mandal S. Lindgren AG. Srivastava AS. Clark AT. Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2010;29:486–495. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owusu-Ansah E. Banerjee U. Reactive oxygen species prime drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crespo FL. Sobrado VR. Gomez L. Cervera AM. McCreath KJ. Mitochondrial reactive oxygen species mediate cardiomyocyte formation from embryonic stem cells in high glucose. Stem Cells. 2010;28:1132–1142. doi: 10.1002/stem.441. [DOI] [PubMed] [Google Scholar]
- 34.Song H. Cha MJ. Song BW. Kim IK. Chang W. Lim S. Choi EJ. Ham O. Lee SY. Chung N. Jang Y. Hwang KC. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 35.Leskelä HV. Risteli J. Niskanen S. Koivunen J. Ivaska KK. Lehenkari P. Osteblast recruiment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun. 2003;311:1008–1013. doi: 10.1016/j.bbrc.2003.10.095. [DOI] [PubMed] [Google Scholar]
- 36.Laitinen A. Nystedt J. Laitinen S. The isolation and culture of human cord blood-derived mesenchymal stem cells under hypoxic conditions. Methods Mol Biol. 2011;698:63–73. doi: 10.1007/978-1-60761-999-4_6. [DOI] [PubMed] [Google Scholar]
- 37.Scheurer SB. Rybak JN. Roesli C. Brunisholz RA. Potthast F. Schlapbach R. Neri D. Elia G. Identification and relative quantification of membrane proteins by surface biotinylation and two-dimensional peptide mapping. Proteomics. 2005;5:2718–2728. doi: 10.1002/pmic.200401163. [DOI] [PubMed] [Google Scholar]
- 38.O'Connell KL. Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997;18:349–359. doi: 10.1002/elps.1150180309. [DOI] [PubMed] [Google Scholar]
- 39.Shevchenko A. Tomas H. Havlis J. Olsen JV. Mann N. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 40.Kinter M. Sherman NE. Protein sequencing and identification using tandem mass spectrometry. In: Kinter M, editor; Sherman NE, editor. Protein Sequencing and Identification using Tandem Mass Spectrometry. John Wiley & Sons Inc.; New York: 2000. pp. 160–163. [Google Scholar]
- 41.UniProt Consortium. The universal protein resource (UniProt) in 2010. Nucleic Acid Res. 2010;38(Database issue):D142–D148. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquinelli G. Tazzari P. Ricci F. Vaselli C. Buzzi M. Conte R. Orrico C. Foroni L. Stella A. Alviano F. Bagnara GP. Lucarelli E. Ultrastructural characteristics of human mesenchymal stromal (stem) cells derived from bone marrow and term placenta. Ultrastruct Pathol. 2007;31:23–31. doi: 10.1080/01913120601169477. [DOI] [PubMed] [Google Scholar]
- 43.An JH. Yang JY. Ahn BY. Cho SW. Jung JY. Cho HY. Cho YM. Kim SW. Park KS. Kim SY. Lee HK. Shin CS. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010;47:140–150. doi: 10.1016/j.bone.2010.04.593. [DOI] [PubMed] [Google Scholar]
- 44.Foster LJ. Zeemann PA. Li C. Mann M. Jensen ON. Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23:1367–1377. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 45.Giusta MS. Andrade H. Santos AV. Castanheira P. Lamana L. Pimenta AM. Goes AM. Proteomic analysis of human mesenchymal stromal cells derived from adipose tissue undergoing osteoblast differentiation. Cytotherapy. 2010;12:478–490. doi: 10.3109/14653240903580270. [DOI] [PubMed] [Google Scholar]
- 46.Zhang AX. Yu WH. Ma BF. Xu XB. Mao FF. Liu W. Zhang JQ. Zhang XM. Li SN. Li MT. Lahn BT. Xiang AP. Proteomic identification of differentially expressed proteins responsible for osteoblast differentiation from human mesenchymal stem cells. Mol Cell Biochem. 2007;304:167–179. doi: 10.1007/s11010-007-9497-3. [DOI] [PubMed] [Google Scholar]
- 47.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smaili SS. Russel JT. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Ca2+ signals in oligodendrocyte progenitors. Cell Calcium. 1999;26:121–130. doi: 10.1054/ceca.1999.0061. [DOI] [PubMed] [Google Scholar]
- 49.Chacon E. Acosta D. Mitochondrial regulation of superoxide by Ca2+: an alternate mechanism for the cardiotoxicity of doxorubicin. Toxicol Appl Pharmacol. 1991;107:117–128. doi: 10.1016/0041-008x(91)90336-d. [DOI] [PubMed] [Google Scholar]
- 50.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop DJ. Horwitz E. Minimal criteria for defining multipotent stromal cells. The international society of cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 51.Felson DT. The epidemiology of osteoarthritis: prevalence and risk factors. In: Kuettner KE, editor; Goldberg VM, editor. Osteoarthritic Disorders. American Academy of Orthopaedic Surgeons; Rosemont: 1995. pp. 13–24. [Google Scholar]