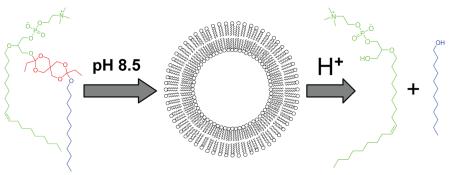
Abstract
The use of pH-sensitive liposomes for acid-triggered site specific drug delivery is one of the more promising approaches to improve the therapeutic index of drugs. Here, we report the synthesis, assembly, and hydrolysis of the first ortho ester phosphocholine (OEPC). The acid hydrolysis of OEPC liposomes consists of a lag phase and a burst phase. The lag time is pH-dependent—the lower the pH, the shorter the time. Upon acid hydrolysis, the OEPC liposomes were transformed into leaky metastable vesicles that rapidly collapsed in the presence of albumin. OEPC, when formulated with cationic lipid, significantly enhanced the transfection efficiency compared with the pH-insensitive formulation.
Low-pH-sensitive liposomes are of particular interest for the site-specific release of therapeutics. Ideally, these liposomes should be relatively stable in the circulation under the physiological pH, but be triggered to release their therapeutic contents upon the exposure to the decreased pH at the target. Numerous efforts have been made toward the ideal pH-sensitive liposome system,1 principally through two different approaches: 1) formulations containing polymorphic lipids or fusion proteins;2 2) lipids containing acid-cleavable component. The first approach has been intensively studied, but may be the least useful for the in vivo application due to the intrinsic limitation such as instability in the presence of serum and rapid clearance from the circulation.
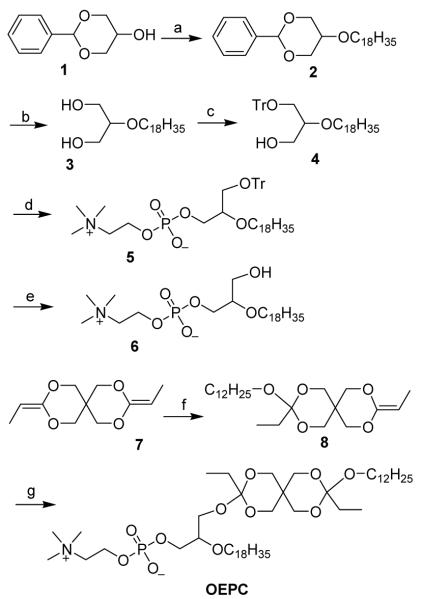
A variety of pH-sensitive lipids have been developed containing different pH-sensitive linkers such as: acetal, ketal, vinyl ether, and ortho ester. 3-5 Of all these linkers, the ortho ester is the most promising one when high pH-sensitivity is desired. Two classes of pH-sensitive cationic lipids have been synthesized containing either a 2,6,7-trioxabicyclo[2.2.2]octane ring or a dioxazocinium ortho ester.6, 7 The cationic head group is cleaved upon the acid hydrolysis of the ortho ester, resulting in the release of the condensed DNA. We developed a convenient one-pot synthesis of polyethylene glycol (PEG)-diortho ester-lipid conjugate (POD).4 This method has been further optimized and applied to the synthesis of POD with different PEG chain length or unsaturated lipid.8, 9 Masson et al. has reported another class of PEG-ortho ester-lipid conjugates containing the monocyclic ortho ester linkage.10 Here, we report the first example of ortho ester phosphocholine (OEPC) and its use to form liposome (Scheme 1).
Scheme 1.
Synthesis of OEPC
We desired to create a pH-sensitive lipid that would mediate a lamellar phase to micelle transition at low pH. The trapping of titratable detergent in lysosome is a well established method to disrupt the lysosomal membrane,11 but vesicles stable in biological fluid can not be prepared from detergents. In this report, the highly pH-sensitive diortho ester functional group was used to conjugate dodecanol to the 1-position of the glycerol backbone, and the 2-position was etherized with an oleyl chain. Theoretically, the acid hydrolysis of the ortho ester will convert OEPC from a double chain amphiphile to a single chain lyso-phosphocholine (lyso-PC), transforming the OEPC liposome into the lyso-PC micelle.
The synthesis of OEPC is straightforward but requires careful attention on handling the highly pH-sensitive diortho ester. 1,3-benzylidene glycerol (1) was etherized with oleyl mesylate to afford 2, and followed by acid hydrolysis to give 2-oleyl glycerol (3). Subsequent tritylation of the 1-hydroxyl group led to 4. The one-pot phosphorylation of 4 and coupling with choline tetraphenyl borate afforded 5. The lyso-PC (6) was obtained after the removal of the trityl group. For a better yield of OEPC, diketene acetal (7) was first treated with slightly excess dodecanol, then reacted directly in large excess (more than 10 fold) with 6. This approach proved to be an effective way to attach ortho ester side chain to the 1-hydroxyl group of the lyso-PC (40% yield).
Since OEPC is designed for the acid triggered drug delivery, we are most interested in the following questions. 1) Can OEPC form liposomes through self-assembly? 2) If OEPC does form liposome, can acid trigger the content release and how fast? 3) Will the liposome be transformed into micelle upon acid hydrolysis? To answer these questions, we hydrated the OEPC film in the pH 8.5 HEPES buffer with 50 mM 8-aminonaphthalene-1,3,6-trisulphonic acid (ANTS) and p-xylene-bispyridinium bromide (DPX) using the freeze-thaw method.12 After extrusion and column purification, vesicles of 154 nm were obtained with quenched ANTS/DPX encapsulated. This is a strong evidence of the formation of OEPC liposomes. To determine the pH-sensitivity of the OEPC liposomes, we incubated the purified vesicles in different pH buffers to monitor the content release (Figure 1). When ANTS is released from the liposome, it is no longer quenched by DPX, resulting in increased fluorescence signal. The release of ANTS from the OEPC liposomes consists of two distinct phases—a lag phase where a small portion of ANTS slowly leaks out, and a burst phase when ANTS is quickly released. The lag time (the transition point from the shallow slope to the steep slope)4, 13 is pH-dependent—the lower the pH, the shorter the time. In Figure 2, we plot the logarithm of the lag time versus the buffer pH and observe a linear correlation (r = 0.999). The pH-dependent leakage of OEPC liposomes is very similar to our previous observation on the POD liposomes but with a relatively shorter lag time at the same pH. This difference may come from the different destabilization mechanisms between the OEPC liposomes and the POD liposomes.
Figure 1.
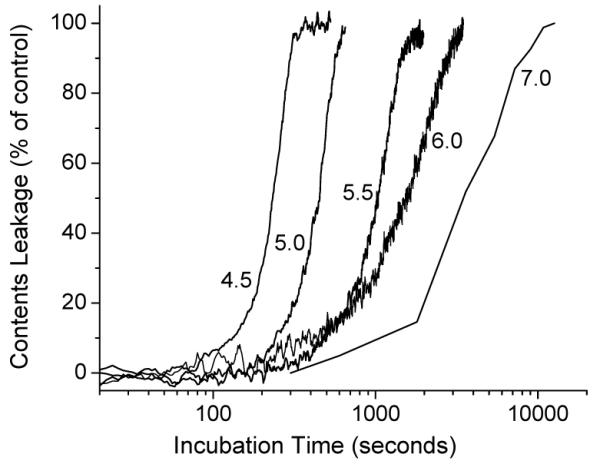
pH-dependent leakage of OEPC liposomes. Percentage of leakage over time in glucuronate buffer (pH 4.5, 5.0, and 5.5) and phosphate buffer (pH 6.0 and 7.0).
Figure 2.
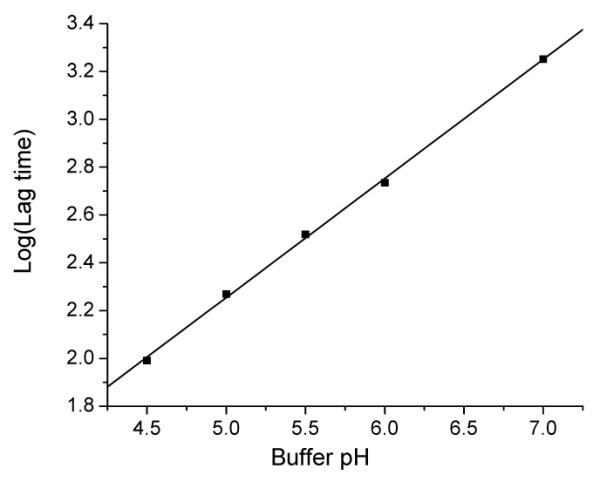
Logarithm of lag time (second) of OEPC liposomes at various pH. Linear regression for the data: Log (Lag time) = −0.236 + 0.498 × pH, r = 0.999, and P < 0.0001.
For the POD liposomes, when the amount of surface POD is lowered to a critical level, the lamellar-to-inverted hexagonal phase transition is initiated in the phosphatidylethanolamine, and the liposomes aggregate and collapse rapidly. As expected, the OEPC liposomes do not spontaneously collapse under the acidic conditions. Moreover, unlike POD-DOPE vesicles, they do not show a concentration dependent release rate at low pH (supporting information). This is the evidence for a non-contact mediated content release.14 When the OEPC liposomes were incubated in the pH 5.2 buffer, the average particle diameter decreased from 154 nm to 130 nm in 10 min, then stabilized for more than 10 h even at pH 1.0. TLC analysis showed that OEPC was completely hydrolyzed to the lyso-PC. However, the hydration of a lipid film of lyso-PC/dodecanol (mole ratio 1:1) only led to micelles instead of liposomes. We then added albumin into the OEPC liposomes at pH 5.2 and pH 8.5, respectively, and found that particles in the pH 5.2 buffer immediately fell apart while those in pH 8.5 buffer were unchanged. Based on these observations, we believe that the acid hydrolysis of OEPC liposomes ultimately leads to leaky metastable vesicles consisting of lyso-PC and dodecanol. When the amount of lyso-PC reaches a critical level, pores form on the surface of the vesicle, resulting in rapid content release. Eventually, the metastable vesicles can be completely destroyed when the lyso-PC and dodecanol are removed from the bilayer by the albumin or the interaction with other in vivo components.
OEPC is a new class ortho ester lipid with a biocompatible phosphocholine headgroup. Highly pH-sensitive and biocompatible, OEPC or its similar derivatives have great potential for the application in drug and gene delivery. For example, when formulated with cationic lipids, OEPC may facilitate the gene release from the acidic endosome to the cytosol in a timely manner, minimizing the lysosomal gene degradation. Our preliminary data showed that OEPC significantly enhanced the in vitro transfection efficiency compared with the pH-insensitive phosphocholine when formulated with cationic lipids (8.24×105 versus 3.95×104 RLU/mg protein).
In summary, we have designed and synthesized a highly pH-sensitive diortho ester phosphocholine (OEPC). The acid hydrolysis of OEPC liposome is fast and pH-dependent, and the liposomes are transformed into leaky metastable vesicles that quickly collapsed upon the interaction with albumin. The application of OEPC and its derivatives on acid-triggered drug and gene delivery is promising.
Supplementary Material
Acknowledgment
This work was supported by NIH EB003008 and GM61851. Zhaohua Huang is partially supported by the UCSF Cystic Fibrosis Foundation. We dedicate the paper to Dr. Jorge Heller at A.P. Pharma (Redwood City, CA) on his 79th birthday for the generous gift of 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5,5]undecane. Thanks are also extended to the UCSF Mass Spectrometry Facility (A.L. Burlingame, Director) supported by NIH NCRR RR01614.
Footnotes
Supporting Information Available: Synthetic and characterization details for all compounds and experimental protocols for liposome studies. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Drummond DC, Zignani M, Leroux J. Prog. Lipid. Res. 2000;39:409–460. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- (2).Simoes S, Moreira JN, Fonseca C, Duzgunes N, de Lima MC. Adv. Drug Deliv. Rev. 2004;56:947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- (3).Guo X, Szoka FC. Accounts Chem. Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- (4).Guo X, Szoka FC. Bioconjug. Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- (5).Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Adv. Drug Deliver. Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- (6).Zhu J, Munn RJ, Nantz MH. J. Am. Chem. Soc. 2000;122:2645–2646. [Google Scholar]
- (7).By K, Nantz MH. Angew. Chem. Int. Edit. 2004;43:1117–1120. doi: 10.1002/anie.200352589. [DOI] [PubMed] [Google Scholar]
- (8).Guo X, Huang Z, Szoka FC. Methods Enzymol. 2004;387:147–152. doi: 10.1016/S0076-6879(04)87009-5. [DOI] [PubMed] [Google Scholar]
- (9).Li W, Huang Z, MacKay JA, Grube S, Szoka FC., Jr. J. Gene Med. 2005;7:67–79. doi: 10.1002/jgm.634. [DOI] [PubMed] [Google Scholar]
- (10).Masson C, Garinot M, Mignet N, Wetzer B, Mailhe P, Scherman D, Bessodes M. J. Control. Release. 2004;99:423–434. doi: 10.1016/j.jconrel.2004.07.016. [DOI] [PubMed] [Google Scholar]
- (11).Firestone RA, Pisano JM, Bonney RJ. J. Med. Chem. 1979;22:1130–1133. doi: 10.1021/jm00195a026. [DOI] [PubMed] [Google Scholar]
- (12).Monnard PA, Oberholzer T, Luisi P. Biochim. Biophys. Acta. 1997;1329:39–50. doi: 10.1016/s0005-2736(97)00066-7. [DOI] [PubMed] [Google Scholar]
- (13).Guo X, MacKay JA, Szoka FC. Biophys. J. 2003;84:1784–1795. doi: 10.1016/s0006-3495(03)74986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ellens H, Bentz J, Szoka FC. Biochemistry. 1985;24:3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.