Abstract
During the past 15 years, an impressive amount of genetic information has become available in the research field of psychiatry, particularly as it relates to attention-deficit/hyperactivity disorder (ADHD). However, the classical clinical approach to ADHD has minimally affected and not significantly been improved by this genetic revolution. It is difficult to predict how long it will take for genetic findings to alter the way clinicians treat patients with ADHD. New medications or treatment protocols may take years to become routine clinical practice. However, when taken together, recent successes in genomics, pharmacogenomics, and genetic epidemiology have the potential (1) to prevent comorbid consequences of ADHD, (2) to individualize therapies for patients with ADHD, and (3) to define new epidemiological policies to aid with the impact of ADHD on society. Here, we present an overview of how genetic research may affect and improve the quality of life of patients with ADHD: as an example, we use the discovery of LPHN3, a new gene in which variants have recently been shown to be associated with ADHD.
Keywords: ADHD, Complex trait, Gene, Genetics, LPHN3, Latrophilin
Human genetics and genomic medicine in today’s world
The field of human genetics, particularly medical genetics and genomic medicine, has become one of the most active, successful, and exciting areas of research in modern medicine. Just as astrophysicists have the Hubble Space Telescope to study astronomy and the Large Hadron Collider to investigate the laws of physics, geneticists now have technology to better understand fundamental aspects of inheritance and human development. This has been used to understand variation of some relatively complex phenotypes (Cadieu et al. 2009), this technology will help to define the many “bricks” we will need to understand and build human complex phenotypes, human behavior being one of them.
Several milestones in the field of genetics are particularly relevant: (1) the completion of the sequencing of the human genome through the Human Genome Project in 2001, the availability of further human genome reference sequences in 2003 (Lander et al. 2001), and the publication of many other genome sequences of several individuals ascertained from diverse populations (Ledford 2010); (2) the completion of the HapMap project to identify genetic similarities and differences between people, with the latest generation HapMap including more than 3.1 million single nucleotide polymorphisms (SNPs) (Manolio and Collins 2009) and a summary database that can explain the remaining ungenotyped variations of the human genome; (3) the discovery of new technologies and methodologies, largely based on advances in nanotechnology, that have greatly reduced the required time and cost of genotyping millions of variants in thousands of individuals; (4) the availability of hundreds of publicly accessible databanks that contain information such as genome sequences, maps, and intra- and interspecies genomic variations; (5) the ability to define potential functionally important variations, such as by characterizing the entire human exome in a small number of individuals to define mutational causes of Mendelian disorders (Choi et al. 2009; Ng et al. 2009); and (6) the ability to perform more refined phenotypic descriptions, whether normal or abnormal, using advance technology, such as magnetic resonance imaging (MRI) in the context of psychiatric conditions (Wong et al. 2008; Kieling et al. 2008).
All of these landmark developments have made the identification of genetic susceptibilities to highly prevalent diseases and complex traits possible, such as type 2 diabetes, various types of cancers, heart disease, Crohn’s disease, bipolar disorder (Manolio et al. 2007; Collins and Manolio 2007), and many others. However, definition of these genomic variants largely implies that a proxy functional variant is the real cause of either the disease or the complex trait. Therefore, a detailed dissection of these associated genomic regions by means of further genetic sequencing and/or functional studies would provide crucial knowledge regarding the physiological causes and potentially effective treatments and methods of prevention of many common diseases (Wong et al. 2008; Manolio et al. 2009).
Attention-deficit/hyperactivity disorder (ADHD)
ADHD, the most common behavioral disorder of childhood, affects 8–12% of children worldwide (Spencer et al. 2007; Arcos-Burgos and Acosta 2007). The disorder is defined as a persistent syndrome characterized by inattention, excessive motor activity, and impulsivity. Affected individuals are at increased risk for poor educational achievement, low income, underemployment, legal difficulties, and impaired social relationships (Spencer et al. 2007; Arcos-Burgos and Acosta 2007). A conservative cost estimate, based on an ADHD prevalence of 5%, established that the cost attributable to ADHD in the United States alone is approximately $42.5 billion per year (Lee et al. 2007). Although ADHD does occur as an isolated disorder in a minority of individuals, it is usually comorbid with other behavioral and emotional disorders, such as oppositional defiant disorder (ODD), conduct disorder (CD), and substance abuse (Jain et al. 2007; Palacio et al. 2004).
ADHD and genetic causes
Even though it is clear that there are multiple environmental risk factors significantly related to the development of ADHD, there is now overwhelming evidence that genetics can help explain most of the variability in terms of susceptibility to ADHD (Pineda et al. 2007; Gizer et al. 2009; Waldman and Gizer 2006). Twin studies, adoption studies, and epidemiological studies including relative risk estimates and segregation analyses have demonstrated that there is a significantly great genetic contribution to ADHD when compared to environmental factors (Biederman et al. 1992; Maher et al. 1999; Lopera et al. 1999; van den Oord et al. 1994; Reiersen et al. 2008; Rasmussen et al. 2002; Neuman et al. 2001; Willcutt et al. 1999).
In fact, several of these studies have estimated that the heritability of ADHD is around 70%, a strikingly high figure when compared to the heritability estimates for many other complex disorders such as diabetes, non-syndromic facial clefting, and dementia (Manolio et al. 2009; Marazita et al. 1983, 1984, 1986). This high heritability, as estimated by several complex segregation analyses, is in complete agreement with a model based upon major gene effects. This strongly suggests an oligogenic model, a scenario in which a small and finite number of genes of moderate, but not necessarily equal, effects are involved (Schliekelman and Slatkin 2002). This is in contrast to a multifactorial threshold model (Elston and Yelverton 1975; Stricker et al. 1995; Iyengar et al. 2004), which incorporates an infinite number of genes with small and equal effects, plus the presence of an environmental trigger. The detection of these quasi-Mendelian, moderate-effect factors has compelled many groups, including our own, to design genetic epidemiology-based protocols to dissect these loci, or chromosomal positions thought to include the genes at play. Such protocols aim to link a disorder to a specific locus, with the power of detection dependent on the relative risk attributable to that locus. The power to detect linkage deteriorates quickly as the number of loci increases (Faraone et al. 2000; Guo and Elston 2000; Risch 1990a, b, c, d).
Family-based versus case–control designs: association and/or linkage of ADHD to candidate genes and genomic regions
The substantial amount of genetic-epidemiological data prompted a large number of studies aimed at linking gene variants with ADHD. Family-based and case–control-based designs were then used to investigate the presence of linkage and/or association of ADHD to either candidate genes or genomic regions. Risch et al. have reviewed the pros and cons of using families that cluster a high number of relatives with ADHD, compared to using ADHD cases and controls ascertained from populations (Risch and Merikangas 1993; Risch and Teng 1998; Risch and Zhang 1995). In summary, although case–control-based studies have a higher statistical power and are easier in terms of recruiting cases, family-based studies avoid the problem of genetic stratification and genetic heterogeneity (Arcos-Burgos et al. 2002).
Following studies similar to those mentioned earlier, either genetic variants or genomic regions were found to be in association and/or linkage with ADHD. Impressive meta-analytical overviews have been performed, and several of these findings showed replication (Gizer et al. 2009; Faraone and Mick 2010; Coghill and Banaschewski 2009; Smith et al. 2009). A summary of several of these genes significantly associated and/or linked to ADHD by meta-analyses is presented in Table 1. As far as we are aware, three genome-wide association studies (GWAS) reports disclosed nominal associations. But none of them reached significance after correcting by multiple testing (Lesch et al. 2008; Elia et al. 2009; Franke et al. 2009).
Table 1.
Candidate genes in ADHD
| Gene | Type of study |
Variant | Chromosomal location |
Cases\trans | Controls\untrans | P value | Odds ratio (IC) | Replicated | Comments and references |
|---|---|---|---|---|---|---|---|---|---|
| DRD4 | C/C | 48 bp VNTR-7R allele | 11 | NA | NA | 1 × 10E−3 | 1.9 (1.5–2.2) | + | Meta-analyses 8 studies (Faraone et al. 1999, 2001) |
| DRD4 | FB | 48 bp VNTR-7R allele | 11 | NA | NA | 2 × 10E−5 | 1.41 (1.2–1.64) | + | Meta-analyses 13 studies (Faraone et al. 1999, 2001) |
| DRD4 | FB and C/C | 48 bp VNTR-7R allele | 11 | NA | NA | 2 × 10E−12 | 1.34 (1.23–1.45) | + | Meta-analyses 33 studies (Thapar et al. 2005) |
| DRD5 | FB and C/C | 148 bp CA(n) microsatellite marker | 4 | NA | NA | 8 × 10E−8 | 1.34 (1.21–1.50) | + | Meta-analyses 9 studies (Li et al. 2006) |
| DRD5 | FB | 148 bp CA(n) microsatellite marker | 4 | NA | NA | 8 × 10E−5 | 1.57 (1.25–1.96) | + | Meta-analyses 5 studies (Maher et al. 2002) |
| DRD5 | FB | 148 bp CA(n) microsatellite marker | 4 | NA | NA | 5 × 10E−5 | 1.24 (1.12–1.38) | + | Meta-analyses 14 studies (Lowe et al. 2004) |
| DDC | C/C | rs6592961 | 7 | 142/109/10a | 288/98/12a | 1 × 10E−5 | 2.22 (1.60–3.08)b | NA | Unique study (Ribases et al. 2009) |
| SLC6A3 (DAT1) | FB | Haplotype inside the gene | 5p13 | 168 | 99 | 3.4 × 10E−5 | 1.95 (1.05–3.63) | – | Friedel et al. (2007) and Brookes et al. (2006) |
| SLC6A4 (5-HTT) | FB | STin2.12 (A12) | 17q11.1 | 56 | 174 | 0.008 | 3.00 (1.53–5.90) | – | Unique study (Banerjee et al. 2006) |
| SLC6A4 (5-HTT) | FB | Promoter 3′UTR SNP | 17q11.1 | 65 | 49 | 0.004 | – | Unique study (Kent et al. 2002) | |
| BDNF | FB | rs6265 (Val66Met) | 11p13 | 10 | 32 | 0.0005 (paternal only) | 3.2 | – | Unique study (Kent et al. 2005) |
| TPH2 | FB | rs1843809 (G-T) | 12q15 | 52 | 22 | 0.0006 | 2.36 | – | Brookes et al. (2006), Sheehan et al. (2005) |
| ARRB2 | FB | Undisclosed | 17p13 | 103 | 66 | 0.004 | 1.56 | – | Unique study (Brookes et al. 2006) |
| PNMT | FB | Undisclosed | 17q21 | 70 | 42 | 0.008 | 1.67 | – | Unique study (Brookes et al. 2006) |
| MAOA | Alleles 3,4,5? | Xp11.23 | NA | NA | 0.0007 | – | – | Unique study (Manor et al. 2002) | |
| AR | C/C | Haplotype CAGL/GGCL | Xq11–12 | 133 | 52 | 0.0001 | – | – | Not replicated in Hispanic Caucasians (Comings et al. 1999) |
| SLC6A4 (5-HTT) | FB | 5-HTTLPR Short allele | 2,774 | 3,652 | 1 × 10E−3 | 1.13 (1.05–1.22) | + | Meta-analyses 15 studies (Thapar et al. 2005) |
Trans/Untrans Transmitted/Untransmitted, C/C = case–control study, FB = family-based study
Genotypes counts
Odds ratio for genotype
LPHN3, a gene recently described to have a high potential for the prevention and treatment of ADHD
For almost 20 years, one of the authors (M.A.B.) has studied highly prevalent conditions (including Alzheimer’s disease, idiopathic epilepsy, autoimmune disorders, and several psychiatric conditions) by collecting extended and multigenerational pedigrees, ascertained from a population exhibiting features of a genetic isolate (Acosta et al. 2004; Arcos-Burgos and Muenke 2002). This population, commonly known as the Paisa Community of Colombia, inhabits the Northeastern region of Colombia, South America, predominantly in the State of Antioquia. A complete demographic description has been presented elsewhere (Arcos-Burgos and Muenke 2002).
In order to identify behavioral vulnerability genes, particularly those related to ADHD, a genetic-epidemiological protocol was established over 10 years ago between the Human Development Section of the Medical Genetics Branch, National Human Genome Research Institute, National Institutes of Health in Bethesda, MD, USA and the University of Antioquia in Medellin, Colombia. Goal of this collaboration was to recruit and study extended and multigenerational families from this Paisa genetic isolate. Our study sample initially included one large multigenerational family and several ADHD nuclear families. After observing the recruited families, we found that ADHD was highly comorbid with other disruptive behaviors (Palacio et al. 2004; Arcos-Burgos et al. 2002). We also performed a genome-wide scan that demonstrated significant genetic linkage of ADHD to several regions located on chromosomes 4q, 5q, 8q, 11q, and 17p (Arcos-Burgos and Acosta 2007; Arcos-Burgos et al. 2004a, b).
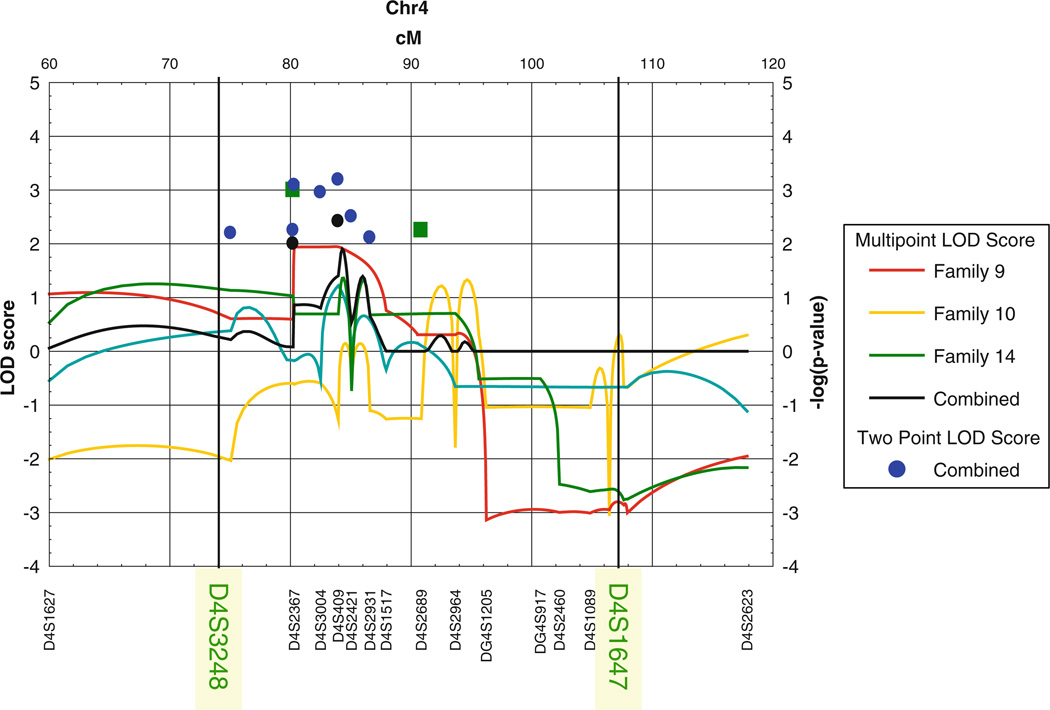
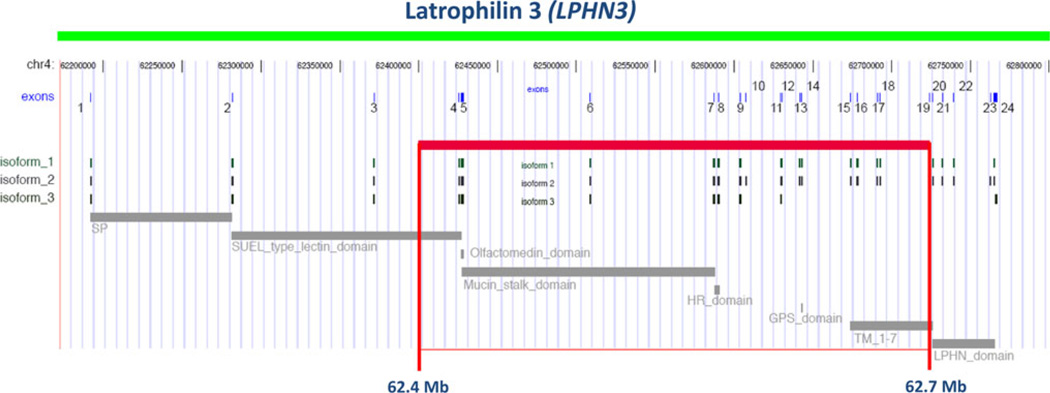
In particular, the region located on chromosome 4q provided a combined LOD score = 4.44 (Jain et al. 2007; Arcos-Burgos et al. 2004, 2010), with the presence of several families with nominal values of linkage to the same area (Fig. 1). The application of fine mapping to these linked families sharpened the linkage signal and revealed new meiotic recombination events in individuals with ADHD, which further narrowed the minimal critical region with the gene to ~20 Mb (Arcos-Burgos et al. 2010). Fine-scale genetic association, with a resolution of ~68 kb, a third of the minimum distance able to provide full coverage in the Paisa population (Carvajal-Carmona et al. 2003; Service et al. 2006), was conducted in both nuclear and large multigenerational families from the Paisa genetic isolate. Areas of interest included those that were gene-rich or that included potential candidate genes, were covered at a higher density (Arcos-Burgos et al. 2010). An empirical linkage disequilibrium map, built from control individuals, demonstrated full coverage of the entire region and excluded the presence of uncovered gaps (Fig. 2) (Arcos-Burgos et al. 2010). A pedigree disequilibrium test (PDT) (Martin et al. 2000) and haplotype-based cladistic analysis (Durrant and Morris 2005; Durrant et al. 2004) were performed. A significant area of association with ADHD was then defined by the single nucleotide polymorphic (SNP) markers rs1901223 and rs1355368 (P = 3.1 × 10−3, marker based; P = 2.7 × 10−5, haplotype based) (Fig. 2) (Arcos-Burgos et al. 2010).
Fig. 1.
Linkage of ADHD to 4q13.2. Screening of 18 extended multigenerational families from the Paisa community of Colombia showed significant linkage of ADHD to chromosome 4q13.2, with a nominal region between markers D4S3248 and D4S1647
Fig. 2.
Location of the haplotype in LD to ADHD. The susceptibility haplotype encompasses exons 4–19 of LPHN3 and contains important functional domains and variable splicing sites for isoforms of the gene
The region of association was located at 62.4–62.7 Mb (UCSC coordinates) on 4q within exons 4 through 19 of latrophilin3 (LPHN3) (Arcos-Burgos et al. 2010). Latrophilin3 is a member of the latrophilin (LPHN) subfamily of G-protein-coupled receptors (GPCRs). Latrophilins have seven transmembrane regions as well as long N-terminal extracellular sequences containing a 19-amino acid signal peptide (GPCR proteolytic site, GPS domain), and a serine/threonine-rich glycosylation region (Sugita et al. 1998; Ichtchenko et al. 1998). Latrophilins1 and 2 serve as receptors for alpha-latrotoxin, a component of the venom of the black widow spider (Latrodectus mactans). Alpha-latrotoxin interacts with neuronal GPCRs to stimulate exocytosis of GABA-containing presynaptic vesicles (Lelianova et al. 1997; Matsushita et al. 1999; Mee et al. 2004; Linets’ka et al. 2002). GABA is an inhibitory neurotransmitter. This suggests a possible role of latrophilin3 in ADHD, the most brain-specific latrophilin (Sugita et al. 1998; Ichtchenko et al. 1998). In fact, other GPCRs, such as DRD4 and DRD5, have been associated directly with ADHD (Gizer et al. 2009; Arcos-Burgos et al. 2010).
Once the study of Paisa families identified a specific region of the LPHN3 gene that was associated with symptoms of ADHD, fine mapping of this region was performed. This allowed to precisely pinpoint potential variants in the DNA code that may alter the gene’s function. In order to validate these findings, we pursued the replication of the study in additional samples from Colombia, Germany, Norway, Spain, and two U.S. populations. The results of these meta-analyses performed in thousands of individuals showed evidence for a significant homogeneous genetic effect for three of the top associated markers inside of the LPHN3 gene (Arcos-Burgos et al. 2010).
Combined efforts of this collaboration revealed potentially functional sequences within the LPHN3 gene that may be considered targets by future studies on the field. This study is not only a critical step to a better understanding of ADHD but it also offers a clear example of how multidisciplinary groups can interact to dissect causes of human disease (Arcos-Burgos et al. 2010).
In addition to the genetic studies, we also carried out pathologic studies of brain tissue specimens as well as brain imaging studies. This showed that a key LPHN3 variant of interest is expressed in brain regions related to attention and activity. Most importantly, the same variant associated with ADHD susceptibility was also associated with the response to stimulant medication (Arcos-Burgos et al. 2010).
Predicting the impact of genetics on ADHD attributable risk
These specific findings are important because they may open a new window of hope for the prevention and treatment of ADHD. However, the findings have been difficult to incorporate into the clinical assessment of patients and the design of population assessment policies by epidemiologists and geneticists. These findings are difficult to interpret because both genetic and phenotypic heterogeneity increases as quickly as new genes associated with ADHD are discovered. Furthermore, the potential presence of non-linear interactions between genes is insufficiently explored, since the number of interactive epistatic models increases exponentially as a function of the number of loci (Slatkin 2008, 2009).
As we described elsewhere (Arcos-Burgos et al. 2010), the interpretation of these association findings must be placed in the context of its potential impact for the clinical and epidemiological practice and caution must be the rule. We used the population attributable risk (PAR) as a measure of epidemiological impact, which provides a figure at a glance about the consequences (prevalence and outcome) of an association between an exposure factor (in this particular case, the LPHN3 common variant conferring susceptibility) and a disease (ADHD) at the population level (Arcos-Burgos et al. 2010). Specifically, the PAR defines the proportion of ADHD cases that could be treated if it were possible to control for the effects of the exposure factor (genetic variant) conferring susceptibility to ADHD. The PAR is a function of the relative risk and the probability of exposure (Pe) given that a person has the disease. Family-based samples provide an odds ratio (OR) instead of relative risk. However, for a highly prevalent disorder, such as ADHD, the OR is not a good estimator of relative risk, discussed in the original manuscript (Arcos-Burgos et al. 2010). We calculated the PAR% for marker rs65511665 (located in the LPHN3 gene) with the case–control-based sample from Norway, as proposed by Hildebrandt et al. (2006). Thus far, the PAR% for the marker rs6551665 in the Norway sample was 8.99 (95% CI = 3.90–14.12). This means that controlling the effect of the LPHN3 common variant conferring susceptibility to ADHD would result in a reduction of ~9% in the ADHD prevalence in the Norwegian population (Arcos-Burgos et al. 2010). Here, we want to emphasize that only replications of this epidemiological finding would determine how important it might be. In the past, we have seen to many statements like this in psychiatric genetics thus far, which all turned out to be exaggerated.
Conclusion
We predict that a better knowledge of how genes interact to produce complex phenotypes will help to further our understanding of the ADHD behavioral spectrum and variation in treatment response.
Acknowledgments
This research was supported by funds of the NHGRI intramural research program, NIH, Bethesda, MD, USA.
Glossary of terms
- Complex phenotype (disorder)
This term attempts to define those phenotypes (an observable characteristic or trait) where genetic effects have been demonstrated to play a role in their causality but where inheritance does not fit patterns of classical Mendelian segregation (the discrete transmission described by Mendel in which a change in one gene leads to a specific disorder). Environmental effects are also assumed to play a role in the genesis of these complex phenotypes.
- Exome
This refers to the 1% of the human genome that is most functionally relevant and most likely to cause observable phenotypes. Comprised of short segments of DNA called exons that code for proteins, the exome provides the genetic blueprint.
- Heritability
This includes the proportion of observed variation in a particular trait (ADHD in our case) that can be attributed to inherited genetic factors, in contrast to environmental factors.
- Major gene effects
This defines genetic variation in accordance with Mendelian models of segregation, (i.e., dominant, codominant, recessive), unlike those models anchored in a multifactorial framework.
- Complex segregation analysis
This is heuristic genetic-epidemiological test outlined by a unified framework that contrasts models combining Mendelian, multifactorial, and random effects.
- Oligogenic model
This pertains to hereditary characteristics produced by one or only a few genes.
- Multifactorial threshold model
This model assumes that (Cadieu et al. 2009) many factors (hence, the term multifactorial) contribute to a disorder; (Lander et al. 2001) the effect of each single factor is small but the effects add to each other; and Ledford (2010) once the additive effects of the factors pass some critical value (the threshold), one becomes affected with the disorder.
- Genetic epidemiology
As outlined by Newton E. Morton, this is a new discipline that incorporates methods found in genetics, epidemiology, and population genetics.
- Genetic stratification
This is the hidden process of micro-differentiation, as determined by changes of the genotype frequencies that human populations suffer because of absence of migration.
- Genetic heterogeneity
This occurs when a specific phenotype can be produced by alterations in independent genes.
- Genome-wide association study (GWAS)
This is an analysis of allelic association for genes throughout a genome.
- Genetic linkage
This describes a biological phenomenon for which two loci (areas) lie near each other on a chromosome and are therefore likely to be inherited together.
- LOD score (logarithm of the odds)
This is a statistical estimate of the genetic linkage between two loci. LOD implies “to the base 10”. For example, a LOD score of three means the odds are a thousand to one in favor of genetic linkage.
- Pedigree disequilibrium test (PDT)
This is a method of analyses for which genetic linkage and association between two loci is tested in pedigrees.
Footnotes
The authors declare no competing financial interests.
References
- Acosta MT, Arcos-Burgos M, Muenke M. Attention deficit/hyperactivity disorder (ADHD): complex phenotype, simple genotype? Genet Med. 2004;6(1):1–15. doi: 10.1097/01.gim.0000110413.07490.0b. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Acosta MT. Tuning major gene variants conditioning human behavior: the anachronism of ADHD. Curr Opin Genet Dev. 2007;17(3):234–238. doi: 10.1016/j.gde.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61(4):233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Lopera F, Pineda D, Palacio JD, Garcia M, et al. Attention-deficit/hyperactivity disorder (ADHD): feasibility of linkage analysis in a genetic isolate using extended and multigenerational pedigrees. Clin Genet. 2002;61(5):335–343. doi: 10.1034/j.1399-0004.2002.610503.x. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Konecki D, Lopera F, Pineda D, Palacio JD, et al. Pedigree disequilibrium test (PDT) replicates association and linkage between DRD4 and ADHD in multigenerational and extended pedigrees from a genetic isolate. Mol Psychiatry. 2004a;9(3):252–259. doi: 10.1038/sj.mp.4001396. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Castellanos FX, Pineda D, Lopera F, Palacio JD, Palacio LG, et al. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. Am J Hum Genet. 2004b;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D, et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.6. (in press) [DOI] [PubMed] [Google Scholar]
- Banerjee E, Sinha S, Chatterjee A, Gangopadhyay PK, Singh M, Nandagopal K. A family-based study of Indian subjects from Kolkata reveals allelic association of the serotonin transporter intron-2 (STin2) polymorphism and attention-deficit-hyperactivity disorder (ADHD) Am J Med Genet B Neuropsychiatr Genet. 2006;141B(4):361–366. doi: 10.1002/ajmg.b.30296. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, et al. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry. 1992;49(9):728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry. 2006;11(10):934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Cadieu E, Neff MW, Quignon P, Walsh K, Chase K, Parker HG, et al. Coat variation in the domestic dog is governed by variants in three genes. Science. 2009;326(5949):150–153. doi: 10.1126/science.1177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Ophoff R, Service S, Hartiala J, Molina J, Leon P, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet. 2003;112(5–6):534–541. doi: 10.1007/s00439-002-0899-8. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA. 2009;106(45):19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill D, Banaschewski T. The genetics of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2009;9(10):1547–1565. doi: 10.1586/ern.09.78. [DOI] [PubMed] [Google Scholar]
- Collins FS, Manolio TA. Merging and emerging cohorts: necessary but not sufficient. Nature. 2007;445(7125):259. doi: 10.1038/445259a. [DOI] [PubMed] [Google Scholar]
- Comings DE, Chen C, Wu S, Muhleman D. Association of the androgen receptor gene (AR) with ADHD and conduct disorder. Neuroreport. 1999;10(7):1589–1592. doi: 10.1097/00001756-199905140-00036. [DOI] [PubMed] [Google Scholar]
- Durrant C, Morris AP. Linkage disequilibrium mapping via cladistic analysis of phase-unknown genotypes and inferred haplotypes in the Genetic Analysis Workshop 14 simulated data. BMC Genet. 2005;6 Suppl 1:S100. doi: 10.1186/1471-2156-6-S1-S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant C, Zondervan KT, Cardon LR, Hunt S, Deloukas P, Morris AP. Linkage disequilibrium mapping via cladistic analysis of single-nucleotide polymorphism haplotypes. Am J Hum Genet. 2004;75(1):35–43. doi: 10.1086/422174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Capasso M, Zaheer Z, Lantieri F, Ambrosini P, Berrettini W, et al. Candidate gene analysis in an on-going genome-wide association study of attention-deficit hyperactivity disorder: suggestive association signals in ADRA1A. Psychiatr Genet. 2009;19(3):134–141. doi: 10.1097/YPG.0b013e32832a5043. [DOI] [PubMed] [Google Scholar]
- Elston RC, Yelverton KC. General models for segregation analysis. Am J Hum Genet. 1975;27(1):31–45. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(1):159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Weiffenbach B, Keith T, Chu MP, Weaver A, et al. Dopamine D4 gene 7-repeat allele and attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(5):768–770. doi: 10.1176/ajp.156.5.768. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC. Toward guidelines for pedigree selection in genetic studies of attention deficit hyperactivity disorder. Genet Epidemiol. 2000;18(1):1–16. doi: 10.1002/(SICI)1098-2272(200001)18:1<1::AID-GEPI1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Hum Genet. 2009;126(1):13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel S, Saar K, Sauer S, Dempfle A, Walitza S, Renner T, et al. Association and linkage of allelic variants of the dopamine transporter gene in ADHD. Mol Psychiatry. 2007;12(10):923–933. doi: 10.1038/sj.mp.4001986. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- Guo X, Elston RC. Two-stage global search designs for linkage analysis II: including discordant relative pairs in the study. Genet Epidemiol. 2000;18(2):111–127. doi: 10.1002/(SICI)1098-2272(200002)18:2<111::AID-GEPI2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hildebrandt M, Bender R, Gehrmann U, Blettner M. Calculating confidence intervals for impact numbers. BMC Med Res Methodol. 2006;6:32. doi: 10.1186/1471-2288-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Sudhof TC. alpha-latrotoxin action probed with recombinant toxin: receptors recruit alpha-latrotoxin but do not transduce an exocytotic signal. EMBO J. 1998;17(21):6188–6199. doi: 10.1093/emboj/17.21.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar SK, Song D, Klein BE, Klein R, Schick JH, Humphrey J, et al. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am J Hum Genet. 2004;74(1):20–39. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Palacio LG, Castellanos FX, Palacio JD, Pineda D, Restrepo MI, et al. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: evidence of pleiotropy and new susceptibility loci. Biol Psychiatry. 2007;61(12):1329–1339. doi: 10.1016/j.biopsych.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kent L, Doerry U, Hardy E, Parmar R, Gingell K, Hawi Z, et al. Evidence that variation at the serotonin transporter gene influences susceptibility to attention deficit hyperactivity disorder (ADHD): analysis and pooled analysis. Mol Psychiatry. 2002;7(8):908–912. doi: 10.1038/sj.mp.4001100. [DOI] [PubMed] [Google Scholar]
- Kent L, Green E, Hawi Z, Kirley A, Dudbridge F, Lowe N, et al. Association of the paternally transmitted copy of common Valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene with susceptibility to ADHD. Mol Psychiatry. 2005;10(10):939–943. doi: 10.1038/sj.mp.4001696. [DOI] [PubMed] [Google Scholar]
- Kieling C, Goncalves RR, Tannock R, Castellanos FX. Neurobiology of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2008;17(2):285–307. viii. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Ledford H. Africa yields two full human genomes. Nature. 2010;463(7283):857. doi: 10.1038/463857a. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Waldman I, Van Hulle CA, Rathouz P, Pelham WE, et al. Association of dopamine transporter genotype with disruptive behavior disorders in an eight-year longitudinal study of children and adolescents. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(3):310–317. doi: 10.1002/ajmg.b.30447. [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, et al. Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem. 1997;272(34):21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, Roser C, Nguyen TT, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm. 2008;115(11):1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Hum Mol Genet. 2006;15(14):2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- Linets’ka MV, Storchak LH, Himmelreich NH. Effect of synaptosomal cytosolic [3H]GABA pool depletion on secretory ability of alpha-latrotoxin. Ukr Biokhim. 2002;Zh 74(3):65–72. [PubMed] [Google Scholar]
- Lopera F, Palacio LG, Jimenez I, Villegas P, Puerta IC, Pineda D, et al. Discrimination between genetic factors in attention deficit. Rev Neurol. 1999;28(7):660–664. [PubMed] [Google Scholar]
- Lowe N, Kirley A, Hawi Z, Sham P, Wickham H, Kratochvil CJ, et al. Joint analysis of the DRD5 marker concludes association with attention-deficit/hyperactivity disorder confined to the predominantly inattentive and combined subtypes. Am J Hum Genet. 2004;74(2):348–356. doi: 10.1086/381561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Moss HB, Vanyukov MM. Segregation analysis of attention deficit hyperactivity disorder. Am J Med Genet. 1999;88(1):71–78. [PubMed] [Google Scholar]
- Maher BS, Marazita ML, Ferrell RE, Vanyukov MM. Dopamine system genes and attention deficit hyperactivity disorder: a meta-analysis. Psychiatr Genet. 2002;12(4):207–215. doi: 10.1097/00041444-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS. The HapMap and genome-wide association studies in diagnosis and therapy. Annu Rev Med. 2009;60:443–456. doi: 10.1146/annurev.med.60.061907.093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Rodriguez LL, Brooks L, Abecasis G, Ballinger D, Daly M, et al. New models of collaboration in genome-wide association studies: the Genetic Association Information Network. Nat Genet. 2007;39(9):1045–1051. doi: 10.1038/ng2127. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor I, Tyano S, Mel E, Eisenberg J, Bachner-Melman R, Kotler M, et al. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7(6):626–632. doi: 10.1038/sj.mp.4001037. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Elston RC, Namboodiri KK, Hames CG. Factors contributing to the variability in serum lipid levels and blood pressure in a large kindred. Am J Epidemiol. 1983;118(6):806–817. doi: 10.1093/oxfordjournals.aje.a113699. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Spence MA, Melnick M. Genetic analysis of cleft lip with or without cleft palate in Danish kindreds. Am J Med Genet. 1984;19(1):9–18. doi: 10.1002/ajmg.1320190104. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Spence MA, Melnick M. Major gene determination of liability to cleft lip with or without cleft palate: a multiracial view. J Craniofac Genet Dev Biol Suppl. 1986;2:89–97. [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet. 2000;67(1):146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: multiply spliced G protein-coupled receptors with differential tissue distribution. FEBS Lett. 1999;443(3):348–352. doi: 10.1016/s0014-5793(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Mee CJ, Tomlinson SR, Perestenko PV, De Pomerai D, Duce IR, Usherwood PN, et al. Latrophilin is required for toxicity of black widow spider venom in Caenorhabditis elegans. Biochem J. 2004;378(Pt 1):185–191. doi: 10.1042/BJ20031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RJ, Heath A, Reich W, Bucholz KK, Madden PAF, Sun L, et al. Latent class analysis of ADHD and comorbid symptoms in a population sample of adolescent female twins. J Child Psychol Psychiatry. 2001;42(7):933–942. doi: 10.1111/1469-7610.00789. [DOI] [PubMed] [Google Scholar]
- Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461(7261):272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio JD, Castellanos FX, Pineda DA, Lopera F, Arcos-Burgos M, Quiroz YT, et al. Attention-deficit/hyperactivity disorder and comorbidities in 18 Paisa Colombian multigenerational families. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1506–1515. doi: 10.1097/01.chi.0000142279.79805.dc. [DOI] [PubMed] [Google Scholar]
- Pineda DA, Palacio LG, Puerta IC, Merchan V, Arango CP, Galvis AY, et al. Environmental influences that affect attention deficit/hyperactivity disorder: study of a genetic isolate. Eur Child Adolesc Psychiatry. 2007;16(5):337–346. doi: 10.1007/s00787-007-0605-4. [DOI] [PubMed] [Google Scholar]
- Rasmussen ER, Neuman RJ, Heath AC, Levy F, Hay DA, Todd RD. Replication of the latent class structure of attention-deficit/hyperactivity disorder (ADHD) subtypes in a sample of Australian twins. J Child Psychol Psychiatry. 2002;43(8):1018–1028. doi: 10.1111/1469-7610.00229. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Res Hum Genet. 2008;11(6):579–585. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribases M, Ramos-Quiroga JA, Hervas A, Bosch R, Bielsa A, Gastaminza X, et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit/hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Mol Psychiatry. 2009;14(1):71–85. doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits. III. The effect of marker polymorphism on analysis of affected relative pairs. Am J Hum Genet. 1990a;46(2):242–253. [PMC free article] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990b;46(2):229–241. [PMC free article] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traitsI. Multilocus models. Am J Hum Genet. 1990c;46(2):222–228. [PMC free article] [PubMed] [Google Scholar]
- Risch N. Genetic linkage and complex diseases, with special reference to psychiatric disorders. Genet Epidemiol. 1990;7(1):3–16. doi: 10.1002/gepi.1370070103. (discussion 7–45) [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas KR. Linkage studies of psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 1993;243(3–4):143–149. doi: 10.1007/BF02190720. [DOI] [PubMed] [Google Scholar]
- Risch N, Teng J. The relative power of family-based and case-control designs for linkage disequilibrium studies of complex human diseases I. DNA pooling. Genome Res. 1998;8(12):1273–1288. doi: 10.1101/gr.8.12.1273. [DOI] [PubMed] [Google Scholar]
- Risch N, Zhang H. Extreme discordant sib pairs for mapping quantitative trait loci in humans. Science. 1995;268(5217):1584–1589. doi: 10.1126/science.7777857. [DOI] [PubMed] [Google Scholar]
- Schliekelman P, Slatkin M. Multiplex relative risk and estimation of the number of loci underlying an inherited disease. Am J Hum Genet. 2002;71(6):1369–1385. doi: 10.1086/344779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service S, DeYoung J, Karayiorgou M, Roos JL, Pretorious H, Bedoya G, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38(5):556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- Sheehan K, Lowe N, Kirley A, Mullins C, Fitzgerald M, Gill M, et al. Tryptophan hydroxylase 2 (TPH2) gene variants associated with ADHD. Mol Psychiatry. 2005;10(10):944–949. doi: 10.1038/sj.mp.4001698. [DOI] [PubMed] [Google Scholar]
- Slatkin M. Genotype-specific recurrence risks as indicators of the genetic architecture of complex diseases. Am J Hum Genet. 2008;83(1):120–126. doi: 10.1016/j.ajhg.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M. Epigenetic inheritance and the missing heritability problem. Genetics. 2009;182(3):845–850. doi: 10.1534/genetics.109.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Mick E, Faraone SV. Advances in genetic studies of attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2009;11(2):143–148. doi: 10.1007/s11920-009-0022-0. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Stricker C, Fernando RL, Elston RC. Linkage analysis with an alternative formulation for the mixed model of inheritance: the finite polygenic mixed model. Genetics. 1995;141(4):1651–1656. doi: 10.1093/genetics/141.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Sudhof TC. alpha-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors G-protein coupling not required for triggering exocytosis. J Biol Chem. 1998;273(49):32715–32724. doi: 10.1074/jbc.273.49.32715. [DOI] [PubMed] [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14(Spec No. 2):R275–R282. doi: 10.1093/hmg/ddi263. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Boomsma DI, Verhulst FC. A study of problem behaviors in 10- to 15-year-old biologically related and unrelated international adoptees. Behav Genet. 1994;24(3):193–205. doi: 10.1007/BF01067187. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev. 2006;26(4):396–432. doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Chhabildas NA, Friedman MC, Alexander J. Psychiatric comorbidity associated with DSM-IV ADHD in a nonreferred sample of twins. J Am Acad Child Adolesc Psychiatry. 1999;38(11):1355–1362. doi: 10.1097/00004583-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Wong ML, Arcos-Burgos M, Licinio J. Frontiers in psychiatric research. Psychiatr Times. 2008;25(7):1–8. [Google Scholar]