Abstract
Lyme disease is caused by infection with the spirochete pathogen Borrelia burgdorferi, which is maintained in nature by a tick-rodent infection cycle 1. A tick-borne murine model 2 has been developed to study Lyme disease in the laboratory. While naíve ticks can be infected with B. burgdorferi by feeding them on infected mice, the molting process takes several weeks to months to complete. Therefore, development of more rapid and efficient tick infection techniques, such as a microinjection-based procedure, is an important tool for the study of Lyme disease 3,4. The procedure requires only hours to generate infected ticks and allows control over the delivery of equal quantities of spirochetes in a cohort of ticks. This is particularly important as the generation of B. burgdorferi infected ticks by the natural feeding process using mice fails to ensure 100% infection rate and potentially results in variation of pathogen burden amongst fed ticks. Furthermore, microinjection can be used to infect ticks with B. burgdorferi isolates in cases where an attenuated strain is unable to establish infection in mice and thus can not be naturally acquired by ticks 5. This technique can also be used to deliver a variety of other biological materials into ticks, for example, specific antibodies or double stranded RNA 6. In this article, we will demonstrate the microinjection of nymphal ticks with in vitro-grown B. burgdorferi. We will also describe a method for localization of Lyme disease pathogens in the tick gut using confocal immunofluorescence microscopy.
Protocol
1. Microinjection of Nymphal Ixodes scapularis Ticks
1. Preparing needles
Fabricate several microinjection needles by heating and pulling 1 mm glass capillary tubes (World Precision Instruments) in a glass micropipette puller device (Narishige). Carefully remove the fragile capillary tubes.
Store pulled needles (with tip facing upward) on adhesive tape in a Petri dish.
2. Preparing B. burgdorferi
Grow B. burgdorferi in BSK culture media 7 until at a concentration of about 107 cells per mL. Spirochetes are counted under a dark-field microscope by use of a Petroff-Hausser counting chamber (Hausser scientific).
Pellet B. burgdorferi by centrifugation at 3,000 x g for 10 minutes at room temperature.
Remove supernatant and completely resuspend the pellet in BSK media by gently passing through a 200 μL sterile microtip to a final concentration of 109 cells per mL. Since the cells are resuspended at high cell density, and could clump together, the cell suspension should be used for microinjection immediately.
3. Preparing ticks
Place clear, double-sided adhesive tape on a glass slide.
Working on a sticky mat, carefully remove nymphal ticks from the container using a small brush.
Place the required number of ticks to be injected on the adhesive tape, ventral side up, facing the same direction.
4. Injecting ticks
Load 5 μL of the B. burgdorferi culture into the pulled capillary needle using a 20 μL microloader pipette tip (Eppendorf). Inspect the needle for trapped air bubbles and reload the needle with bacterial culture, if necessary.
View the tick under the dissecting binocular microscope and focus on the tick's anal aperture area. Gently touch the tip of the capillary needle in order to break the tube where the diameter is slightly smaller than that of the tick's anal aperture, forming the microinjection needle. This step is critical as any attempt to inject ticks with a non-appropriate needle tip causes injury and possible death of the injected tick.
Place the immobilized ticks under the dissecting binocular microscope and focus on the anal aperture, which is covered by two movable anal plates. Using fine forceps, gently touch and apply very mild pressure to any area near anal aperture. This will allow separation of the anal plates and opening of anal pore that connects to the gut. Carefully insert the tip of the needle slightly into the anal aperture through the forced opening of the anal plates. Needle insertion should be kept to a minimum as the glass tip could damage the semitransparent hindgut that connects to the rectum. Using a microinjector equipped with automated foot control (Eppendorf), inject B. burgdorferi solution using the following parameters: 1,000 hectopascals (hPa) injection pressure, 0.2 seconds injection time and 8 hPa compensation pressure. Each tick receives a single injection.
Following microinjection, the ticks should behave similar to the pre-injection state. For example, the tick should crawl in response to stimuli.
We usually allow the ticks to recover for 48 hours in an environmental chamber set at 24°C with 16 hours/8 hours light/dark photoperiod regimen and 95% humidity. If a chamber is not available, injected ticks could be stored at room temperature in moist conditions within an air-tight container, such as a regular desiccator chamber. If necessary, recovery time of the ticks could also be shortened to a few hours before used for feeding on mice.
2. B. burgdorferi Localization by Confocal Immunofluorescence Microscopy
1. Dissecting ticks
Place a droplet of phosphate buffered saline (PBS) on a clean glass slide for dissection and another on a poly-L-lysine coated slide (Sigma) for microscopy. Prepare another glass slide with double-sided adhesive tape.
Remove the tick from the container and place on double-sided adhesive tape using a small brush.
View and focus the tick in the dissection microscope. Place a sharp razor blade on the tick between the first and second pairs of legs. Press down firmly cutting the tick in two pieces allowing access to the abdomen. Immediately, submerge the abdomen in a droplet of PBS.
Using very fine forceps, grab the dorsal and ventral exoskeleton around the site of the cut. Carefully pull the dorsal shield up and away from the tick exposing the brownish colored gut diverticula. Be careful to keep the dissecting ticks under the PBS at all times.
The semi-translucent salivary gland bundles are located on either side of the anterior region of the gut, and can be removed at this point, if desired. Holding the remaining exoskeleton in place with forceps, carefully pull the gut out from the abdomen.
Gently clean the gut by removing any trapped tissues (for example, trachea).
Using the tip of fine forceps, quickly transfer the tick gut in a droplet of PBS on a poly-L-lysine glass slide. Separate the gut into smaller pieces using fine blades or gently pressing with the tip of fine forceps. Carefully aspirate excess PBS around the gut tissues.
Allow the gut tissues to air dry at room temperature.
Fix the tick gut by submerging in acetone for 10 minutes. Allow to air dry at room temperature. Slides can be stored at this step for months at -20°C in an air-tight container.
2. Staining
Using tissue paper, remove excess moisture around the tissue and draw a circle around the dried tick gut by a pap-pen or any hydrophobic barrier lining device. This will help to retain the staining solutions during subsequent incubation steps.
Cover the tick gut with one or a few drops of blocking buffer (0.05% Tween-20, 5% goat serum in PBS) for 30 minutes at room temperature. The serum used for blocking depends on the source of host animal for the antibody. Do not allow the slide to dry from this point forward.
Slowly aspirate the blocking buffer. Incubate the gut with appropriate primary and/or secondary antibody solutions. We use fluorescein isothiocyanate (FITC)- labeled anti-B. burgdorferi antibody (Kirkegaard & Perry Laboratories) at a 1:100 dilution in blocking buffer for 1 hour at room temperature. Cover the container with aluminum foil to limit exposure to light.
Remove the antibody solution by gentle aspiration. Incubate the gut with a fluorescent dye to label tick tissues, such as 4',6-diamidino-2-phenylindole (DAPI) or propidium iodide. We normally use 20 μg/ mL propidium iodide (Sigma) in PBS for 5 min. at room temperature.
Wash 3 times with 0.05% Tween-20 in PBS.
Mount the slide in buffered glycerol containing an antifade reagent such as Slowfade (Invitrogen) and carefully cover with a glass coverslip. Slides can be stored at this step for a few months at 4°C within an air-tight container. Image and localize B. burgdorferi under a confocal microscope.
3. Representative Results
Position of a nymphal tick for microinjection and an image representing B. burgdorferi localization in the tick gut is presented in Figure 1.
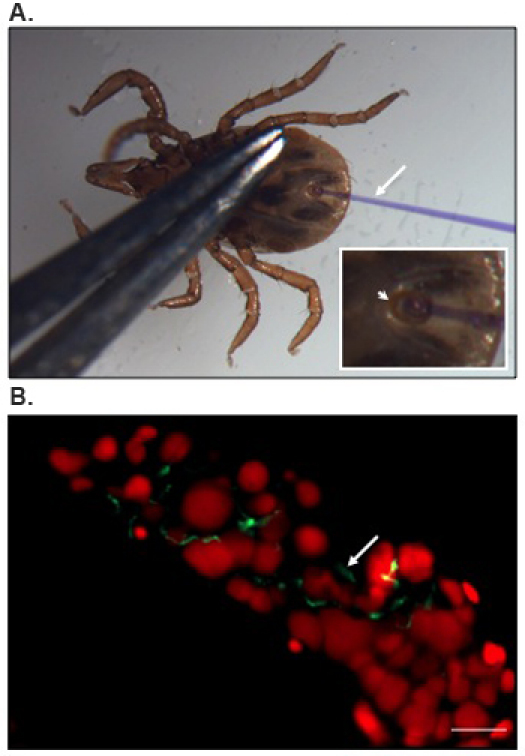 Figure 1. Microinjection and localization of B. burgdorferi into the tick gut.
(A) Ventral view of a nymphal I. scapularis tick positioned for microinjection. Anal aperture (arrowhead) with inserted microinjection needle (arrow) is shown under magnification in the inset. A fine forcep is used to apply gentle pressure to the body, which allowed separation of the anal plates and opening of the anal pore for microinjection. The needle is filled with a solution of Coomassie brilliant blue to enhance visibility. (B) Representative results of confocal immunofluorescence imaging of B. burgdorferi within tick gut. Anterior region of a gut diverticulum is shown. Gut nuclei and spirochetes (arrow) are labeled with propidium iodide (red color) or FITC-conjugated anti-B. burgdorferi (green color), respectively. Bar = 20 μm.
Figure 1. Microinjection and localization of B. burgdorferi into the tick gut.
(A) Ventral view of a nymphal I. scapularis tick positioned for microinjection. Anal aperture (arrowhead) with inserted microinjection needle (arrow) is shown under magnification in the inset. A fine forcep is used to apply gentle pressure to the body, which allowed separation of the anal plates and opening of the anal pore for microinjection. The needle is filled with a solution of Coomassie brilliant blue to enhance visibility. (B) Representative results of confocal immunofluorescence imaging of B. burgdorferi within tick gut. Anterior region of a gut diverticulum is shown. Gut nuclei and spirochetes (arrow) are labeled with propidium iodide (red color) or FITC-conjugated anti-B. burgdorferi (green color), respectively. Bar = 20 μm.
Discussion
Here we demonstrate a microinjection-based procedure for rapid and efficient infection of nymphal Ixodes ticks with the bacterial pathogen B. burgdorferi. We also describe a confocal immunofluorescence procedure for the detection of B. burgdorferi in the tick gut in situ. Although our demonstration involves nymphal gut, similar procedures are also applicable for other developmental stages of ticks, such as larva or adults 8,9. However, due to their smaller size, the technique may be relatively challenging for use in larvae, but should be well applicable for use in adult ticks. Other methods of artificial infection of ticks with B. burgdorferi have been developed, such as feeding through glass capillary tubes 10 or by immersion in the culture medium 11. These methods are efficient, simpler and relatively inexpensive. However, these procedures rely on relatively uncontrolled transfer of B. burgdorferi into individual ticks and thus potentially are limited in generating cohorts of infested ticks with equal pathogen burdens. The latter shortcoming could be substantially overcome by more controlled delivery procedures, such as microinjection. In our laboratory, we routinely employ this procedure to transfer B. burgdorferi into ticks with infection rates of nearly 100%, and most ticks survive the procedure. Injected ticks can be kept in the laboratory for several weeks to months, or immediately allowed to engorge on mice and transmit B. burgdorferi infection. The efficiency and kinetics of B. burgdorferi transmission from microinjected ticks are similar to that of naturally-infected ticks, and therefore, artificial tick infection procedures are likely to assist in our efforts to study tick-borne Lyme borreliosis. Additionally, similar microinjection techniques have also been applied for related experimental purposes, for example, RNA interference-mediated genetic manipulation of ticks 6,9,12.
Microinjection of immature stages of ticks is a relatively delicate procedure. It is thus important to verify that the ticks injected with B. burgdorferi are healthy before proceeding to the next set of experiments. Post-injected ticks that have retracted legs and are unresponsive to stimuli, such as exhaled breath or touching with a brush, should not be used for further experiments. These are most likely dead or on the verge of dying from the injection trauma. We have found that the size of the needle tip and injection parameters are the two critical factors in the procedure, as larger needle tips and injection volumes could potentially rupture the gut wall resulting in high tick mortality. It is also important to note that the microinjection settings described here are primarily optimized for injecting B. burgdorferi suspended in BSK media. Other biological materials, such as antibodies or concentrated RNA solutions, could differ in fluid viscosity. For other materials, the optimal microinjection settings, primarily the injection pressure and injection time, need to be determined empirically.
Disclosures
No conflicts of interest declared.
Acknowledgments
We sincerely thank members of the Pal laboratory for assistance with the preparation of this demonstration. This study was supported by PHS grants AI076684 and AI080615 from NIH/NIAID.
References
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Diego C, Philipp MT. In: Borrelia, Molecular Biology, Host Interaction and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic Press; 2010. pp. 353–405. Chapter 14. [Google Scholar]
- Pal U. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang X, Kumar M, Pal U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J Infect Dis. 2009;200:1318–1330. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S. A tick antioxidant facilitates the Lyme disease agent's successful migration from the mammalian host to the arthropod vector. Cell Host Microbe. 2007;2:7–18. doi: 10.1016/j.chom.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater AH, Sonenshine DE, Hynes WL, Ceraul S, DeSilva A. Glass capillary tube feeding: a method for infecting nymphal Ixodes scapularis (Acari: Ixodidae) with the Lyme disease spirochete Borrelia burgdorferi. J Med Entomol. 2002;39:285–292. doi: 10.1603/0022-2585-39.2.285. [DOI] [PubMed] [Google Scholar]
- Policastro PF, Schwan TG. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol. 2003;40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- Fuente dela, Kocan J, M K, Almazan C, Blouin EF. RNA interference for the study and genetic manipulation of ticks. Trends Parasitol. 2007;23:427–433. doi: 10.1016/j.pt.2007.07.002. [DOI] [PubMed] [Google Scholar]


