Abstract
In a perspective of regenerative medicine, multipotent human neural progenitor cells (hNPCs) offer a therapeutic advantage over pluripotent stem cells in that they are already invariantly “neurally committed” and lack tumorigenicity. However, some of their intrinsic properties, such as slow differentiation and uncontrolled multipotency, remain among the obstacles to their routine use for transplantation. Although rodent NPCs have been genetically modified in vitro to overcome some of these limitations, the translation of this strategy to human cells remains in its early stages. In the present study, we compare the actions of 4 basic helix-loop-helix transcription factors on the proliferation, specification, and terminal differentiation of hNPCs isolated from the fetal dorsal telencephalon. Consistent with their proneural activity, Ngn1, Ngn2, Ngn3, and Mash1 prompted rapid commitment of the cells. The Ngns induced a decrease in proliferation, whereas Mash1 maintained committed progenitors in a proliferative state. As opposed to Ngn1 and Ngn3, which had no effect on glial differentiation, Ngn2 induced an increase in astrocytes in addition to neurons, whereas Mash1 led to both neuronal and oligodendroglial specification. GABAergic, cholinergic, and motor neuron differentiations were considerably increased by overexpression of Ngn2 and, to a lesser extent, of Ngn3 and Mash1. Thus, we provide evidence that hNPCs can be efficiently, rapidly, and safely expanded in vitro as well as rapidly differentiated toward mature neural (typically neuronal) lineages by the overexpression of select proneural genes.
Introduction
The properties of neural stem cells—including self-renewal, multipotency, and engraftability—have made these cells attractive tools for cell and/or molecular replacement therapies in a range of central nervous system (CNS) pathologies. In rodents, stem-cell based interventions have been demonstrated to reverse the course in a number of models of neurodegenerative diseases, myelin disorders, and traumatic injuries [1–6]. On the basis of these advances, efforts have been made to identify and isolate neural stem and progenitor cells from humans, including from blastocysts [7], fetal organs [8–13], and adult tissues [14]. More recently, human neural stem cells have been also derived from induced pluripotent stem cells [15]. All of these stem cell types provide valuable sources of transplantation material, each with advantages and disadvantages, and offer the potential for designing therapeutic paradigms for a variety of pathologies. In particular, fetal tissue–derived multipotent human neural progenitor cells (hNPCs), such as those used in the present study, offer a therapeutic advantage over pluripotent stem cells in that they are already invariantly “neurally committed” and lack tumorigenicity after grafting.
However, whether derived from embryonic, fetal, or adult material, human neural stem and progenitor cells share some species-related properties that have been recognized as potential obstacles to their use in clinical trials. First, hNPCs differentiate much slower than their rodent counterparts [16–18]. As a consequence, prolonged proliferation was observed in vivo and eventually led to graft overgrowth [19,20], with deleterious consequences on the host tissue cytoarchitecture. Second, hNPCs undergo decreased neurogenesis upon expansion in vitro [21,22], which might limit the potential benefit for neuronal replacement. Finally, the heterogeneity of their progeny may lead to undesired side effects because of the presence of unintended donor-derived cell types after transplantation, particularly from pluripotent stem cell sources, but even from multipotent tissue-derived somatic stem cell starting material. Therefore, whatever the source, restraining hNPC proliferation potential and specifying their differentiation toward a desired phenotype represent major prerequisites for regenerative therapy.
Several strategies have been tested to overcome these obstacles, including prospective isolation of desired cell types [23–26] and commitment of hNPCs toward the relevant lineage by epigenetic stimulation [12,13,16,19,27–31]. A strategy that has more recently emerged consists of genetic induction of neural stem cell differentiation toward a particular phenotype. Thus, in rodents, the specification of diverse cell types, such as dopaminergic neurons [32–36], GABAergic neurons [37,38], motor neurons [39,40], or oligodendrocyte progenitor cells (OPCs) [41], was induced by forced expression of various genetic determinants, including basic helix-loop-helix (bHLH) transcription factors, which are known to regulate crucial steps during CNS and peripheral nervous system (PNS) development [42,43]. In human cells, similar approaches have been tested, albeit to a lesser extent. OPCs were specified from fetal hNPCs through Olig2 overexpression, which resulted in a 2-fold increase in myelin generation after transplantation into a mouse dysmyelination model [44]. Moreover, regionally specified mesencephalic hNPCs could be redirected toward a neuronal phenotype by genetic manipulation with the acheate-scute-related transcription factor ASCL1 [2]. However, the variety of paradigms under which hNPCs were genetically modified (eg, after isolation from different brain regions, through transfection or transduction using different viral vectors, with different genes) did not allow a direct comparison of the different proneural gene effects on hNPC development in a well-controlled cellular context.
In the present study, we provide a comparative analysis of the effects of overexpression of the bHLH transcription factors Ngn1, Ngn2, Ngn3, and Mash1 on the fate of human telencephalic NPCs expanded in vitro. Together with variable effects on cell proliferation, enhanced proneural activity of all these genes promoted a rapid cell specification favoring neuronal production. The actions of each of the bHLH transcription factors were a bit different, allowing us to devise a systematic protocol for their rational combined use in providing the best product for translational studies. This work, therefore, provides insights into the manipulation of fetal hNPCs and may facilitate the design of a variety of adequate paradigms for the treatment of CNS pathologies.
Materials and Methods
Tissues
Human fetuses were obtained after legal abortion according to the recommendations of the Agence de la Biomedecine with written consents of the patients. The age of the samples was determined by crown-to-rump length measurement by ultrasound scanning and by observation of fingers and toes development. The collection procedure was performed as previously described [8].
Cell culture
Primary cultures were initiated from the human fetal cortex (HFC) of 2 independent fetuses (6 and 7 weeks of gestation), named HFC6 and HFC7. Briefly, after removal of the meninges, telencephalic vesicles were open and ganglionic eminences were separated from the cortical leaf. This latter was cut into small pieces and single-cell suspensions were obtained by incubation in dissociation solution (0.05% trypsin, 0.1% glucose, and 0.5 nM ethylenediaminetetraacetic acid) for 20 min at 37°C, followed by mechanical trituration. Dissociated cells were seeded at the density of 106 cells/T75 flask and grown as floating clusters in a medium consisting of a 1:1 mixture of Dulbecco's modified Eagle's medium–F12 (Invitrogen) supplemented with 1% N supplement (Invitrogen), 0.5% B27 (Invitrogen), 25 μg/mL insulin (Sigma-Aldrich), 6 mg/mL glucose (Sigma-Aldrich), 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Invitrogen), 20 ng/mL basic fibroblast growth factor (bFGF) (Sigma-Aldrich), and 20 ng/mL epidermal growth factor (EGF) (Sigma-Aldrich). Fresh medium was added twice a week and cells were passaged every 2 weeks by incubation in dissociation solution.
Vector design and production
Murine Ngn1, Ngn2 and Mash1, rat Ngn3, and LacZ cDNAs were inserted between the BamH1 and XhoI restriction sites of the lentiviral plasmid pFlap-woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) [45], under the control of the ubiquitous murine phosphoglycerate kinase (PGK) promoter. Final constructions were noted pFlap-PGK-Ngn1-WPRE, pFlap-PGK-Ngn2-WPRE, pFlap-PGK-Ngn3-WPRE, pFlap-PGK-Mash1-WPRE, and pFlap-PGK-LacZ-WPRE. Recombinant lentiviral particles were generated by transient transfection of HEK293T cells using the calcium phosphate precipitation method. Cells were cotransfected with the vector plasmid (pTrip-PGK-Ngns/mash1-WPRE), the transcomplementation plasmid (p8.9), and the envelope plasmid encoding the vesicular stomatitis virus envelope glycoprotein (pVSV) as previously described [45]. The medium was replaced 12 h after transfection and collected 36 h later. Supernatants were treated with DNase and passed through a 0.45-μm filter. Viral particles were then concentrated by ultracentrifugation (90 min, 22,000 rpm, rotor SW28) and resuspended in 1× phosphate-buffered saline (PBS; Gibco). Total particle concentration of viral stocks was quantified by human immunodeficiency virus-1 p24 enzyme-linked immunosorbent assay (Beckman Coulter). Final stocks were noted as VSV-PGK-Ngn1, VSV-PGK-Ngn2, VSV-PGK-Ngn3, VSV-PGK-Mash1, and VSV-PGK-LacZ.
Human fetal cell transduction
After 160 days in vitro (P8–P9), HFC6 and HFC7 cells were dissociated and seeded in 24-well plates supplied with 12-mm-diameter glass coverslips and coated with gelatin (25 μg/cm2; Merk) and laminin (1.7 μg/cm2; Sigma). Cells were seeded at the density of 1.2×105 cells/well in N medium supplemented with 10 ng/mL bFGF (terminated N+F medium). Four days later, the medium was replaced by 400 μL N+F fresh medium containing 50 ng p24 of viral vectors (day 1). For each cell line, 6 groups were established as follows: 1 control group consisting of nontransduced hNPCs (n=24 wells); 1 control group consisting of hNPCs transduced with the VSV-PGK-LacZ vector (n=24); 4 “experimental groups” consisting of hNPCs subjected to a single transduction with either the VSV-PGK-Ngn1 vector (n=24), the VSV-PGK-Ngn2 vector (n=24), the VSV-PGK-Ngn3 vector (n=24), or the VSV-PGK-Mash1 vector (n=24). On days 2 and 4, the medium was replaced by fresh N+F medium. On day 5, cells were fixed in 4% paraformaldehyde for 30 min at room temperature.
Immunocytochemistry
Human cell characterization was performed using the following primary antibodies: mouse monoclonal anti-human nestin (Clone 10C2; Chemicon; Cat. No. MAB5326; 2 μg/mL), mouse monoclonal anti-MAP5 (Clone AA6; Sigma; Cat. No. M4528; 1/400 dilution of ascites fluid), mouse monoclonal anti-β3-tubulin (Clone SDL.3D10; Sigma; Cat. No. T8660; 1/400 dilution of ascites fluid), mouse monoclonal anti-MAP2 (Clone AP20; Chemicon; Cat. No. MAB3418; 1.25 μg/mL), rabbit polyclonal anti-glail fibrillary acidic protein (GFAP) (Dako; Cat. No. Z0334; 1/400 dilution of antisera), mouse monoclonal A2B5 [American Type Culture Collection (ATCC); 1/2 dilution of hybridoma supernatant], mouse monoclonal O4 (ATCC; 1/10 dilution of hybridoma supernatant), rat monoclonal anti-serotonin (Clone YC5/45; Chemicon; Cat. No. MAB352; 1/50 dilution of culture supernatant), rabbit polyclonal anti-dopamine β-hydroxylase (DBH) (Chemicon; Cat. No. AB1538; 1/200 dilution of antisera), rabbit polyclonal anti-choline acetyl transferase (ChAT; Chemicon; Cat. No. AB143; 1/100 dilution of antisera), rabbit polyclonal anti-Hb9/HLXB9 (Abcam; Cat. No. ab12028; 5 μg/mL), mouse monoclonal anti-glutamate decarboxylase 67 (GAD67) (Clone 1G10.2; Chemicon; Cat. No. AB143; 2 μg/mL), and rabbit polyclonal anti-gamma aminobutyric acid (GABA; Sigma; Cat. No. A2052; 1/500 dilution of buffered aqueous solution). Proliferation was assessed using the rabbit polyclonal anti-Ki67 (Abcam; Cat. No. ab15580; 1/400 dilution of antisera). Briefly, after fixation, cells were incubated in a blocking solution (2% normal goat serum, 0.25% gelatin, and 0.1% Triton X-100 in PBS) and then with primary antibodies diluted in the blocking solution overnight at 4°C. Corresponding secondary antibodies were incubated 2 h at room temperature in the blocking solution and Hoescht 33342 was used to counterstain nuclei. Coverslips were then mounted in moviol solution. Immunofluorescence was visualized with a Zeiss Axioplan 2 fluorescence microscope.
Cell counting
All experiments were independently performed on HFC6 and HFC7 cells. Each staining was performed in triplicate on 3 independent glass coverslips. For each coverslip, 10 independent fields were counted, consisting of a total of at least 3,000 cells. Positive cell number was counted using the ImageJ software and expressed as a percentage of total cell number, as determined by Hoechst-positive nuclei counting.
Statistical analysis
All data in this study are presented as mean±standard deviations. Data were analyzed by Student's t-tests, performed using the SigmaStat software. The t-test results and P-values are presented in Supplementary Table S1 (Supplementary Data are available online at www.liebertonline.com/scd).
Results
Human neural progenitors isolated from the fetal cortex constitute a heterogeneous population of neural stem cells and committed progenitors
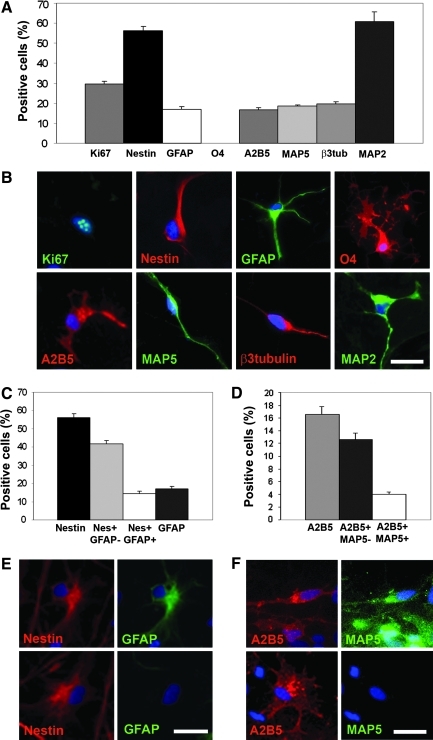
Neural progenitors were isolated from the HFC of 2 fetuses (6 and 7 weeks of age, respectively) and grown in vitro as neurospheres for 8–9 passages. At that time, cell populations were characterized by immunocytochemistry using a set of markers specific for the different neural types and lineages (Fig. 1B), including nestin for immature neuroepithelial-like cells, MAP5 for early neuroblasts, β3-tubulin for late neuroblasts/immature neurons, MAP2 for postmitotic, more terminally differentiated neurons, GFAP for astrocytes, A2B5 for early OPCs and neuroblasts, and O4 for oligodendrocytes. Cell proliferation was estimated using the Ki67 marker. Because some of these markers can have expression patterns that extend beyond one neural cell type, often dual marker expression was used in this study to clarify specific neural cell types along the differentiation continuum. In particular, distinction was made between Nestin+ cells that expressed GFAP and those that did not (Fig. 1E). Although it is now well established that GFAP is expressed by adult rodent neural stem cells in vivo [46], this protein is not expressed by immature neuroepithelial-like progenitor during early fetal development [47]. This was also the case in our study, wherein cells from both the 6- and 7-week-old cortex did not express GFAP at the time of isolation (not shown) but began to do so after amplification in vitro. By that time, numerous Nestin+ cells coexpressed Ki67 (not shown), but virtually none of the GFAP+ cells did so, confirming that GFAP+ cells were not immature, self-renewing neuroepithelial-like cells. Therefore, cells that were Nestin+ but GFAP− were designated as immature, undifferentiated NPCs, whereas cells that were Nestin+ but also GFAP+ were deemed to be astrocytes. Similarly, A2B5+/MAP5+ cells were designated as neuroblasts, whereas A2B5+ cells that were MAP5− were deemed to be OPCs.
FIG. 1.
The HFC7 hNPC population is composed of immature neuroepithelial-like cells and committed progenitors from the 3 main CNS lineages. (A) Proportions of cells expressing various markers in the HFC7 hNPC population after amplification in vitro (passages 8), expressed as a percentage of total Hoechst+ nuclei±SEM. HFC7 cells are a heterogeneous population composed of Ki67+ proliferating cells as well as Nestin+ neuroepithelial-like cells, glial fibrillary acidic protein (GFAP) astrocytes, A2B5+, MAP5+, and β3 tubulin+ neuroblasts, and MAP2+ mature neurons. Extremely rare (<0.005%) O4+ oligodendrocytes were detected among HFC7 cells. (B) Immunofluorescent staining illustrating typical morphology of cells expressing each marker tested in A. (C) Among the Nestin+ cell population, a majority corresponds to Nestin+/GFAP− neuroepithelial-like cells, whereas less than one-third corresponds to Nestin+/GFAP+ astrocytes. Note that, among total GFAP+ astrocyte population, the vast majority coexpresses nestin. (D) Among the A2B5+ cell population, ∼¼ corresponds to A2B5+/MAP5+ neuroblasts, whereas ¾ are A2B5+/MAP5− OPCs. (E) Double-immunofluorescent staining against nestin (red) and GFAP (green), showing a Nestin+/GFAP+ astrocyte (upper cases) and a Nestin+/GFAP−neuroepithelial-like cell (lower cases). (F) Double-immunofluorescent staining against A2B5 (red) and MAP5 (green), showing a A2B5+/MAP5+ neuroblast with typical bipolar morphology (upper cases) and a A2B5+/MAP5− OPC with more flat, multipolar morphology (lower cases). Scale bars: 30 μm. HFC, human fetal cortex; hPNC, human neural progenitor cell; CNS, central nervous system; SEM, standard error of the mean; OPC, oligodendrocyte progenitor cell.
Nontransduced human fetal cortical cells from the 7-week-old fetus (HFC7 cells) constituted a heterogeneous cell population (Fig. 1A–F) comprising numerous proliferating cells (29.5%±1.4% of Ki67+ cells), a large proportion of immature, undifferentiated NPCs (41.8%±1.8% Nestin+/GFAP− cells), as well as committed cells from either the neuronal lineage (18.5%±0.4% of MAP5+ and 4.0%±0.4% of A2B5+/MAP5+ neuroblasts; 19.6%±1.2% of β3-Tubulin+ immature neurons and 60.9%±4.9% of MAP2+ mature neurons), the astroglial lineage (17.1%±1.4% of GFAP+ astrocytes), or the oligodendroglial lineage (12.6%±1.0% of A2B5+/MAP5− OPCs and extremely rare—<0.005%—O4+ oligodendrocytes). Because there are cells that are in transition between immature and mature states, it is possible that some of these would be double counted, allowing for the possibility of exceeding 100%.
The nontransduced HFC6 cell population displayed a phenotype very similar to the HFC7 cell population (Supplementary Fig. S1A–C), with 29.1%±2.2% of proliferating cells, a large proportion of neuroepithelial-like cells (41.7%±0.6% of Nestin+/GFAP− cells), and committed cells from the 3 main lineages of the CNS (23.1%±4.0% of MAP5+ neuroblasts, 27.9%±1.6% of β3-Tubulin+ immature neurons, 44.2%±1.4% of MAP2+ postmitotic neurons, 28.3%±1.3% of GFAP+ astrocytes, and 8.1%±0.5% of A2B5+/MAP5− OPCs). Nevertheless, the slightly higher proportion of neuroepithelial-like cells and immature neuroblasts in the HFC6 cell population, as well as the lower proportion of postmitotic neurons, compared with HFC7, attested to a slightly less mature global phenotype, possibly related to the respective ages of the fetuses used in these experiments.
The various bHLH proneural genes produce different effects on hNPC proliferation
Cells from HFC7 and HFC6 populations were transduced with a VSV-PGK-LacZ vector. Transduction with this control vector did not induce any significant change in the expression of most markers tested (Supplementary Fig. S2). However, a slight decrease in the proportion of Ki67+ proliferating cells (from 29.5%±1.4% to 22.6%±0.5%; P=0.01) and in the proportion of Nestin+/GFAP− immature cells (from 41.7%±0.6% to 38.5%±0.8%; P=0.03) was observed in the HFC7 and HFC6 cell populations, respectively (Supplementary Fig. S2). Therefore, to get rid of any phenotypic changes that may be attributable to transduction by itself, hNPCs transduced with the VSV-PGK-LacZ vector were used as controls to assess the effects of proneural gene overexpression. All Student's t-tests and P-values are presented in Supplementary Table S1. Overexpression of the proneural genes Ngn1, Ngn2, Ngn3, and Mash1 was induced in HFC6 and HFC7 cell populations by lentiviral transduction with the VSV-PGK-Ngn1, VSV-PGK-Ngn2, VSV-PGK-Ngn3, and VSV-PGK-Mash1 vectors. Dot blot analysis attested to the transduction efficiency of each vector (Supplementary Fig. S3).
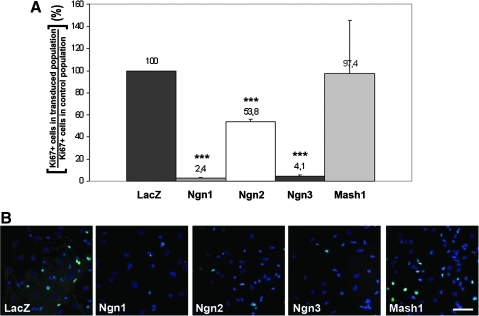
Ngn1 and Ngn 3 overexpression induced a dramatic decrease in the proliferation of HFC7 cells, as shown by quantifying of Ki67 expression (Fig. 2A, B). Four days after transduction, the proportion of Ki67+ cells underwent a 97.6% and 95.9% decrease after overexpression of Ngn1 and Ngn3, respectively (P<0.001 in both cases). A similar, albeit less pronounced, effect was observed after Ngn2 overexpression, with a 46.2% decrease in the proportion of Ki67+ cells (P<0.001). In contrast, Mash1 overexpression did not modify the proportion of proliferating cells (P=0.965). Similar tendencies were observed after overexpression of the Ngns and of Mash1 in HFC6 cell population (Supplementary Fig. S4 and Supplementary Table S1). Altogether, these results indicate that the various bHLH proneural genes produce distinct effects on human neural progenitor proliferation.
FIG. 2.
Overexpression of Ngn1, Ngn2, and Ngn3 in HFC7 cell population decreases the proportion of proliferating cells, whereas Mash1 does not affect proliferation. (A) Impact of proneural gene overexpression on proliferation, expressed as the relative quantity of Ki67+ proliferating cells in transduced populations (LacZ, Ngn1, Ngn2, Ngn3, and Mash1) reported on the quantity of Ki67+ proliferating cells in the control LacZ population. Proliferation underwent a highly significant decrease after Ngn1, Ngn2, and Ngn3 overexpression, whereas it was not affected by Mash1 overexpression, highlighting different roles of Ngns and Mash1 in hNPC proliferation. Student's t-test: ***P<0.001. (B) Immunofluorescent staining against Ki67 (green), illustrating hNPC proliferation in control populations (LacZ) and after overexpression of Ngn1, Ngn2, Ngn3, and Mash1. Scale bar: 50 μm.
Ngn1 overexpression induces hNPC neuronal specification, although not their full neuronal differentiation, without affecting glial differentiation
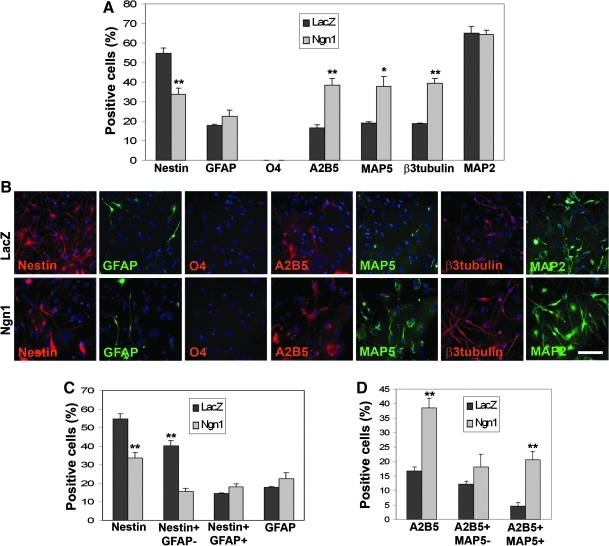
Four days after transduction of HFC7 cells, Ngn1 overexpression induced a highly significant decrease in the proportion of Nestin+ cells (from 54.7%±2.7% to 33.6%±3.1%; P=0.007) (Fig. 3A, B), which was strictly related to the decrease of Nestin+/GFAP− neuroepithelial-like cells (P<0.001) and did not reflect any significant change in the proportion of Nestin+/GFAP+ astrocytes (P=0.125) (Fig. 3C). In contrast, Ngn1 overexpression consistently affected the proportion of neuroblasts, as shown by the increase in MAP5+ (from 19.0%±0.5% to 37.7%±5.1%; P=0.022) and β3-Tubulin+ (from 18.8%±0.2% to 39.4%±2.5%; P=0.001) cell proportions (Fig. 3A, B). Moreover, an increase in A2B5+ cell proportion was also observed (from 16.6%±1.2% to 38.5%±3.4%; P=0.004), which reflected a highly significant increase in the proportion of A2B5+/MAP5+ neuroblasts (P=0.007) but no significant changes in the proportion of A2B5+/MAP5− OPCs (P=0.274) (Fig. 3D). Noticeably, the proportion of postmitotic neurons was not affected 4 days after transduction with the Ngn1-encoding vector, as shown by the absence of significant effect on the MAP2+ cell population (P=0.873), suggesting that Ngn1 overexpression displays an effect on neuronal specification, but this action alone does not result in full neuronal maturation; additional intracellular signaling processes appear to be required. Similar results were obtained after transduction of the HFC6 cells with the VSV-PGK-Ngn1 vector (Supplementary Fig. S5A–C; Supplementary Table S1). Thus, Ngn1 overexpression induced a commitment of neuroepithelial immature cells toward the neuronal lineage (although not to their full differentiation), without affecting glial differentiation.
FIG. 3.
Ngn1 displays its proneural activity on HFC7 cells without affecting glial pathways. (A) Proportions of HFC7 cells from different neural lineages at 4 days after transduction with the VSV-PGK-LacZ and VSV-PGK-Ngn1 vectors. Overexpression of Ngn1 induced a very significant decrease in the proportion of Nestin+ cells, together with a very significant increase in the proportions of A2B5+ cells and β3 Tubulin+ neuroblasts, and a significant increase in the proportion of MAP5+ neuroblasts. However, it did not affect the proportions of GFAP+ astrocytes, O4+ oligodendrocytes, and MAP2+ mature neurons. (B) Immunofluorescent staining against the various markers tested in A, after hNPC transduction with the VSV-PGK-LacZ (upper cases) and VSV-PGK-Ngn1 (lower cases) vectors. (C) The decrease in the proportion of Nestin+ cells reflected a very significant decrease in Nestin+/GFAP− neuroepithelial cells exclusively. Ngn1 overexpression did not affect the proportions of Nestin+/GFAP+ astrocytes. (D) The increase in the proportion of A2B5+ cells reflected a very significant increase in A2B5+/MAP5+ neuroblast proportion exclusively. Ngn1 overexpression did not affect the proportion of A2B5+/MAP5− OPCs. Student's t-test: *P<0.05; **P<0.01. Scale bar: 50 μm. VSV, vesicular stomatitis virus envelope glycoprotein; PGK, phosphoglycerate kinase.
Ngn2 overexpression affects both neuronal and astroglial differentiation pathways
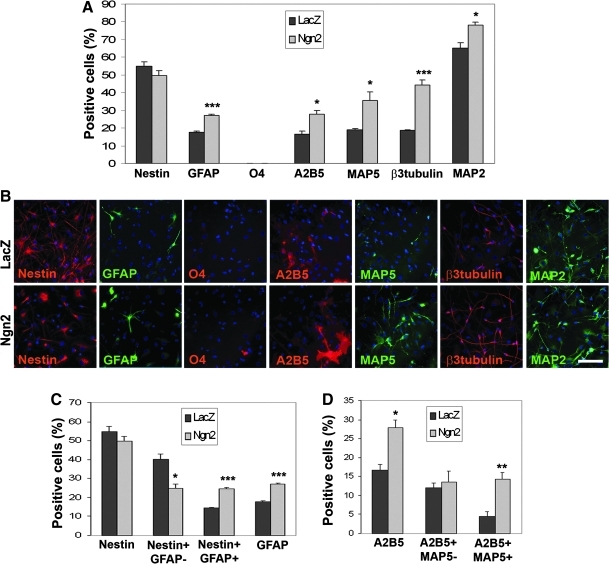
After transduction of the HFC7 cells with the VSV-PGK-Ngn2 vector (Fig. 4), no significant changes were observed in the proportion of Nestin+ cells (P=0.247) (Fig. 4A, B). However, this apparent lack of change in Nestin expression masks the more refined analysis that the proportion of Nestin+/GFAP− neuroepithelial progenitors decreased (from 40.3%±2.5% to 24.9%±2.2%; P=0.01), whereas the proportion of Nestin+/GFAP+ astrocytes increased (from 14.4%±0.3% to 24.7%±0.6%; P<0.001) (Fig. 4C). Neuronal specification was also induced by Ngn2 overexpression, as shown by an increase in MAP5+ (from 19.0%±0.5% to 35.7%±4.8%; P=0.026), β3-tubulin+ (from 18.8%±0.2% to 44.4%±2.8%; P<0.001) and A2B5+ (from 16.6%±1.6% to 27.8%±2.2%; P=0.014) cells, which was only related to the increase of A2B5+/MAP5+ neuroblasts (from 4.6%±1.2% to 14.3%±1.6%; P=0.008) (Fig. 4D). Moreover, the MAP2+ cell proportion also increased after Ngn2 overexpression (from 65.0%±3.3% to 78.1%±1.9%; P=0.027) (Fig. 4A, B), indicating that this factor plays a role in both neuronal specification and differentiation. In contrast, no effect on A2B5+/MAP5− OPCs was observed (P=0.671) (Fig. 4D), indicating that Ngn2 did not affect the oligodendroglial differentiation pathway. Similar results were obtained after transduction of HFC6 cells with the VSV-PGK-Ngn2 vector (Supplementary Fig. S6A–C and Supplementary Table S1). Thus, Ngn2 overexpression induced a decrease in neuroepithelial immature cells, attended by commitment toward both the neuronal and astroglial lineages, without affecting the oligodendroglial lineage.
FIG. 4.
Ngn2 overexpression affects both neuronal and astroglial pathways in HFC7 cells. (A) Proportions of HFC7 cells from different neural lineages at 4 days after transduction with the VSV-PGK-LacZ and VSV-PGK-Ngn2 vectors. Overexpression of Ngn2 did not affect the global proportions of Nestin+ cells but induced a highly significant increase in the proportions of GFAP+ astrocytes and β3 Tubulin+ neuroblasts, together with a significant increase in the proportions of A2B5+ cells, MAP5+ neuroblasts, and MAP2+ mature neurons. (B) Immunofluorescent staining against the various markers tested in A, after hNPC transduction with the VSV-PGK-LacZ (upper cases) and VSV-PGK-Ngn2 (lower cases) vectors. (C) The global lack of changes in the proportion of Nestin+ cells contrasted with the effects of Ngn2 overexpression on the neuroepithelial and astroglial cell populations. Overexpression of Ngn2 induced a very significant decrease in the proportion of Nestin+/GFAP− neuroepithelial cells, along with a highly significant increase in the proportion of Nestin+/GFAP+ astrocytes. (D) The increase in the proportion of A2B5+ cells reflected a very significant increase in the proportion of A2B5+/MAP5+ neuroblasts exclusively. Ngn1 overexpression did not affect the proportion of A2B5+/MAP5-OPCs. Student's t-test: *P<0.05; **P<0.01; ***P<0.001. Scale bar: 50 μm.
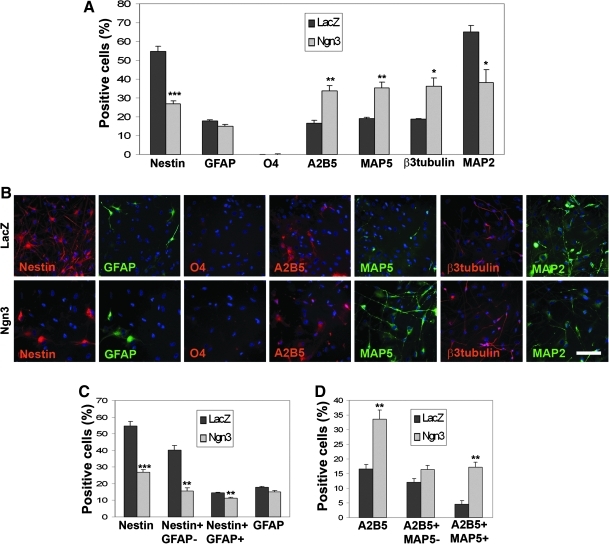
Ngn3 overexpression induces neuronal specification of hNPCs without affecting glial differentiation
In the HFC7 cell population, Ngn3 overexpression induced a highly significant decrease in the Nestin+ cell proportion (from 54.7±2.7 to 26.9%±1.6%; P<0.001) (Fig. 5A, B), affecting both the Nestin+/GFAP− neuroepithelial progenitor cell population (from 40.3%±2.5% to 15.6%±1.9%; P=0.001) and the Nestin+/GFAP+ astroglial cell population (from 14.4±0.3% to 11.3%±0.3%; P=0.002) (Fig. 5C). However, no significant effect was observed on the GFAP+ cell population (P=0.076). Ngn3 overexpression also induced neuronal specification, as shown by an increase in MAP5+ (from 19.0%±0.5% to 35.3%±3.0%; P=0.006), β3-Tubulin+ (from 18.8%±0.2% to 36.4%±4.2%; P=0.014), and A2B5+/MAP5+ (from 4.6%±1.2% to 17.2%±1.7%; P=0.004) cell proportions (Fig. 5A–B, D). In contrast, transduction of HFC7 cells with the VSV-PGK-Ngn3 vector induced a dramatic decrease in MAP2+ cells (from 65.0%±3.3% to 38.0%±7.1%; P=0.026). Finally, no significant effect of Ngn3 overexpression was observed on A2B5+/MAP5− OPCs (P=0.078) (Fig. 5D). Similar effects were observed after Ngn3 overexpression in HFC6 cells (Supplementary Fig. S7A–C and Supplementary Table S1). Therefore, these results indicate that Ngn3 overexpression induced a specification of immature cells toward the neuronal lineage, without affecting the astroglial and oligodendroglial pathways.
FIG. 5.
Ngn3 displays its proneural activity on HFC7 cells without affecting glial pathways. (A) Proportions of HFC7 cells from different neural lineages at 4 days after transduction with the VSV-PGK-LacZ and VSV-PGK-Ngn3 vectors. Overexpression of Ngn3 induced a highly significant decrease in the proportion of Nestin+ cells, together with a very significant increase in the proportions of A2B5+ cells and MAP5+ neuroblasts, and a significant increase in β3 Tubulin+ neuroblasts. However, it did not affect the proportions of GFAP+ astrocytes and O4+ oligodendrocytes. Note that the proportion of MAP2+ mature neurons underwent a significant decrease, which may reflect either neuronal death or dedifferentiation. (B) Immunofluorescent staining against the various markers tested in A, after hNPC transduction with the VSV-PGK-LacZ (upper cases) and VSV-PGK-Ngn3 (lower cases) vectors. (C) The decrease in the proportion of Nestin+ cells reflected a very significant decrease in the proportions of both Nestin+/GFAP-neuroepithelial cells and Nestin+/GFAP+ astrocytes. However, the global proportion of GFAP+ astrocytes was not affected by Ngn3 overexpression. (D) The increase in the proportion of A2B5+ cells reflected a very significant increase in the proportion of A2B5+/MAP5+ neuroblasts. However, Ngn3 overexpression did not affect the proportion of A2B5+/MAP5− OPCs. Student's t-test: *P<0.05; **P<0.01; ***P<0.001. Scale bar: 50 μm.
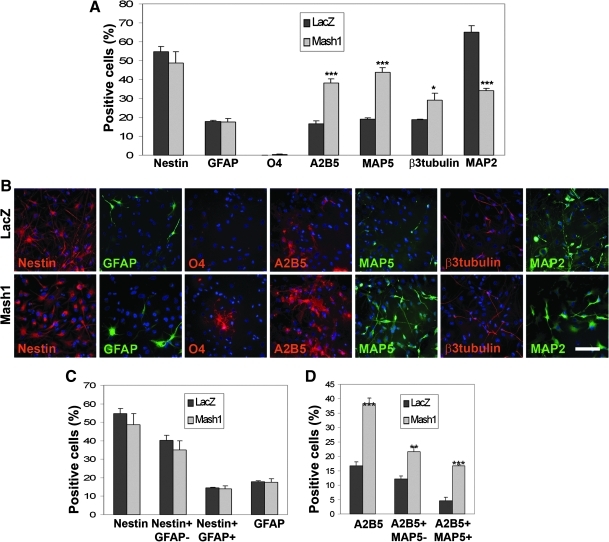
Mash1 overexpression promotes both neuronal and oligodendroglial specification of hNPCs
In HFC7 cell population, Mash1 overexpression did not induce any significant change in Nestin+ and GFAP+ cell populations (P=0.419 and 0.939, respectively) (Fig. 6A, B). Among the Nestin+ cells, neither the Nestin+/GFAP-neuroepithelial cells nor the Nestin+/GFAP+ astrocytes were affected (P=0.380 and 0.775, respectively) (Fig. 6C). However, Mash1 overexpression induced consistent neuronal specification, as shown by the increase in MAP5+ (from19.0%±0.5% to 43.7%±2.5%; P<0.001), β3-Tubulin+ (from 18.8%±0.2% to 29.2%±3.7%; P=0.049), and A2B5+/MAP5+ (from 4.6%±1.2% to 16.6%±0.4%; P<0.001) neuroblast proportions. Most interestingly, the global increase in A2B5+ cells (from 16.6%±1.6 to 38.3%±2.0%; P<0.001) was not only related to the increase in neuroblasts, but also reflected a very significant increase in the proportion of A2B5+/MAP5− OPCs (from 12.1%±1.2% to 21.6%±1.6%; P=0.009) (Fig. 6D). Moreover, scarce O4+ oligodendrocytes appeared among the transduced cells (Fig. 6B). Similar results were obtained after transduction of the HFC6 cell population (Supplementary Fig. S8A–C). However, the effects of Mash1 overexpression on neuronal terminal differentiation were not clear, because a highly significant decrease in the proportion of MAP2+ postmitotic neurons was observed among HFC7 cells (from 65.0%±3.3% to 34.0%±1.3%; P<0.001), whereas no significant effects were highlighted on HFC6 cells (P=0.642). Thus, our results indicate that Mash1 overexpression did not affect the proportions of immature neuroepithelial-like cells and astrocytes, but displayed a concomitant effect on neuronal and oligodendroglial specification in vitro.
FIG. 6.
Mash1 overexpression in HFC7 cells promotes both neuronal and oligodendroglial specification. (A) Proportions of HFC7 cells from different neural lineages at 4 days after transduction with the VSV-PGK-LacZ and VSV-PGK-Mash1 vectors. Overexpression of Mash1 did not affect the global proportions of Nestin+ cells and GFAP+ astrocytes, but induced a very significant increase in the proportions of A2B5+ cells, a highly significant increase in the proportion of MAP5+ neuroblasts, and a significant increase in the proportion of β3 Tubulin+ neuroblasts. Most insterestingly, scarce O4+ oligodendrocytes appeared as soon as 4 days after Mash1 overexpression. Note that the proportion of MAP2+ mature neurons underwent a highly significant decrease, which may reflect either neuronal death or dedifferentiation. (B) Immunofluorescent staining against the various markers tested in A, after hNPC transduction with the VSV-PGK-LacZ (upper cases) and VSV-PGK-Mash1 (lower cases) vectors. (C) Among the Nestin+ cells, neither the proportion of Nestin+/GFAP− neuroepithelial cells nor that of Nestin+/GFAP+ astrocytes was affected by Mash1 overexpression. (D) The increase in the proportion of A2B5+ cells reflected both a highly significant increase in the proportion of A2B5+/MAP5+ neuroblasts and a very significant increase in the proportion of A2B5+/MAP5− OPCs. Therefore, Mash1 overexpression induced both neuronal and oligodendroglial specification from HFC7 cells. Student's t-test: *P<0.05; **P<0.01; ***P<0.001. Scale bar: 50 μm.
Ngns and Mash1 overexpression produce distinct effects on terminal differentiation of hNPCs
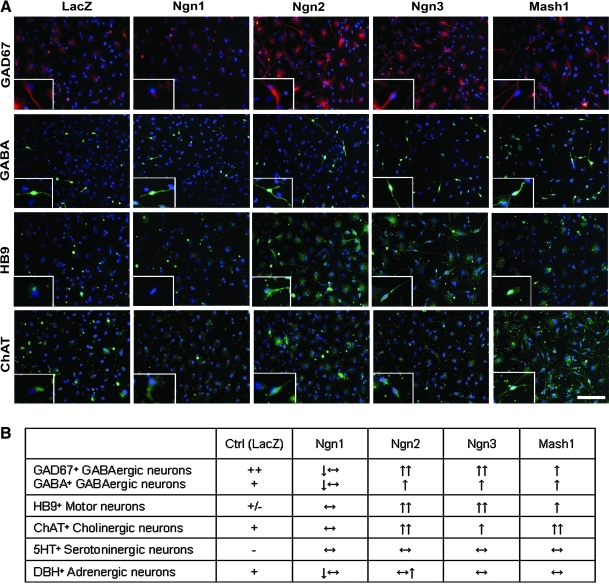
To assess the abilities of Ngns and Mash1 overexpression to promote neuronal subtype specification, we further characterized the phenotype of the transduced human fetal neural cells using a panel of neuronal subclass-specific antibodies, including anti-serotonin (5HT) to detect serotoninergic neurons, anti-DBH for adrenergic neurons, anti-ChAT for cholinergic neurons, anti-HB9 for motor neurons, and anti-GAD67 and anti-GABA for GABAergic neurons.
Among the control HFC7 cell population transduced with the VSV-PGK-LacZ vector, a variety of neuronal subtypes were identified, including GABAergic, adrenergic, cholinergic, and motor neurons (Fig. 7A, B). However, the relative proportions of these different subclasses were very different, with a predominance of GABA+ and GAD67+ GABAergic neurons and a very low proportion of the other subtypes. Serotoninergic neurons were not detected at all.
FIG. 7.
Overexpression of Ngns and Mash1 promotes neuronal subtype specification. (A) Immunofluorescent labeling against the neuronal subtype-specific markers GAD67 (red, first line), GABA (green, second line), HB9 (green, third line), and ChAT (green, fourth line) in hNPC populations transduced with the VSV-PGK-LacZ, VSV-PGK-Ngn1, VSV-PGK-Ngn2, VSV-PGK-Ngn3, and VSV-PGK-Mash1 vectors. Control hNPCs (LacZ) spontaneously gave rise to GAD67+/GABA+ GABAergic, ChAT+ cholinergic, and HB9+ motor neurons. Overexpression of Ngn1 did not promote hNPC differentiation into any of these lineages. On the contrary, overexpression of Ngn2 strongly increased the expression of GAD67, HB9, and ChAT and, to a lesser extent, of GABA. Ngn3 strongly increased the expression of GAD67 and HB9 and, to a lesser extent, of GABA and ChAT. Finally, Mash1 increased the expression of both GAD67 and GABA, as well as HB9, but displayed its strongest effect on the expression of ChAT. Scale bar: 200 μm. (B) Semiquantitative evaluation of the effects of Ngn1, Ngn2, Ngn3, and Mash1 overexpression on terminal neuronal differentiation of hNPCs, compared with control cells transduced with the VSV-PGK-LacZ vector. (+/−) <1% positive cells; (+) 1%–20% positive cells; (++) 20%–50% positive cell; (↔) no effect; (↓↔) <10% positive cell decrease; (↔↑) <10% positive cell increase; (↑) 10%–50% positive cell increase; (↑↑) >50% positive cell increase.
Overexpression of the proneural genes did not favor exclusive terminal phenotypes, but rather produced relative variations in the proportions of cells expressing the different markers tested as well as variable intracellular intensities of expression (Fig. 7A, B). Thus, although no significant effects of proneural gene overexpression were apparent on the serotoninergic and adrenergic neuron populations (not shown), major effects were noted on the other neuronal subtypes (Fig. 7A, B). Thus, Ngn2 overexpression dramatically increased the proportions of GAD67+ GABAergic neurons, HB9+ motor neurons, and ChAT+ cholinergic neurons. Ngn3 overexpression had similar effects on the GAD67+ and HB9+ populations, but only induced a moderate increase in ChAT+ neurons. Mash1 overexpression increased the proportions of GAD67+ GABAergic neurons and HB9+ motor neurons, but to a lesser extent than Ngn2 and Ngn3. Moreover, as did Ngn2, Mash1 also consistently increased the proportions of ChAT+ neurons. Finally, Ngn1 had only moderate or even no apparent effect on the different neuronal populations (Fig. 7B).
Discussion
Human neural stem cells represent a substantial donor source for potential cell-based therapy of a number of CNS diseases, provided that some of their features, such as prolonged proliferation and multipotent differentiation, can be better understood and mastered to avoid graft overgrowth and undesired cell progeny after transplantation. In the present study, we provide a unique in vitro paradigm under which fetal hNPCs from the dorsal telencephalon were transduced with proneural gene-encoding vectors, thus allowing the direct comparison of the effects of Ngn1, Ngn2, Ngn3, and Mash1 on hNPC differentiation.
Proneural genes encode transcription factors of the bHLH class that are both necessary and sufficient to promote neuronal specification from neuroepithelial cells during CNS and PNS development [42,43]. In the telencephalon, Mash1 and Ngns are expressed in dorsal territories and together account for the specification of all cortical neuronal progenitors, whereas Mash1 is the only known proneural gene expressed in the ventral telencephalon and participates in ventral neuronal progenitor emergence [48–50]. Along with their proneural activity, bHLH transcription factors also participate in neuronal subtype specification [48,51,52]. However, accumulating evidence suggested that proneural genes are not sufficient to specify neuronal identity by themselves [40] and that the selection of a particular cell fate ultimately results from the integration of multiple extrinsic and intrinsic signals [40,53–55]. In vitro, the spatiotemporal emergence of neurons and glia could therefore be reproduced in rodent spinal cord neural stem cells [40] through the combinatorial action of bHLH proneural genes and other determinants known to specify the positional identity of multipotent progenitor, such as Pax6, Olig2, and Nkx2.2 [56].
In human systems, very few studies have addressed the role of bHLH transcription factors in cell fate choice. Overexpression of Mash1 was shown to enhance neuronal production from regionally specified hNPCs [2] and overexpression of Olig2 promoted oligodendrogenesis from fetal hNPCs both in vitro and in vivo after grafting [44]. These data suggested that the fate of hNPCs can be redirected toward a desired phenotype by genetic modifications. In our study, overexpression of Ngn1, Ngn2, Ngn3, and Mash1 in human cortical fetal NPCs induced a dramatic increase in the proportions of neuronal progeny in vitro. Our data thereby highlight an evolutionary conserved role of these genes and confirm their proneural activity in a human system. Most interestingly, although human NPCs are known to differentiate slower than their rodent counterparts [17,31,47], proneural gene overexpression drastically accelerated their neuronal commitment and a 2–3-fold increase in neuroblasts was observed as soon as 4 days after lentiviral transduction. Our strategy, therefore, provides a means of rapidly committing hNPC differentiation, which may help overcome both delayed differentiation and undesired cell type generation after grafting.
Consistent with the ability of some bHLH transcription factors to promote cell-cycle exit [57–59], the Ngn1-, Ngn2-, and Ngn3-driven neuronal specification of hNPCs was accompanied by a dramatic decrease in the proportions of proliferating cells at 4 days after transduction. On the contrary, Mash1 overexpression did not seem to affect cell proliferation. This observation is in agreement with former studies that pointed to a divergence in the effects of Ngns and Mash1 on cell-cycle exit in various cellular contexts [49,50,60,61]. In vitro, overexpression of Ngn1 in neural crest stem cells [49] and of Ngn2 in CNS progenitors [61] have been shown to promote a more rapid cell-cycle withdrawal and earlier neuronal differentiation than Mash1, which, in contrast, maintained progenitor populations in a proliferative state. Significant differences in the ability of Ngn2- and Mash1-expressing cells to incorporate BrdU were also highlighted in the mouse telencephalon and spinal cord [50,60]. The mechanisms underlying these divergences have been proposed to be related to a greater sensitivity of Mash1, when compared with Ngns, to Notch-mediated inhibition of neurogenesis [49,58,62]. Here, we show that Ngns were able to override the mitogenic effects of bFGF and to rapidly downregulate proliferation of hNPCs. This was especially true for Ngn1 and Ngn3, which, by 4 days posttransduction, induced an almost complete inhibition of hNPC proliferation, in parallel with a considerable decrease in immature Nestin+/GFAP− neuroepithelial-like cells. On the contrary, Mash1 overexpression maintained the progenitors in a proliferative state and had little (HFC6) or no effect (HFC7) on the pool of Nestin+/GFAP− cells. Thus, in a therapeutic perspective, Ngns may be used when limited proliferation and rapid neuronal commitment are required, whereas Mash1 may allow, together with a rapid neuronal restriction of hNPC differentiation potential, a prolonged proliferation and possibly extended migration of the committed progenitors.
We further evaluated the ability of each factor tested to favor neuronal subtype specification. In contrast to studies suggesting that GABAergic differentiation was the major, if not the only, default differentiation pathway for hNPCs of various CNS origins [21,63,64], we showed that, even after amplification, fetal cortical hNPCs were able to spontaneously give rise to a variety of neuronal subtypes in vitro, including GABAergic, adrenergic, cholinergic, and motor neurons. After proneural gene overexpression, transduced hNPCs gave rise to the same range of neuronal subtypes, suggesting that Ngns and Mash1 were not sufficient to redirect hNPC differentiation toward one specific neuronal fate in vitro. This is consistent with studies performed in rodents, which indicated that the combinatorial action of proneural genes with other genetic determinants was required for the specification of various neuronal subtypes [40,57,61]. Nevertheless, our results suggest that Ngns and Mash1 share a proneural activity on hNPCs in vitro and each of these genes also displays its proper ability to interact with other factors to specify different neuronal subtypes at different rates and following different patterns. Thus, Ngn2 overexpression simultaneously promoted GABAergic, cholinergic, and motor neuron emergence from hNPC cultures, whereas Ngn3 preferentially promoted GABAergic and motor neuron production and displayed a much weaker effect on cholinergic neurons. Mash1 also promoted GABAergic, cholinergic, and motor neuron specification, but its effect was less obvious than that of Ngn2 and 3, with both a lower proportion of positive neurons and a weaker expression of the markers tested, which was probably related to the particular ability of this factor to maintain the progenitors in an immature, proliferative state. Finally, Ngn2, Ngn3, and Mash1 had no effect on adrenergic and serotoninergic neuron production, whereas Ngn1 had little or no effect on all lineages tested. Ultimately, the generation of a specific neuronal subtype may require a combination of different strategies, including genetic induction of neuronal commitment, as was performed here, and either addition of other transcription factors [35,40,61,65] or epigenetic manipulation of the cells [13,29,66].
Most interestingly, Ngn2 and Mash1 not only promoted neuronal commitment of the hNPCs but also seemed to play a role in glial pathways. Thus, Ngn2 increased astrocyte generation in 2 independent experiments from HFC7 and HFC6 cells, whereas Mash1 promoted the emergence of A2B5+/MAP5− oligodendroglial progenitors, along with scarce O4+ oligodendrocytes. Previous studies highlighted the dual role of Mash1 in the generation of neurons and oligodendrocytes within the developing forebrain and spinal cord [40,67,68]. In our study, although hNPCs were isolated at a time when human OPCs have not yet emerged within the dorsal telencephalon [47], they were able to produce oligodendrocytes through Mash1 overexpression. This suggests that, beyond its proneural activity, Mash1 was able to act as an instructive factor for human OPC specification and to override the intrinsic properties of the cells. This observation may be of crucial importance for the design of therapeutic strategies in myelin diseases. Indeed, cell transplantation in these pathologies has long been hampered by the difficulty to derive oligodendroglial cells from human neural stem/progenitor cells [69]. Although Olig2 promoted OPC emergence from fetal hNPCs [44], overexpression of both Olig2 and Mash1 in rodent NSC clones gave rise to higher levels of oligodendrocytes than Olig2 overexpression alone [40]. This suggests that overexpression of both these genes in human NPCs may significantly yield more oligodendrocytes than Olig2 or Mash1 alone, which may, therefore, consistently improve the potential of hNPCs to reverse the course of demyelinating diseases.
The role of Ngn2 in the astroglial lineage is less clear. Our results are at variance with previous studies reporting that in vitro overexpression of Ngns promoted the exclusive differentiation of multipotent or pluripotent stem cells into neurons [57,70,71] and that overexpression of Ngn2 in spinal cord–derived rodent NPCs dramatically reduced astroglial differentiation and astroglial-mediated side effects after transplantation in a model of spinal cord trauma [39]. During telencephalon development, the timing of astrogliogenesis is regulated in part by accumulation of gliogenic cytokines, including CNTF, LIF, and cardiotrophin-1 [72]. These factors, secreted by newborn neurons during late neurogenesis, activate the JAK-STAT signaling pathway [29,73,74], which ensures, when sufficient levels of cytokines are accumulated, the transition from neurogenesis to astrogliogenesis [72,75]. In our study, among the genes tested, Ngn2 was the most efficient in promoting a clear maturation of neurons and may, therefore, have prompted the accumulation of sufficient amounts of cytokines to promote a negative feedback loop and subsequent generation of astrocytes. Moreover, along with their ability to maintain neural stem cells in an undifferentiated state, bFGF and EGF have been shown to promote the acquisition by cortical progenitors of a competence to respond to cytokine signaling and differentiate into astrocytes [55,76,77]. The fact that hPNCs were amplified as neurospheres in the presence of these growth factors may also have induced a greater sensitivity of hNPCs to cytokine-induced astroglial differentiation. However, evidence has been provided that Ngns, in parallel to their proneural activity, inhibit the generation of astrocytes by at least 2 complementary mechanisms that intervene downstream cytokine signaling [71,78,79]. Ngns induce the sequestration of the gliogenic complex SMAD1/CBP/P300 away from the GFAP promoter and repress components of the JAK/STAT pathway [71]. These mechanisms are highly dependent on the levels of expression of Ngns, which, upon downregulation, play a permissive role in astrogliogenesis. However, in our study, Ngn2 expression was under the control of the ubiquitous PGK promoter, which yielded high levels of transgene expression, and it is, therefore, very unlikely that Ngn levels of expression were at some point low enough to promote astrocyte generation. Finally, maintaining NPCs as 3-dimensional neurospheres—wherein a large degree of unobservable cellular cross-talk occurs—may yield results that would not be seen when the cells were maintained in monolayer. Thus, the reason why Ngn2 overexpression yielded astrocyte production in human fetal telencephalic NPC cultures remains to be answered in future studies.
Conclusion
CNS-derived multipotent somatic stem cells present an advantage over pluripotent stem cells [7,15] in that they are already “neurally committed” and lack tumorigenicity after grafting. Their major barrier to widespread use has been rapid expandability and scalability. Here, we provide evidence that human neural stem/progenitor cells can be efficiently, rapidly, and safely expanded in vitro as well as rapidly differentiated toward mature neural (typically neuronal) lineages by the overexpression of select proneural genes. Our study further highlights the individual roles of Ngn1, Ngn2, Ngn3, and Mash1 in hNPC proliferation, neuronal versus glial commitment, and terminal differentiation. Deciphering these aspects of hNPC development constitutes a crucial step toward the establishment of large-scale, valuable donor cell sources and may allow the design of experimental models for the treatment of a range of CNS diseases.
Supplementary Material
Acknowledgments
The authors thank Dr. A. Baron-Van Evercooren for the generous gift of A2B5 and O4 antibodies. This work was supported by the Centre National de la Recherche Scientifique (CNRS) and Université Pierre et Marie Curie. A.S. and E.Y.S. were supported by grants from the Sanford Children's Research Center, Children's Neurobiological Solutions, and National Institutes of Health (USA). D.B. was supported by grants from European Leucodystrophy Association (ELA), Association Pour l'Education Thérapeutique et la Réadaptation des Enfants Infirmes moteurs Cérébraux (APETREIMC), La Fondation Motrice, and the Institut pour la Recherche sur la Moelle épinière et l'Encéphale (IRME).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Goldman SA. Windrem MS. Cell replacement therapy in neurological disease. Philos Trans R Soc Lond B Biol Sci. 2006;361:1463–1475. doi: 10.1098/rstb.2006.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SU. de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 3.Rosser AE. Zietlow R. Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007;20:688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- 4.Lee JP. Jeyakumar M. Gonzalez R. Takahashi H. Lee PJ, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 5.Snyder EY. Taylor RM. Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 6.Yandava BD. Billinghurst LL. Snyder EY. “Global”. cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reubinoff BE. Itsykson P. Turetsky T. Pera MF. Reinhartz E, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 8.Buc-Caron MH. Neuroepithelial progenitor cells explanted from human fetal brain proliferate and differentiate in vitro. Neurobiol Dis. 1995;2:37–47. doi: 10.1006/nbdi.1995.0004. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter MK. Cui X. Hu ZY. Jackson J. Sherman S, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158:265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- 10.Palmer TD. Schwartz PH. Taupin P. Kaspar B. Stein SA, et al. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 11.Svendsen CN. ter Borg MG. Armstrong RJ. Rosser AE. Chandran S, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 12.Vescovi AL. Parati EA. Gritti A. Poulin P. Ferrario M, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- 13.Redmond DE., Jr. Bjugstad KB. Teng YD. Ourednik V. Ourednik J, et al. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson PS. Perfilieva E. Bjork-Eriksson T. Alborn AM. Nordborg C, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Hu BY. Weick JP. Yu J. Ma LX. Zhang XQ, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu BY. Du ZW. Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izrael M. Zhang P. Kaufman R. Shinder V. Ella R, et al. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Roy NS. Cleren C. Singh SK. Yang L. Beal MF, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 19.Aubry L. Bugi A. Lefort N. Rousseau F. Peschanski M, et al. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keene CD. Chang RC. Leverenz JB. Kopyov O. Perlman S, et al. A patient with Huntington's disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol. 2009;117:329–338. doi: 10.1007/s00401-008-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain M. Armstrong RJ. Tyers P. Barker RA. Rosser AE. GABAergic immunoreactivity is predominant in neurons derived from expanded human neural precursor cells in vitro. Exp Neurol. 2003;182:113–123. doi: 10.1016/s0014-4886(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 22.Wright LS. Prowse KR. Wallace K. Linskens MH. Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Keyoung HM. Roy NS. Benraiss A. Louissaint A., Jr. Suzuki A, et al. High-yield selection and extraction of two promoter-defined phenotypes of neural stem cells from the fetal human brain. Nat Biotechnol. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- 24.Roy NS. Wang S. Harrison-Restelli C. Benraiss A. Fraser RA, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida N. Buck DW. He D. Reitsma MJ. Masek M, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Windrem MS. Schanz SJ. Guo M. Tian GF. Washco V, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu BY. Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu BY. Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johe KK. Hazel TG. Muller T. Dugich-Djordjevic MM. McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 30.Perrier AL. Tabar V. Barberi T. Rubio ME. Bruses J, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SC. Wernig M. Duncan ID. Brustle O. Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 32.Chung S. Sonntag KC. Andersson T. Bjorklund LM. Park JJ, et al. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH. Auerbach JM. Rodriguez-Gomez JA. Velasco I. Gavin D, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 34.Kim JY. Koh HC. Lee JY. Chang MY. Kim YC, et al. Dopaminergic neuronal differentiation from rat embryonic neural precursors by Nurr1 overexpression. J Neurochem. 2003;85:1443–1454. doi: 10.1046/j.1471-4159.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 35.Park CH. Kang JS. Kim JS. Chung S. Koh JY, et al. Differential actions of the proneural genes encoding Mash1 and neurogenins in Nurr1-induced dopamine neuron differentiation. J Cell Sci. 2006;119:2310–2320. doi: 10.1242/jcs.02955. [DOI] [PubMed] [Google Scholar]
- 36.Sakurada K. Ohshima-Sakurada M. Palmer TD. Gage FH. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult brain. Development. 1999;126:4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- 37.Muramatsu D. Sato Y. Hishiyama S. Miyamoto Y. Hisatsune T. Transplantation of GABAergic neurons into adult mouse neocortex. Exp Neurol. 2005;194:1–11. doi: 10.1016/j.expneurol.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Oishi K. Watatani K. Itoh Y. Okano H. Guillemot F, et al. Selective induction of neocortical GABAergic neurons by the PDK1-Akt pathway through activation of Mash1. Proc Natl Acad Sci USA. 2009;106:13064–13069. doi: 10.1073/pnas.0808400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofstetter CP. Holmstrom NA. Lilja JA. Schweinhardt P. Hao J, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 40.Sugimori M. Nagao M. Bertrand N. Parras CM. Guillemot F, et al. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development. 2007;134:1617–1629. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- 41.Copray S. Balasubramaniyan V. Levenga J. de Bruijn J. Liem R, et al. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006;24:1001–1010. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- 42.Bertrand N. Castro DS. Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 43.Ross SE. Greenberg ME. Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 44.Maire CL. Buchet D. Kerninon C. Deboux C. Baron-Van Evercooren A, et al. Directing human neural stem/precursor cells into oligodendrocytes by overexpression of Olig2 transcription factor. J Neurosci Res. 2009;87:3438–3446. doi: 10.1002/jnr.22194. [DOI] [PubMed] [Google Scholar]
- 45.Zennou V. Serguera C. Sarkis C. Colin P. Perret E, et al. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol. 2001;19:446–450. doi: 10.1038/88115. [DOI] [PubMed] [Google Scholar]
- 46.Doetsch F. Caille I. Lim DA. Garcia-Verdugo JM. Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 47.Buchet D. Garcia C. Deboux C. Nait-Oumesmar B. Baron-Van Evercooren A. Human neural progenitors from different foetal forebrain regions remyelinate the adult mouse spinal cord. Brain. 2011;134:1168–1183. doi: 10.1093/brain/awr030. [DOI] [PubMed] [Google Scholar]
- 48.Fode C. Ma Q. Casarosa S. Ang SL. Anderson DJ, et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 49.Lo L. Dormand E. Greenwood A. Anderson DJ. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- 50.Parras CM. Schuurmans C. Scardigli R. Kim J. Anderson DJ, et al. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cau E. Gradwohl G. Fode C. Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- 52.Horton S. Meredith A. Richardson JA. Johnson JE. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci. 1999;14:355–369. doi: 10.1006/mcne.1999.0791. [DOI] [PubMed] [Google Scholar]
- 53.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 54.Panchision DM. McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12:478–487. doi: 10.1016/s0959-437x(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 55.Qian X. Shen Q. Goderie SK. He W. Capela A, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 56.Shirasaki R. Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- 57.Farah MH. Olson JM. Sucic HB. Hume RI. Tapscott SJ, et al. Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development. 2000;127:693–702. doi: 10.1242/dev.127.4.693. [DOI] [PubMed] [Google Scholar]
- 58.Ma Q. Kintner C. Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 59.Novitch BG. Chen AI. Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 60.Helms AW. Battiste J. Henke RM. Nakada Y. Simplicio N, et al. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HJ. Sugimori M. Nakafuku M. Svendsen CN. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007;203:394–405. doi: 10.1016/j.expneurol.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 62.Chitnis A. Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122:2295–2301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- 63.Kabos P. Kabosova A. Neuman T. Blocking HES1 expression initiates GABAergic differentiation and induces the expression of p21(CIP1/WAF1) in human neural stem cells. J Biol Chem. 2002;277:8763–8766. doi: 10.1074/jbc.C100758200. [DOI] [PubMed] [Google Scholar]
- 64.Yan J. Xu L. Welsh AM. Hatfield G. Hazel T, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roybon L. Hjalt T. Christophersen NS. Li JY. Brundin P. Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, Msx1, Ngn2, and Pitx3. J Neurosci. 2008;28:3644–3656. doi: 10.1523/JNEUROSCI.0311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu P. Tarasenko YI. Gu Y. Huang LY. Coggeshall RE, et al. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 67.Parras CM. Galli R. Britz O. Soares S. Galichet C, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parras CM. Hunt C. Sugimori M. Nakafuku M. Rowitch D, et al. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci. 2007;27:4233–4242. doi: 10.1523/JNEUROSCI.0126-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buchet D. Baron-Van Evercooren A. In search of human oligodendroglia for myelin repair. Neurosci Lett. 2009;456:112–119. doi: 10.1016/j.neulet.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 70.Falk A. Holmstrom N. Carlen M. Cassidy R. Lundberg C, et al. Gene delivery to adult neural stem cells. Exp Cell Res. 2002;279:34–39. doi: 10.1006/excr.2002.5569. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y. Nadal-Vicens M. Misono S. Lin MZ. Zubiaga A, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 72.Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Barnabe-Heider F. Wasylnka JA. Fernandes KJ. Porsche C. Sendtner M, et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–265. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 74.Bonni A. Sun Y. Nadal-Vicens M. Bhatt A. Frank DA, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 75.Rajan P. McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burrows RC. Wancio D. Levitt P. Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 77.Viti J. Feathers A. Phillips J. Lillien L. Epidermal growth factor receptors control competence to interpret leukemia inhibitory factor as an astrocyte inducer in developing cortex. J Neurosci. 2003;23:3385–3393. doi: 10.1523/JNEUROSCI.23-08-03385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nieto M. Schuurmans C. Britz O. Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401–413. doi: 10.1016/s0896-6273(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 79.Tomita K. Moriyoshi K. Nakanishi S. Guillemot F. Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. Embo J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.