Abstract
Cytoreductive surgery and empirical combination chemotherapy have improved 5-year survival for ovarian cancer patients, but have not increased the overall rate of cure. Poor outcomes relate, at least in part, to late diagnosis and to the persistence of dormant ovarian cancer cells that have resisted conventional drugs. Increased understanding of the molecular, cellular and clinical biology of ovarian cancer must be translated into personalized therapy with conventional and targeted agents as well as personalized detection of high-grade cancers in early stages. Different strategies will be required to treat low-grade and high-grade serous cancers as well as other histotypes. Activating mutations of Ras and Raf can be targeted in low-grade cancers. Activation of the PI3K pathway—PI3Kness—and inactivation of BRCA function—BRCAness—can be targeted in high-grade lesions. Inhibition of multiple pathways will be required. Sensitivity of primary cancers to paclitaxel and platinum can be modulated by inhibiting kinases and other molecules that regulate the cell cycle. Dormant ovarian cancer cells may depend upon autophagy, cytokines and growth factors for survival. Early detection can utilize two stage strategies where rising serum biomarker levels prompt imaging in a small fraction of women. Screening can be personalized by taking into account each woman's baseline biomarker levels.
Keywords: ovarian cancer, genomics, early detection, biomarkers, personalized therapy, targeted therapy
current management of ovarian cancer
Progress in preventing, detecting and treating ovarian malignancy has been influenced by the fact that epithelial ovarian cancer is neither a common nor a rare disease. The lifetime risk is 1 in 70 and the prevalence is 1 in 2500 for postmenopausal women >50 years of age. In the United States in 2010, some 21 880 women developed ovarian cancer and 13 850 died from the disease [1]. With a limited number of patients to participate in clinical trials, progress in the clinic has been gradual, but significant. With the increasing use of cytoreductive surgery and combination chemotherapy, 5-year survival has improved from 37% in 1974–1976 to 46% during 1999–2005 (P < 0.05) [2].
cytoreductive surgery
The surgical management of ovarian cancer has been based on the belief that the removal of as much cancer as possible benefits the patient, even when complete resection is not possible. In retrospective studies, the size of tumor nodules remaining after initial surgery has correlated with prognosis. Prospective randomized trials of immediate cytoreductive surgery have been difficult to perform and results of trials with delayed cytoreduction have provided conflicting results. Better outcomes have, however, been documented when initial cytoreductive surgery is performed by specially trained gynecologic oncologists who subsequently provide optimal chemotherapy.
combination chemotherapy
Ovarian cancer is a chemoresponsive but less frequently chemocurable disease. Combination chemotherapy has improved significantly over the last three decades, based on empirical trials as new drugs have become available. In the 1960s and 1970s, single alkylating agents produced a 20%–30% response rate with few complete responses. With the advent of platinum-based chemotherapy, the response rate improved to 70% and a significant fraction of women survived for 5 years. In subsequent studies, empirical combinations of cytotoxic drugs have been given at maximally tolerated dosage in an attempt to eliminate cancer cells that are resistant to single agents. Initially, a combination of cisplatin and cyclophosphamide was utilized. Subsequently, platinum compounds were combined with paclitaxel. In primary and in recurrent disease, improved progression-free and overall survival has been observed with platinum compounds and taxanes when compared with treatment with cisplatin and cyclophosphamide or with platinum compounds alone. Carboplatin was shown to be less neurotoxic and emetogenic than cisplatin. Comparison of carboplatin and paclitaxel to carboplatin and docetaxel demonstrated that the latter combination produced less neuropathy but greater myelotoxicity.
Several other drugs can produce regression of epithelial ovarian cancers, including pegylated liposomal doxorubicin (PLD), gemcitabine and topotecan. Each of these agents has been combined with paclitaxel and/or carboplatin, in combinations of two or three drugs. In the Gynecologic Oncology Group GOG 182 (ICON5) study, a five-arm trial of different doublets and triplets was carried out [3]. At the end of the trial, the addition of other drugs to carboplatin and paclitaxel did not improve progression-free or overall survival. Many believe that GOG 182 should be a turning point in ovarian cancer research, where clinical investigators stop treating the ‘average’ ovarian cancer patient using empirical combinations of active drugs hoping for better outcomes. In the future, smaller trials must be conducted in selected subsets of patients using drugs and biological agents that target the specific biologic abnormalities found in their particular cancers, driven by distinctive genetic or epigenetic changes. Entry into such trials can be based on analysis of specimens obtained during cytoreductive surgery, but evaluation of response is likely to require fresh biopsies during treatment to determine impact on signaling pathways within each tumor. Reorienting clinical research to treat the ‘individual’ ovarian cancer patient would be an important step toward personalizing and optimizing the management of this disease.
intraperitoneal treatment
One approach to improving patient outcomes has been to deliver chemotherapy intraperitoneally (i.p.). In a meta-analysis of six randomized studies, i.p. administration of chemotherapy has proven superior to intravenous administration [4]. However, i.p. therapy is appropriate only for optimally cytoreduced patients where chemotherapy within the peritoneal cavity can penetrate small tumor nodules. There is a substantial pharmacological advantage, with a >10-fold increase in the concentration of the drug bathing a tumor on the peritoneal surface. Up to 16 months improvement in overall survival has been observed [5]. Despite these apparent advantages, i.p. therapy is still not widely accepted and additional confirmatory trials are underway. Intraperitoneal therapy is inconvenient and sometimes poorly tolerated, but current data do suggest that we should provide i.p. therapy to a greater fraction of optimally cytoreduced patients. In the future, the genomic analysis of primary cancer specimens might be used to identify patients who would most benefit from i.p. therapy.
recurrent disease
The majority of ovarian cancer patients experience disease recurrence. If progression-free survival is >6 months, retreating with carboplatin-based chemotherapy provides a response rate of 30% to >50%, depending upon the duration of the progression-free interval. Single agents have then been administered sequentially, including pegylated liposomal doxorubicin, topotecan, gemcitabine, vinorelbine, etoposide, hexamethylmelamine and bevacizumab, producing response rates of 15%–30%. Some combinations are more effective than single agents for platinum-sensitive disease. In the ICON4 trial, carboplatin and paclitaxel have been shown to provide superior progression-free and overall survival compared with carboplatin alone. Combinations of carboplatin and gemcitabine or PLD and trabectedin have improved progression-free survival, but not overall survival, over carboplatin alone or PLD alone. Recent studies suggest that PLD and carboplatin are actually superior to carboplatin and paclitaxel in prolonging progression-free survival, but data regarding overall survival are not yet available. Given the many different agents that can be utilized, we need effective biomarkers to predict response or lack of response to conventional drugs, permitting personalized therapy for recurrent disease. The identification of biomarkers may require prospective trials, in that retrospective data generally utilize combinations of drugs and are difficult to interpret. Once identified, biomarkers might also be used to personalize primary therapy.
monitoring disease recurrence
Serum biomarkers have been used to detect disease recurrence. A doubling of CA125 outside the normal range detects recurrent ovarian cancer in 70% of patients with a lead time of 3–4.8 months. Rising CA125 has often been used as a cost-effective tool to trigger more expensive imaging studies. Over the last decade, studies have shown that rising CA125 within the normal range has up to 94% specificity for detecting recurrence with a mean lead time of 6 months (range 2.8–17 months). The value of detecting recurrent disease for any cancer depends critically on the effectiveness of therapy. Gestational trophoblastic disease provides the paradigm, where human chorionic gonadotropin—a very sensitive and specific biomarker—can detect recurrent disease that can be cured with chemotherapy in a very large fraction of cases. Combination chemotherapy for recurrent ovarian cancer is not curative, but can prolong progression-free and overall survival, as detailed above.
In clinical practice, patients in complete clinical response with normal CA125 and normal imaging studies have generally been monitored with CA125 every 3 months for several years on the assumption that smaller volumes of recurrent disease would respond more effectively to chemotherapy. One recent study has challenged the value of earlier detection of recurrent disease in a randomized trial that initiated treatment when CA125 doubled outside the normal range or when patients became symptomatic [6]. CA125 detected recurrent disease 4.8 months before symptomatic recurrence, but there was no survival advantage for earlier treatment. Unfortunately, the trial required 9 years to complete, and during that interval standards for the use of CA125 [7, 8] and for the treatment of recurrent disease had changed [9]. Technically, the study arms were not stratified for the amount of residual disease following cytoreductive surgery, one of the most important prognostic factors. Whether patients were consistently restaged with computerized tomography following chemotherapy was not specified. Of potentially greater importance, treatment was at the discretion of the individual physicians participating in the study. Despite the results of ICON4, which became available during the study [10], some 66% of patients did not receive paclitaxel chemotherapy and 25% were started after a delay of >1 month or never treated. Consequently, only 25% of participants were treated promptly with therapy that would prolong survival. Only 7% of patients underwent secondary surgical cytoreduction, a procedure associated with improved survival in many studies and that is most feasible with small-volume disease. Consequently, most patients did not receive optimal state-of-the-art treatment by current standards. Based on these data, one can only conclude that earlier initiation of suboptimal treatment was not effective.
Although two prospective studies are currently underway, the use of secondary cytoreductive surgery is based on retrospective reviews. Fleming et al. have recently reported 74 patients who underwent secondary cytoreductive surgery that was optimal (<0.5 cm) in 41 and suboptimal in 33 [11]. Optimal cytoreduction was associated with longer disease-free survival (19 versus 12 months) and longer overall survival (47 versus 23 months, P < 0.0001). Patients who attained optimal secondary cytoreduction went to surgery sooner after a twofold rise in CA125 from nadir (5.3 versus 16.4 weeks). Each week delay after the first CA125 elevation correlated with a 3% increased chance of suboptimal surgery.
Whether or not to monitor with CA125 should be discussed with each patient, pointing out that the benefit is uncertain and that therapy for recurrent disease is not curative but can prolong survival. Some patients will not want additional aggressive therapy and may want to avoid the anxiety associated with monitoring. Many patients will want to be monitored to increase the odds for optimal secondary cytoreductive surgery, and to provide time to utilize multiple conventional and novel therapies should disease recur. On average, women with ovarian cancer only survive 12–18 months following symptomatic relapse. A small fraction survives up to a decade after responding to multiple drugs individually and in combination. Some 2–3 months is required to test each of the seven currently available agents with activity against ovarian cancer. Waiting for symptomatic recurrence, particularly when those symptoms relate to intestinal obstruction, will limit the number of agents that can be given and the chance for longer survival. Rustin's trial does, however, underline the need for more effective therapy. At present there are >400 new agents being developed to treat cancer. Combinations will almost certainly be required. In the United States <4% of patients enter trials and only half of ovarian cancer patients may have readily measurable disease. Waiting for symptomatic recurrence is likely to further reduce the number of women willing and capable of participating in clinical trials, further slowing progress.
current outcomes
In summary, no chemotherapeutic regimen has proven superior to a combination of platinum and a taxane for primary therapy. Intraperitoneal therapy can benefit a fraction of patients with small volumes of disease after surgery. Monitoring for disease recurrence with CA125 and imaging studies is an option that should be discussed with each patient. Recurrent disease cannot be cured, but survival can be significantly prolonged with combination therapy. If one considers all stages, at present ∼50% of women survive 5 years with optimal treatment. Despite improvement in 5-year survival, long-term survival for advanced-stage disease has not changed and is still no more than 20%–30%. This relates to at least two factors: late diagnoses and dormant drug-resistant cancer cells.
personalizing management of ovarian cancer through translational research
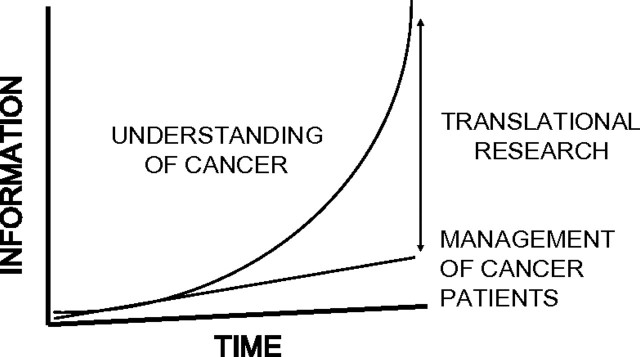
If we are to improve outcomes for women with ovarian cancer, translational research must be prioritized and accelerated. Over the last three decades our understanding of ovarian cancer at the molecular and cellular level has increased exponentially. Our management of cancer patients has also improved significantly, but more linearly (Figure 1). The challenge in this next decade is to make our progress in the clinic look more like our progress in the laboratory. This will require the identification of multiple molecular abnormalities that can be targeted in human ovarian cancer or used for earlier diagnosis, the improvement of predictive models and biomarkers, and the development of appropriate therapeutic agents and assays in the laboratories of academic cancer centers, Biotech and Pharma. Translation of these new agents to the clinic must be accompanied by the design and implementation of intelligent trials that are hypothesis driven and that include biopsies, monitoring and imaging to facilitate planning the next step in translation, regardless of outcome. Finally, insights from clinical trials and human samples must be returned to the laboratory to iterate more effective treatment and earlier diagnosis.
Figure 1.
The challenge for translational research. Over the last two decades of cancer research, progress in the laboratory has been exponential, whereas progress in the clinic has been more gradual. Through translational research we have the opportunity to accelerate progress in the clinic (courtesy of Dr Gordon Mills).
In recent years, targeted therapy guided by biomarkers has had a significant impact on other forms of cancer. Trastuzumab (Herceptin) has enhanced response to chemotherapy and increased survival of patients whose breast cancers exhibit amplification and overexpression of the HER2 receptor [12]. Imatinib has improved survival not only in chronic myelogenous leukemia (CML) with a BCR-Abl translocation [13], but also in gastrointestinal stromal tumors with c-KIT mutations [14]. Mutations in exons 19 and 21 of epidermal growth factor receptor (EGFR) have predicted long-term response to erlotinib in lung cancer [15]. Recent results from phase I trials at the MD Anderson Cancer Center suggest that matching mutations that activate signaling pathways with appropriately targeted drugs can improve response rates, time to progression and overall survival [16]. Among 161 patients where one mutational aberration was matched with targeted therapy, the CR + PR rate was 29% compared with 8% in 152 patients without matching (P = 0.0001) and 6% in 438 patients without molecular testing. A similar approach might be based on the distinctive biology of ovarian cancer.
biology of ovarian cancer
origin
A great deal has been learned about the biology of ovarian cancers. In the past, ovarian cancers have been thought to arise from the cells that cover the ovarian surface or the epithelial cells that line subserosal inclusion cysts. Recently, it has become apparent that histologically identical cancers can arise from endometriosis, the peritoneal surface or from the fimbriae of the fallopian tube. The fallopian tube may be particularly important in hereditary ovarian cancers [17]. During ovulation, the fimbriae cover the ovary to facilitate ova passing into the fallopian tube, where they might be fertilized before entering the uterus. Mutant TP53 can be detected in fimbriae and is associated with in situ and invasive serous cancers of the tube. Up to 80% of familial ovarian cancers arising in carriers of BRCA1/2 mutations could arise from the fallopian tube, in that many of them are primary peritoneal disease that implants upon or coats the ovary, rather than growing from it. A significant fraction of sporadic high-grade serous cancers that coat the ovary could also arise from the fallopian tube. In many institutions, this is up to 20% of cancers.
pattern of metastasis
Ovarian cancers exhibit a distinctive pattern of metastasis. Like other epithelial malignancies, ovarian cancers can metastasize through lymphatics, in this case to lymph nodes at the renal hilum. Ovarian cancer cells can also metastasize hematogenously. As patients are living longer with their disease, clinicians are encountering parenchymal brain and lung metastases that were seldom observed decades ago. Most frequently, however, ovarian cancers spread over the peritoneal surface and implant, forming a myriad of tiny nodules on the visceral and parietal peritoneum.
hereditary and sporadic
Some 10%–15% of ovarian cancers arise in the setting of a strong family history of the disease that tracks from generation to generation. Most hereditary cases are associated with BRCA1 or BRCA2 germline mutations that predispose to breast and ovarian cancers, but mismatch repair defects have also been associated with ovarian cancer as well as uterine and colon cancer in the human nonpolyposis colon cancer syndrome. In rare cases, ovarian cancers have been found in Li–Fraumeni kindreds that carry germline mutations of p53 [18]. Some 85%–90% of ovarian cancers are sporadic. Risk factors for sporadic ovarian cancer include age >40 years and a life history of persistent ovulation. Early menarche, late menopause and nulliparity are all associated with more persistent ovulation as well as an increased incidence of ovarian cancer. One of the best kept secrets in the gynecologic community is that use of oral contraceptives for as long as 5 years decreases the risk of ovarian cancer in later life by 50%. At high dosage, oral contraceptives can suppress ovulation and the progestin in oral contraceptives may purge premalignant cells from the ovary. The association of ovarian cancer with persistent ovulation is consistent with the possibility that spontaneous mutation occurs during the proliferation of epithelial cells to repair ovulatory defects.
clonality and heterogeneity
The majority of ovarian cancers arise from the progeny of single cells. Studies from several laboratories, including our own, indicate that >90% of ovarian cancers are clonal, where the primary cancer and metastases contain identical p53 mutations, X chromosome inactivation and patterns of loss of heterozygosity [19]. Despite an origin from single cells, ovarian malignancies exhibit substantial heterogeneity at a molecular and cellular level within the same primary cancer and among cancers from different women. Cancers differ in the fraction of cycling cells from 1% to 90%, and also differ in histotype and grade.
histotype
While ovarian cancers are thought to develop from flattened epithelial cells, transformed cells develop into serous, mucinous, clear cell and endometrioid histotypes resembling the mucosa of fallopian tubes, endocervix, endometrium and glycogen-filled rests within the vagina, respectively. During normal gynecologic development the HOXA genes are sequentially expressed in fallopian tube (HOXA9), endometrium (HOXA10) and endocervix (HOXA11). The expression of different HOXA genes has been observed in ovarian cancers of different histotypes [20]. Forced expression of HOXA9, HOXA10 and HOXA11 in partially transformed ovarian surface epithelial cells produces cancer cells with serous, endometrioid or mucinous histotypes.
Histotype matters. Serous, mucinous, endometrioid and clear cell cancers are distinct entities with different genetic abnormalities, gene expression profiles and sensitivities to chemotherapy. Serous and undifferentiated cancers are most prevalent, and dominate most clinical studies. In the future, separate trials will be required for each different histotype, as they respond to different conventional agents and express different targets for molecular therapies. Trials of targeted therapy in gastrointestinal and endometrial cancers may identify relevant drugs that can be tested in the less common ovarian histotypes. Recent discoveries in clear cell cancer suggest that changes in chromatin remodeling may provide a target, as described below.
grade
Perhaps the most important distinction for the management of ovarian cancer exists between high- and low-grade serous carcinomas [21]. Type I or low-grade carcinomas are most frequently identified in early stage and progress slowly. Low-grade cancers tend to be resistant, but not refractory, to platinum-based therapy during primary treatment. They frequently have activating Ras mutations, inactivating PTEN mutations and express the insulin-like growth factor receptor (IGFR). Almost all low-grade cancers have wild-type TP53. Some 90% of serous cancers are high grade or type II. Type II cancers tend to present at advanced stage and are more aggressive but also more responsive to platinum-based therapy. These invariably have TP53 mutations. When BRCA1 or BRCA2 mutations occur in ovarian cancers they are generally found in high-grade serous cancers. Activation of the PI3K pathway occurs in a significant minority of high-grade cancers.
personalizing therapy for low-grade ovarian cancers
Among low-grade cancers, only a few mutations occur frequently. Some 20% of low-grade serous ovarian cancers have mutations of Ras or Raf [22]. Among clear cell ovarian cancers, mutations have been found in ARID1A, a gene involved in chromatin remodeling, in 50% of cases and mutations of the PPP2R1A phosphatase in 7% [23, 24].
Different strategies have been developed for targeting low-grade and high-grade cancers. A phase II trial has been conducted by the GOG using the mitogen-activated protein kinase (MEK) inhibitor AZD6244 in recurrent low-grade serous ovarian cancers. Data are being analyzed, but multiple responses have been observed with this targeted therapy in a setting where conventional chemotherapy produces few, if any, responses in recurrent lesions. A PR occurred in a low-grade cancer with KRAS mutation. Given the cross-talk between MEK and PI3K downstream of receptor tyrosine kinases, the inhibition of both pathways may be required to induce apoptosis. A randomized phase II trial of the MEK inhibitor AZD6244 and an AKT inhibitor (MK2206) is planned. As low-grade ovarian cancers express IGFR, studies are also being undertaken with a humanized anti-IGFR antibody [25].
One additional strategy could take advantage of the persistence of wild-type TP53 in most low-grade cancers. Nutlin-2 is a small-molecular-weight inhibitor that fits into the pocket where wild-type TP53 binds to MDM, a molecule required for the rapid degradation of TP53 through the ubiquitin-proteasome pathway [26]. Inhibition of the MDM–TP53 interaction results in the increased expression of wild-type TP53, inhibiting tumor growth and inducing apoptosis.
personalizing therapy for high-grade ovarian cancers
the ‘omics’ of high-grade ovarian cancers
The Cancer Genome Atlas (TCGA) Research Network has sequenced coding genes in exomes from 316 high-grade serous ovarian cancers [27]. Some 96% had somatic mutations of p53 and germline or somatic mutations of BRCA1 or BRCA2 were found in 20%. Six additional genes were recurrently mutated in ≤5% of high-grade serous ovarian cancers, including RB1, NF1, FAT3, CSMD3, GABRA6 and CDK12, although FAT3 and GABRA6 did not appear to be expressed and consequently may not be relevant to ovarian oncogenesis. Other activating or inactivating mutations were uncommon. Copy number abnormalities were much more common. Across the chromosomes, 5 areas of recurrent copy number gain and 22 areas of loss occurred in >50% of 489 high-grade ovarian cancers. Substantially greater genetic instability was observed in ovarian cancer than in glioblastoma multiforme. Sixty-three regions of focal amplification were detected, including the MYC oncogene, cyclin E (CCNE1), and the Mds and Evi1 complex (MECOM) in >20% of cases. Other genes amplified in smaller fractions of ovarian cancers include KRAS, AKT1, HER2, HER3, BCL2L1, the receptor for activated C kinase (ZMYND8), the TP53 target gene IRF2BP2, the DNA-binding protein inhibitor ID4, the embryonic development protein PAX8 and the telomerase catalytic subunit TERT. At least 22 potential therapeutic targets were identified among the amplified genes. Fifty focal deletions were also identified, with homozygous deletion of PTEN, RB1 and NF1 tumor suppressor genes in ≥2% of ovarian cancers. Reduced expression and increased promoter methylation, consistent with epigenetic silencing, was found in 168 genes. The BRCA1 promoter was hypermethylated and silenced in 11.5% of ovarian cancers. Overall, ∼50% of high-grade cancers exhibit homologous recombination defects, if one includes tumors with epigenetic silencing, inactivating mutations of BRCA1 and BRCA2, amplification or mutation of EMSY, focal deletion or mutation of PTEN, hypermethylation of RAD51C, mutation of ATM or ATR, and mutation of Fanconi anemia genes.
signaling pathways in high-grade ovarian cancers
In high-grade ovarian cancers, a number of signaling abnormalities have been observed. In the TCGA dataset [27], the RB1 pathway that regulates the cell cycle was deregulated in 67% of high-grade ovarian cancers. Notch signaling was altered in 22%, and PI3K and/or Ras signaling was deregulated in 45%. In contrast to low-grade ovarian cancers, Ras or Raf mutations that activate Ras/MAP signaling are rare in high-grade lesions. KRAS is amplified in only 11% of cancers. Activating mutations of PIK3CA, inactivating mutations of PTEN and amplification of AKT are also uncommon events (<5%). In addition to activating mutations or amplification of signaling molecules, autocrine and paracrine signaling through tyrosine kinase growth factor receptors can stimulate both the PI3K and Ras pathways [28]. Activation of EGFR (HER1) and FMS by their respective ligands stimulates the Ras/MAP pathway preferentially. Amplification of HER2 or HER3 in a small fraction (<5%) of ovarian cancers and activation of IGFR by IGF-1 stimulates the PI3K pathway preferentially. The interleukin (IL)-6 receptor is expressed by the majority of ovarian cancers. Autocrine or paracrine activation by IL-8 can stimulate translocation to Stat3 to the nucleus, inducing the expression of a number of genes required for cancer cell proliferation, drug resistance and angiogenesis. The EDG4 receptor is expressed by the majority of ovarian cancer cells and its ligand, LPA, is produced by the cancer cells and found at high concentrations in the tumor microenvironment and in the circulation. TP53 function is lost in nearly all high-grade ovarian cancers. Wild-type TP53 represses the expression of FOXM1, which is up-regulated in 87% of high-grade ovarian cancers [27]. FOXM1 is a transcription factor that regulates a number of proliferation-related targets, including AURKB, CCNB1, BIRC5, CDC25 and PLK1 that are overexpressed in ovarian cancers in the absence of increased DNA copy number, consistent with transcriptional regulation.
integrating the signaling matrix
While several individual pathways have been shown to be dysregulated, signaling within ovarian tumors is even more complex. Signaling pathways are influenced by juxtacrine and paracrine signals from adjacent cancer cells and the surrounding stroma, as well as by hypoxia, low pH and nutrient deprivation that are often found within tumors. Within cancer cells, there is a matrix of signaling molecules. Cross-talk between pathways can produce paradoxical effects on cancer cell proliferation, motility, invasion and survival. Given this complexity, bioinformatic systems and biological analysis have been applied to model tumor behavior. New technologies have been utilized to understand the heterogeneity of ovarian cancers. Gene expression array analysis of 285 serous and endometrioid ovarian cancers collected by the Australian Ovarian Cancer Study Group identified four subgroups of high-grade cancers [29]. Ovarian cancers with a high stromal response or with mesenchymal transition and a low immune response exhibited a worse prognosis than cancers with a high immune signature or a low stromal response. In the TCGA analysis of gene expression using three different platforms, four subgroups were identified—immunoreactive, differentiated, proliferative and mesenchymal—but these did not correlate with survival [27]. Reverse-phase protein arrays (RPPA) permit the direct analysis of signaling proteins and their state of phosphorylation. Unsupervised clustering of RPPA data for 150 signaling proteins and their derivatives in >300 ovarian cancers at the MD Anderson Cancer Center has identified four groups exhibiting: (i) a ‘stromal’ signature; (ii) cyclin E2 and kit overexpression; (iii) activation of kinase signaling through Ras/MAP and PI3K; and (iv) estrogen receptor (ER) positivity (Gordon Mills, personal communication). The survival of patients with an ER-positive phenotype is significantly better than those with activation of PI3K.
PI3Kness
Activation of the PI3K pathway is observed in more than one-third of high-grade serous ovarian cancers. As outlined above, a small fraction of cancers contain activating mutations of PIK3CA, inactivating mutations of PTEN or amplification of AKT, and a larger fraction exhibit autocrine/paracrine growth factor stimulation. PI3K activity prevents apoptosis and increases drug resistance. PI3K inhibitors slow the growth of human ovarian cancer xenografts and enhance paclitaxel response. Currently, inhibitors of mTOR, AKT and PI3K are being evaluated in a variety of tumor types. In addition to mutational analysis, expression array and proteomic signatures are being developed to identify potential candidates among ovarian cancer patients.
BRCAness
As detailed above, while only 10%–15% of ovarian cancer patients carry BRCA1 or BRCA2 mutations in their germline, ∼50% of ovarian cancers exhibit a defect in the homologous recombination repair of DNA. Poly-ADP-ribose polymerase (PARP) is required for base excision repair, a second form of DNA repair. PARP inhibitors preferentially affect cells with decreased homologous recombination repair, where loss of both repair pathways proves lethal. An objective response rate of >40% has been observed when PARP inhibitors have been used as single agents to treat ovarian cancers that arise in patients with germline BRCA1/2 mutations. Additional trials of PARP inhibitors are anticipated in patients whose cancers exhibit ‘BRCAness’ in the absence of germline mutations [30]. As PARP inhibitors are myelotoxic, combination with other cytotoxic drugs has often not been well tolerated.
synthetic lethality
Ultimately, targeting a single molecular defect is unlikely to produce long-term regression in a significant fraction of ovarian cancers. The remarkable efficacy of imatinib in CML is thought to be related to the leukemic cells becoming ‘addicted’ to strong, persistent Abl signaling produced by a single dominant BCR-Abl translocation with the concomitant loss of redundant survival pathways that prevent programmed cell death when Abl is inhibited. Most solid tumors, including ovarian cancers, have multiple genetic abnormalities and retain redundant survival pathways. In the GOG 170 series, most single targeted therapies have a response rate of <10% in recurrent ovarian cancer using standard phase II designs with unselected patients. The matching of cancers with activated signaling pathways to particular drugs might improve these response rates, but it will also be necessary to seek combinations of drugs that exert ‘synthetic lethality’, i.e. drugs that are inactive individually, but lethal for cancer cells and not for normal cells when used in combination.
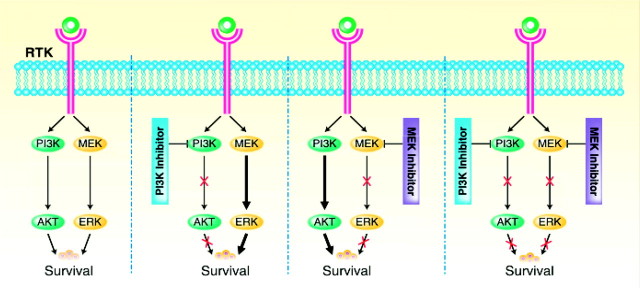
In cancers where ligand-induced activation of receptor tyrosine kinases stimulates both PI3K and MEK signaling, the inactivation of either PI3K or of MEK fails to affect cancer cell survival. The simultaneous inhibition of both pathways, however, produces synthetic lethality (Figure 2). In seeking targets for synthetic lethality, some survival pathways may be constitutively activated, whereas others may become activated only after inhibiting a dominant oncogenic pathway. Biopsies may be required not only before, but also during targeted therapy to identify second survival pathways that could be inhibited to produce synthetic lethality. Inhibitory RNA (RNAi) against both oncogenic and survival pathways could produce synthetic lethality where neither alone is sufficient. This approach could identify combinations of novel targets, but could also identify targets that enhance conventional therapy.
Figure 2.
Synthetic lethality produced by inhibiting the PI3K and MEK signaling pathways. Inhibition of PI3K or MEK signaling individually fails to exert toxicity, whereas inhibiting both pathways kills cancer cells (courtesy of Dr David Gershenson). RTK, receptor tyrosine kinase.
enhancing sensitivity to conventional chemotherapy
During primary therapy, 70% of ovarian cancers respond to platinum compounds, but only 42% respond to taxanes based on the results of the GOG 132 study [31]. High-throughput siRNA screens have indentified targets that regulate taxane sensitivity. Microtubule stability and sensitivity to paclitaxel can be increased by knocking down several different kinases [32]. Centrosome separation can be inhibited and paclitaxel sensitivity enhanced by knocking down SIK2 [33]. A testis-specific protein, ACRBP, has also been found in ovarian cancers and to regulate taxane sensitivity [34].
Knockdown of copper exporters has increased the sensitivity to platinum compounds [35]. While conventional small-molecular-weight inhibitors could be developed, RNAi might also be used as a therapeutic agent. The use of neutral liposomes or chitosan has permitted the delivery of siRNA in xenografts inhibiting tumor growth and this is now being prepared for clinical trials at the MD Anderson Cancer Center [36].
antiangiogenic therapy for ovarian cancer
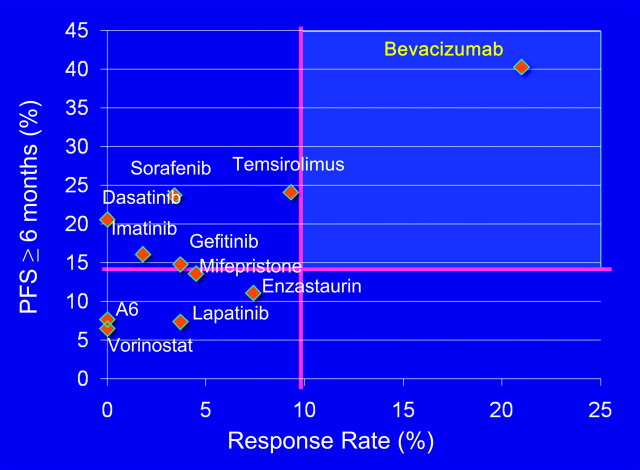
Angiogenesis provides another important target. Tumor nodules cannot grow to >1 mm in size without developing their own blood supply. Ovarian cancers produce multiple factors that stimulate angiogenesis, including VEGF, IL-8 and bFGF [28]. Bevacizumab (Avastin) is a humanized monoclonal antibody that neutralizes VEGF, a peptide growth factor required for the migration and proliferation of endothelial cells that line tumor vessels. Treatment with bevacizumab produces a 15%–21% response rate in recurrent ovarian cancers when used alone or in combination with low-dose oral cyclophosphamide [37]. Disease stability for 6 months has been observed in 40% of cases. In the GOG 170 series, all of the other single targeted agents produced a <10% response rate and controlled disease for 6 months in <25% of cases (Figure 3). In phase II studies of targeted agents for ovarian cancer, bevacizumab is clearly in a class of its own. Bevacizumab has been generally well tolerated, but has produced severe hypertension in up to 15% of patients, cerebrovascular accidents in 2%–3%, reversible proteinuria in 10%, poor wound healing and intestinal perforation in 5%, often in the setting of more than three previous courses of chemotherapy and partial small bowel obstruction. Four randomized trials have been undertaken; two are in first line (GOG 218 and ICON7) and two in recurrent platinum-sensitive disease (OCEANS and GOG 213) [38]. In GOG 218, the administration of bevacizumab during treatment with carboplatin and paclitaxel and for 15 months thereafter prolonged progression-free survival by 3.8 months. Median overall survival was not affected. In ICON7, concurrent treatment with bevacizumab, carboplatin and paclitaxel followed by 9 months maintenance also significantly improved progression-free survival. In recurrent disease, the administration of bevacizumab with carboplatin and gemcitabine followed by maintenance bevacizumab prolonged progression-free survival by 4 months. Results have not yet been reported for GOG 213.
Figure 3.
Impact of targeted therapy in the Gynecologic Oncology Group (GOG) 170 trials. When the fraction of responses and the fraction of patients with stable disease for 6 months are considered, treatment with bevacizumab has the best outcome (courtesy of Dr Robert Coleman).
In addition to endothelial cells, pericytes contribute to the stability of tumor vessels and can also be targeted for more effective antivascular therapy. Whereas endothelial cells require VEGF and bear VEGF receptors, pericytes bear platelet-derived growth factor receptors (PDGFR) and depend upon PDGF produced by endothelial and cancer cells. Dual function tyrosine kinase inhibitors can inhibit both vascular endothelial growth factor receptors and PDGFR, potentiating chemotherapy-induced apoptosis of endothelial cells and pericytes as well as tumor cells. A phase II trial of a dual function tyrosine kinase inhibitor, vandetinib (ZD6474), in combination with docetaxel is being carried out by the Southwest Oncology Group (United States) and accrual should be completed by the end of 2011.
Given the expense and potential toxicity of bevacizumab and other antiangiogenic agents, predictive biomarkers to identify patients likely to respond or not respond are a critical unmet need.
eliminating dormant ovarian cancer cells
Approximately 70% of ovarian cancer patients can be placed in complete clinical remission with cytoreductive surgery and chemotherapy using carboplatin and paclitaxel. Second-look surgery has documented small deposits of residual disease in nearly half of patients following apparently successful chemotherapy. All but 20% of advanced-stage patients will relapse in an average of 18 months, but some patients will relapse after 4 years, presumably related to the persistence of dormant ovarian cancer cells. Factors controlling dormancy are not well understood.
Our laboratory has studied an imprinted tumor suppressor gene ARHI (DIRAS3) that may have an important role in inducing dormancy in ovarian cancers [39, 40]. ARHI is a 26 kDa GTPase that has 50%–60% homology to Ras and Rap, but also has an N-terminal extension that reverses Ras function. ARHI is a maternally imprinted tumor suppressor gene that is down-regulated in 60% of ovarian cancers, which is associated with decreased progression-free survival. The re-expression of ARHI at physiologic levels inhibits proliferation, motility and xenograft growth, triggers autophagy, and induces tumor dormancy [41].
Autophagy has an important function in normal cellular physiology. Damaged mitochondria and endoplasmic reticulum are engulfed by double membrane vesicles that fuse with lysosomes, creating autophagosomes that digest and degrade both proteins and lipids, releasing amino acids and fatty acids that can generate ATP. Autophagy also has an important role during normal development. The survival of mammals in the first 24 h postpartum depends upon autophagy in the liver and heart. Autophagy has a dual and opposing role in cancer cells, acting as a suppressor of oncogenesis, but also as a survival mechanism for transformed cancer cells. Genetically engineered mice that are hemizygous for beclin, a critical inducer of autophagy, develop breast cancers more frequently and at an earlier interval than wild-type mice [42]. Once cancers develop, however, autophagy may favor the survival of cancer cells by providing essential energy and carbon precursors in the nutrient-poor microenvironment found in many tumors.
The re-expression of ARHI induces autophagy and growth arrest in SKOv3 ovarian cancer cells both in culture and in nu/nu murine xenografts [40]. In xenografts, cancer cells remain dormant, but grow promptly when ARHI levels are reduced. If mice are treated with chloroquine, a functional inhibitor of autophagy, while ovarian cancer cells are dormant, outgrowth of tumors is significantly delayed when ARHI levels are reduced. In culture, 90% of autophagic cancer cells die within 3 days, but can be rescued by treatment with survival factors found at the xenograft site, including VEGF, IL-8 and IGF-1. These observations are consistent with a model where dormant ovarian cancer cells require low levels of autophagy to generate ATP in order to survive in an avascular, nutrient-poor environment. Survival factors produced both by the tumor and the microenvironment, including VEGF, IL-8 and IGF-1, prevent autophagic death in dormant cells. Experiments are underway to test combinations of chloroquine to inhibit autophagy and specific antibodies to neutralize survival factors. The clinical relevance of this model is supported by the observation made in collaboration with Dr Douglas Levin at the Memorial Sloane Kettering Cancer Center that ARHI is expressed by <40% of ovarian cancers at primary surgery, but in 80%–90% of ovarian cancer nodules that persist at second-look operations.
personalizing detection of early-stage disease
rationale and requirements for screening
One of the major reasons that patients succumb to ovarian cancer is that the disease is diagnosed late. When ovarian cancer is detected while still confined to the ovaries (stage I), ≥90% of patients can be cured with currently available therapy. Disease that has spread from the pelvis can be cured in only ≤20%. Only 25% of ovarian cancers are currently diagnosed in stage I. The detection of preclinical disease at an earlier stage might improve survival, although this needs to be proven, as the current diagnosis of early-stage disease may detect cancers with a more indolent course and may fail to include more aggressive cancers.
There are stringent requirements for ovarian cancer screening in the community. Even in the postmenopausal population that is at greatest risk, the prevalence of ovarian cancer is 40/100 000 or 1 in 2500. In order to achieve a positive predictive value of 10%, i.e. no more than 10 operations for each case of ovarian cancer detected, high sensitivity is required (>75%), but very high specificity is essential (>99.6%).
CA125 and transvaginal sonography
In the past, three different approaches have been used to screen for epithelial ovarian cancers, including sonography, serum/plasma or urine markers and two stage strategies using abnormal levels of serum biomarkers to prompt sonography. Soon after the discovery of CA125 [43], our group studied sera from a patient with acquired hypogammaglobulinemia who subsequently developed ovarian cancer [44]. Sera had been saved to monitor globulin levels. When we analyzed serial serum specimens for CA125, we found that biomarker levels remained within normal limits for several years prior to diagnosis, but rose abruptly ∼1 year prior to diagnosis of her ovarian cancer. CA125 began to increase linearly on a log scale, suggesting that there might be a lead time for early detection. In other studies, CA125 has been elevated for 10–60 months prior to diagnosis. Elevated CA125 (>35 U/ml) has exhibited a sensitivity of 50%–60% for stage I disease.
Specificity can be improved by combining serum biomarkers with transvaginal sonography (TVS). Both specificity and sensitivity can be improved by sequential monitoring. Steven Skates has studied preclinical specimens from patients with ovarian cancer. In patients with malignant disease, CA125 rises persistently, whereas in patients with benign disease, CA125 can be elevated, but the values tend to remain constant. Consequently, rising CA125 values are associated with ovarian cancer and stable CA125 values, even when elevated, are associated with benign conditions. A Bayesian computer algorithm has been developed that estimates the risk of ovarian cancer based on change-point analysis during annual sequential monitoring of CA125 over time. This is ‘personalized’ screening, in that each woman serves as her own control.
UKCTOCS trial
Professors Ian Jacobs and Usha Menon have undertaken a large, 200 000 woman study of screening in postmenopausal women at average risk in the UK (UKCTOCS) [45]. Some 100 000 women serve as controls and undergo surveillance by their family physician with annual pelvic examination; ∼50 000 have annual TVS; and ∼50 000 have annual CA125 with <2% for TVS based on a rising CA125. Steven Skates has developed a risk-of-ovarian-cancer algorithm, which uses change-point analysis to identify women likely to have ovarian cancer based on a rising CA125. After an annual CA125 determination, women judged to be at normal risk are asked to return in 1 year for CA125. Women with clearly increased risk are referred for TVS and consultation with a gynecologic oncologist. If the TVS is abnormal, surgery is performed. If screened women are at intermediate risk, they return in 3 months and CA125 is repeated. If risk is not altered, women return in 1 year. If risk is further elevated, women proceed to gynecologic oncology consultation, TVS and potentially to surgery.
In an analysis of the prevalence phase of the UKCTOCS study, 48% of cancers found by screening were in stage I–II, doubling the detection of early-stage disease. CA125 followed by TVS detected 89% of the ovarian cancers. CA125 followed by TVS prompted 2.8 operations per case of ovarian cancer diagnosed compared with 36.2 operations per case of ovarian cancer diagnosed with annual TVS alone. With annual TVS in all patients, a number of benign lesions were found, but it was not possible to rule out malignancy and a larger number of operations were performed. Importantly, the prevalence of ovarian cancer was twice the incidence, consistent with a 2-year lead time and the feasibility of an annual screen.
MD Anderson Cancer Center SPORE trial
In collaboration with Dr Karen Lu and the MD Anderson Cancer Center Ovarian SPORE study, a screening trial has been conducted at six sites in the United States using the strategy in the third arm of the UKCTOCS trial [46]. In this study, postmenopausal women at average risk for developing ovarian cancer have annual CA125 monitoring and are referred to surgery if the risk of ovarian cancer is elevated based on Steven Skates’ algorithm. The MD Anderson Cancer Center trial is adequately powered to test the specificity and positive predictive value of the screen, but not to detect improved survival. This study is testing the feasibility of screening in the United States and has also developed a serum, plasma and urine bank over multiple years, which has permitted the identification and validation of additional biomarkers.
Over the last 9 years, 10 679 samples have been obtained from 3252 postmenopausal women at conventional risk. Less than 0.9% has been referred for ultrasound after each annual screening and 2.6% over multiple years on study. Nine operations have been prompted by the algorithm and have detected five cases of ovarian cancer—two borderline IA and IC, IC, and IIB invasive high grade—and one case of endometrial cancer. One borderline tumour was not detected but no invasive cancers were missed. No more than three operations will be required to detect each case of ovarian cancer using this strategy.
multimarker panels
In 20% of ovarian cancers, CA125 is not expressed at the tissue level [47]. Consequently, using the Skates’ algorithm, only 80% of ovarian cancers could be detected. Greater sensitivity might be achieved with multiple markers, provided that specificity is not compromised. In collaboration with Anna Lokshin at the University of Pittsburgh we used a Luminex assay to evaluate 96 biomarkers in sera from patients with stage I/II ovarian cancer, in healthy women and in patients with benign pelvic tumors [48]. The 12 most promising markers were chosen. An algorithm was used to create a diagnostic model, based on a set of sera from 139 patients with stage I/II ovarian cancer, 149 patients with stage III/IV ovarian cancer and 1102 healthy women.
The multimarker panel that provided the highest diagnostic power for both early-stage and late-stage disease was comprised of four biomarkers (CA125, HE4, CEA and soluble vCAM). This panel detected early-stage disease with 86% sensitivity at 98% specificity. When applied to an independently collected validation set consisting of sera from 44 patients with early-stage ovarian cancer, 124 with late-stage ovarian cancer and 929 healthy women, the four biomarkers exhibited 82% sensitivity at 98% specificity. A new algorithm has been developed to include the four biomarkers over time. A new screening trial will be initiated to test the specificity and positive predictive value of the four-biomarker algorithm. The four marker assays will be developed with laboratory-on-a-chip nanotechnology, permitting one-stop screening at the point of service. A CA125 assay has already been established with nanotechnology. A nano biochip will permit tests for ovarian cancer in 30 min with a fingerstick of blood. This would permit the development of a screening center where women might have a fingerstick and within 30 min know if they should come back in a year or obtain an ultrasound on the same day.
autoantibodies
Very small serous cancers of the ovary or fallopian tube may evoke autoantibodies before protein markers are elevated. In collaboration with Origene, blood from 38 early-stage and 26 late-stage ovarian cancer patients and 37 healthy women has been tested for autoantibodies against 10 464 human proteins expressed in human cells, and placed in a protein array. Autoantibodies to 159 proteins were found in serum from 58% of early-stage and 65% of late-stage ovarian cancer patients. In early-stage ovarian cancer, autoantibodies were found in 42%, CA125 was elevated in 51% and either occurred in 71%. In healthy controls, autoantibodies were seen in 14%. Of the six early-stage patients encountered in the MDACE SPORE screening trial, autoantibodies were found in three at the time of diagnosis. Autoantibodies were also detected 12 months prior to diagnosis in two of the six patients.
prospects for early detection
In summary, the early detection of ovarian cancer could have a major impact on the disease. Two stage strategies are likely to be most effective. Multiple serum markers will be needed for an optimal initial stage. If the UKCTOCS trial shows a survival advantage, the MD Anderson Cancer Center SPORE trial has demonstrated feasibility in the United States. New MD Anderson Cancer Center SPORE studies will evaluate potentially more sensitive and more convenient screening strategies.
conclusion
Considering the data cited in this review, it is likely that over the coming years we will identify combinations of targeted and conventional agents that will significantly improve the survival of ovarian cancer patients and we will also validate techniques for early detection. In addition, it may be possible to develop novel strategies to enhance sensitivity to paclitaxel and carboplatin, as well as to eliminate dormant drug-resistant ovarian cancer cells.
funding
This work was supported by funds from the MD Anderson SPORE in Ovarian Cancer NCI P50 CA83639, the MD Anderson CCSG NCI P30 CA16672, the National Foundation for Cancer Research, the Ovarian Cancer Research Fund, and philanthropic support from Golfers Against Cancer, the Tracey Jo Wilson Foundation, the Mossy Foundation, The Zarrow Foundation and Stuart and Gaye Lynn Zarrow NCI 1R01 CA135354-01.
disclosures
Robert C. Bast receives royalties for the discovery of CA125 and serves on scientific advisory boards for Fujurebio Diagnostics, Vermillion, Inc. and Illumina, Inc.
Acknowledgments
Drs Gordon Mills, David Gershenson and Robert Coleman kindly provided figures for this review.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Bookman M, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer InterGroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess LM, Benham-Hutchins M, Herzog TJ, et al. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Int J Gynecol Cancer. 2007;17:561–570. doi: 10.1111/j.1525-1438.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 6.Rustin GJ, van der Burg ME, Griffin CL, et al. MRC OV05/EORTC 55955 investigators. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–1163. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 7.Santillan A, Garg R, Zahurak ML, et al. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. J Clin Oncol. 2005;23:9338–9343. doi: 10.1200/JCO.2005.02.2582. [DOI] [PubMed] [Google Scholar]
- 8.Wilder JL, Pavlik E, Straughn JM, et al. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation. Gynecol Oncol. 2003;89:233–235. doi: 10.1016/s0090-8258(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 9.Bast RC., Jr Commentary: CA125 and the detection of recurrent ovarian cancer: a reasonably accurate biomarker for a difficult disease. Cancer. 2010;116:2850–2853. doi: 10.1002/cncr.25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 11.Fleming ND, Cass I, Walsh CS, et al. CA125 surveillance increases optimal respectability at secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecol Oncol. 2011;121:249–252. doi: 10.1016/j.ygyno.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Callahan R, Hurvitz S. Human epidermal growth factor receptor-2-positive breast cancer: current management of early, advanced, and recurrent disease. Current Opinion Obstet Gynecol. 2011;23:37–43. doi: 10.1097/gco.0b013e3283414e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbour E, Cortes J, Kantarjian H. Long-term outcomes in the second-line treatment of chronic myeloid leukemia: a review of tyrosine kinase inhibitors. Cancer. 2011;117:897–906. doi: 10.1002/cncr.25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essaf M, Cooper K. Imatinib as adjuvant therapy for gastrointestinal stromal tumors: a systematic review. Int J Cancer. 2011;128:2202–2214. doi: 10.1002/ijc.25827. [DOI] [PubMed] [Google Scholar]
- 15.Mok TS, Zhou Q, Leung L, et al. Personalized medicine for non-small cell lung cancer. Expert Rev Anticancer Ther. 2010;10:1601–1611. doi: 10.1586/era.10.76. [DOI] [PubMed] [Google Scholar]
- 16.Tsinmberidou AM. Personalized medicine in a phase I trials program: the M.D. Anderson initiative. J Clin Oncol. 2011;29(18S) 787s (Abstr CRA2500) [Google Scholar]
- 17.Mehra K, Mehrad M, Ning G, et al. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci. 2011;3:625–634. doi: 10.2741/e275. [DOI] [PubMed] [Google Scholar]
- 18.Berek JS, Friedlander ML, Bast RC., Jr . Ovarian cancer. In: Hong WK, Bast RC Jr, Hait W, et al., editors. Holland–Frei Cancer Medicine. 8th edition. Shelton, CT: PMPH: 2010. pp. 1344–1375. [Google Scholar]
- 19.Jacobs IJ, Kohler MF, Wiseman R, et al. Clonal origin of epithelial ovarian carcinoma: analysis by loss of heterozygosity, p53 mutation and X chromosome inactivation. J Natl Cancer Inst. 1992;84:1793–1798. doi: 10.1093/jnci/84.23.1793. [DOI] [PubMed] [Google Scholar]
- 20.Cheng W, Liu J, Yoshida H, et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11:531–537. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]
- 21.Kurman RJ, Shih IEM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong KK, Tsang YT, Deavers MT, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McConechy MK, Anglesio MS, Kalloger SE, et al. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J Pathol. 2011;223:567–573. doi: 10.1002/path.2848. [DOI] [PubMed] [Google Scholar]
- 25.King ER, Zu Z, Tsang YT, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol. 2011;123:13–18. doi: 10.1016/j.ygyno.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima K, Konopleva M, McQueen T, et al. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nature Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 30.Bast RC, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol. 2010;28:3545–3548. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- 31.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:106–115. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 32. Ahmed AA, Wang X, Lu Z et al. Modulating microtubule stability enhances the cytotoxic response of cancer cells to paclitaxel. Cancer Res 2011; 71: 5806–5817. [DOI] [PMC free article] [PubMed]
- 33.Ahmed AA, Lu Z, Jennings NB, et al. SIK2 is a centrosome kinase required for bipolar spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell. 2010;18:109–121. doi: 10.1016/j.ccr.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehurst AW, Xie Y, Purinton SC, et al. Tumor antigen acrosin binding protein normalizes mitotic spindle function to promote cancer cell proliferation. Cancer Res. 2010;70:7652–7661. doi: 10.1158/0008-5472.CAN-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangala LS, Zuzel V, Schmandt R, et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15:3770–3780. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecot CV, Calin GA, Coleman RL, et al. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledermann JA, Raja FA. Targeted trials in ovarian cancer. Gynecol Oncol. 2010;119:151–156. doi: 10.1016/j.ygyno.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Eskander RN, Randall LM. Bevacizumab in the treatment of ovarian cancer. Biologics. 2011;5:1–5. doi: 10.2147/BTT.S13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Xu F, Fang X, et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci USA. 1999;96:214–219. doi: 10.1073/pnas.96.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu Y, Luo R, Lu Z et al. Biochemistry and biology of ARHI (DIRAS3), an imprinted tumor suppressor gene whose expression is lost in ovarian and breast cancers. In Balch WE, Der C, Hall A (eds), Methods in Enzymology, Vol. 407: Regulators and Effectors of Small GTPases. Part D. Ras Proteins. 2006; 455–467. [DOI] [PubMed]
- 41.Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bast RC, Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. New Engl J Med. 1983;309:883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 44.Bast RC, Jr, Siegal FP, Runowicz C, et al. Elevation of serum CA 125 prior to diagnosis of an epithelial ovarian carcinoma. Gynecol Oncol. 1985;22:115–120. doi: 10.1016/0090-8258(85)90015-0. [DOI] [PubMed] [Google Scholar]
- 45.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 46.Lu KH, Skates S, Bevers T, et al. A prospective U.S. ovarian cancer screening study using the risk of ovarian cancer algorithm (ROCA) Proc Am Soc Clin Oncol. 2010:28. (Abstr 5003) [Google Scholar]
- 47.Rosen D, Wang L, Atkinson JN, et al. Potential markers that complement expression of CA 125 in epithelial ovarian cancer. Gynecol Oncol. 2005;99:267–277. doi: 10.1016/j.ygyno.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 48.Yurkovetsky Z, Lomakin A, Skates S, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–2166. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]