Abstract
Phagocytic cells such as alveolar macrophages and lung dendritic cells (LDCs) continuously sample antigens from the alveolar spaces in the lungs. LDCs, in particular, are known to migrate to the lung draining lymph nodes (LDLNs) where they present inhaled antigens to T cells initiating an appropriate immune response to a variety of immunogens1,2. To model interactions between the lungs and airborne antigens in mice, antigens can be administered intranasally1,3,4, intratracheally5 or as aerosols6. Delivery by each route involves distinct technical skills and limitations that need to be considered before designing an experiment. For example, intranasal and aerosolized exposure delivers antigens to both the lungs and the upper respiratory tract. Hence antigens can access the nasal associated lymphoid tissue (NALT)7, potentially complicating interpretation of the results. In addition, swallowing, sneezing and the breathing rate of the mouse may also lead to inconsistencies in the doses delivered. Although the involvement of the upper respiratory tract may be preferred for some studies, it can complicate experiments focusing on events specifically initiated in the lungs. In this setting, the intratracheal (i.t) route is preferable as it delivers test materials directly into the lungs and bypasses the NALT. Many i.t injection protocols involve either blind intubation of the trachea through the oral cavity or surgical exposure of the trachea to access the lungs. Herein, we describe a simple, consistent, non-surgical method for i.t instillation. The opening of the trachea is visualized using a laryngoscope and a bent gavage needle is then inserted directly into the trachea to deliver the innoculum. We also describe procedures for harvesting and processing of LDLNs and lungs for analysis of antigen trafficking by flow cytometry.
Protocol
1. Before the process, prepare and gather the following items
Please refer to the image in Figure 1a) and 1b) for building a wooden platform to restrain the mouse during the procedure.
Digestion mix for lymph nodes - HBSS + 1.25mg/ml Collagenase TypeIV or 2.5 mg/ml Collagenase D
Digestion mix for lungs - HBSS + 1 mg/ml Collagenase
Dilute latex beads (1:20) in PBS for intraperitoneal injection to visualize the LDLNs
Note: LDLNs are small and hard to find. To learn how to find them, inject 200μl of the 1:20 diluted red fluorescent latex beads i.p into the mouse. The peritoneum drains to these LDLNs8. 24 hpi, expose the thoracic cavity of the mouse as described above. The LDLNs will appears pink from the beads that drained from the peritoneal cavity.
Red Blood Cell (RBC) Lysis Buffer - 0.15M NH4Cl, 10mM KHCO3, 0.1 mM Na2EDTA in 1L dH20. Adjust pH to 7.2-7.4 and store at room temperature.
70μm or 100μm cell strainer
Stock solution of 0.5M EDTA, pH = 8.0
HBSS without CaCl2 and MgCl2
Scissors and blunt-ended forceps
Laryngoscope
Gavage needle (22 gauge) bent at an angle (Figure 2)
Styrofoam board and pins for spreading the mouse
Top pan balance
Aniline Blue Solution - 10mg/ml in H2O
Note: We will be using this solution for demonstration of i.t injection technique.
2. Preparation and administration of mouse anesthetic
Prepare a mixture of Xylazine and Ketamine in sterile PBS so that the final concentrations are 2mg/ml and 10mg/ml respectively.
Weigh the mouse on a top pan balance.
Calculate the dose required for each mouse. Dose at Xylazine (2-4 mg/kg) and Ketamine (70-80 mg/kg) body weight using appropriate volume of the prepared mixture and inject intaperitoneally. Each mouse strain may require optimization of anesthetic. Mice receiving too much anesthetic may have a reduced respiration rate that could be fatal after inoculum is instilled into the lungs.
- After 3-5 minutes, observe the mouse for the effects of the anesthetic. Check for the following signs to confirm that the mouse is fully anesthetized:
- The breathing rate should slow down.
- The mouse should not stretch its arm when picked up by the neck.
- There should not be any response when the hind limbs are stimulated. If these criteria are not met, wait for a few minutes and check again before proceeding to the next step.
3. Intratracheal injection
Prepare the innoculum in 50μl saline. Fill a 1ml syringe fitted with a sterile bent gavage needle with the innoculum. The syringe should be loaded with a 100μl air pocket behind the innoculum to ensure that all of the fluid is instilled into the lung.
Place the mouse on the angled wooden platform hanging by its incisors on the wire and gently restrain it in place with a piece of ribbon.
Turn on the laryngoscope with your left hand (if you are right handed) and grab a pair of blunt ended forceps. Use the tip of laryngoscope and the forceps to gently pry open the mouth.
With the forceps, pull the tongue out and hold it to the side. Guide the laryngoscope blade towards the back of the mouth. Keep the laryngoscope pressed down very gently at a 90° angle until you see the opening of the trachea. Hold the laryngoscope in place. Care should be taken when pulling the tongue from the mouth and this should be done gently so no tissue damages occurs.
With your other hand, take the 1ml syringe containing the innoculum, fitted with the bent gavage needle with your right hands and insert the needle into the trachea until the bend in the needle is by the front incisors. Push the plunger evenly to deliver the innoculum. The innoculum should not bubble. Pull the needle out of the trachea as soon as possible. Mice will suffocate and die if the trachea is blocked for too long. Hold the mouse upright for a few seconds to allow innoculum to be inhaled into the lungs.
Take the mouse out of the platform and place it near a heating lamp. Do not leave mice under direct heat for extended periods of time. The mouse should be fully awake within 30 min after the procedure (recovery time may vary based on strains). Mice should be observed diligently during this time and should not be allowed to get too hot or too cold, this could also put the mice into respiratory distress leading to death.
4. Harvesting and processing Lung draining lymph nodes and lungs for flow cytometry
Sacrifice mice using the desired method as per the AVMA euthanasia guidelines. This may vary depending upon individual harvest outcomes. Administration of a lethal does of sodium pentobarbital is an example of an appropriate euthanasia procedure. We have not seen negative pulmonary effects of euthanasia by CO2.
Pin the arms and legs on a dissection board. Make an incision along the middle of the ventral side with scissors. Gently pull the skin and expose the thoracic wall muscles.
Make incisions through the thoracic wall muscles to expose the abdominal organs.
Hold the tip of sternum with the forceps and puncture the diaphragm. Cut the diaphragm along the sides of the rib cage.
With the scissors parallel to the dissection board surface, cut through the thoracic cavity along the dorso-ventral line on both sides.
Slightly lift the rib cage up. On the right side of the heart, just underneath the thymus, look for two lymph nodes. A slightly larger third lymph node is located underneath a small arterial blood vessel that runs perpendicular to the trachea. Harvest lymph nodes into 1 ml of lymph node digestion mix.
After harvesting the lymph nodes, hold the heart with a pair of forceps and grab the lungs with another. Harvest lungs into 3ml of ice cold PBS in a 6-well plate.
Separate the lobes of the lungs, aspirate PBS and chop the lobes into small pieces using forceps and scissors. Add 3ml of the digestion mix for lungs. Keep the 6-well plate on ice during this step.
Incubate the lymph nodes for 25-30 min and lungs for 30 min at 37°C. Add EDTA to a final concentration of 10mM to each of the digestion mixtures to stop the reaction and place on ice.
Using a 3ml syringe, pass pieces of lungs through an 18GA needle 3 times. Mash the pieces of the lungs and the LNs through 70-100μm cell strainers using the back end of a 1ml syringe plunger. Wash the cell strainers with 10ml of HBSS without Ca2+ and Mg2+. Centrifuge at 500 x g for 5 min.
Resuspend the pellet in 5 ml of RBC lysis buffer and incubate for 3 min at room temperature. Add 10ml of HBSS without Ca2+ and Mg2+ to quench lysis. Centrifuge at 500 x g for 5 min. Bring up the lung and LDLN single cell suspensions to 10ml and 5ml respectively. Count live cells using a hemacytometer by trypan blue exclusion. Cells are now ready to stain for flow cytometry. 1 lobe of the lung might be sufficient for flow cytometry. Other lobes can be used for other experimental questions within the same mouse such as homogenates for cytokines or tissue for histology.
5. Representative Results:
LDCs and alveolar macrophages in the lungs acquire antigen from the alveolar spaces of the lungs immediately following i.t injection. Figure 3 shows percentage of CD11c+ (expressed on LDCs and alveolar macrophages) cells that are positive for fluorescently labeled Bacillus anthracis spores, 30 min post i.t injection. At this time, ~15.9% of CD45+CD11c+ cells in the lung single cell suspension harbored Bacillus anthracis spores (Figure 3, lower panels). For further characterization of CD11c+ cells in the lungs, refer to Kim and Braciale4. Fluorescently labeled beads are routinely used to assess trafficking of particulate antigens to LDLNs9. To assess this transport from lungs to LDLNs, fluorescent latex beads were injected i.t with LPS and LDLNs were analyzed at 24 hpi. About 4% of CD11chi cells in the LDLNs harbored fluorescent beads at 24hpi (Figure 4). These representative experiments show that this protocol can be efficiently used to track cells that acquire inhaled particulate antigens in the lungs and subsequently traffic them to the LDLNs.
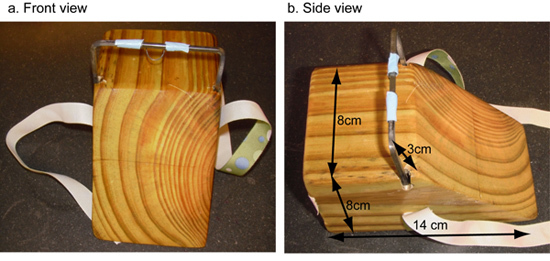 Figure 1. Wooden platform for restraining mice during i.t injection. A wooden block was cut at an angle and metal bars were fitted, front view figure 1a) and side view figure 1b). A small piece of wire was wound around the metal bar to anchor the front incisors of the mouse. A piece of ribbon was attached to the platform to tie around the mouse to keep it in place during the procedure.
Figure 1. Wooden platform for restraining mice during i.t injection. A wooden block was cut at an angle and metal bars were fitted, front view figure 1a) and side view figure 1b). A small piece of wire was wound around the metal bar to anchor the front incisors of the mouse. A piece of ribbon was attached to the platform to tie around the mouse to keep it in place during the procedure.
 Figure 2. Gavage needle for i.t injection. A gavage needle (right) was bent at an angle of ˜135°(left) for i.t injection.
Figure 2. Gavage needle for i.t injection. A gavage needle (right) was bent at an angle of ˜135°(left) for i.t injection.
 Figure 3. Percentage of all CD11c+ cells in the lungs that harbor i.t injected GFP expressing Bacillus anthracis (Sterne-p6GFP), 5hrs post challenge. AJ mice were challenge with 1x108 GFP- Bacillus anthracis spores and the lungs were analyzed at 5hrs post infection. The dot plots show percentage of CD11c+ lung cells that are positive for Bacillus anthracis.
Figure 3. Percentage of all CD11c+ cells in the lungs that harbor i.t injected GFP expressing Bacillus anthracis (Sterne-p6GFP), 5hrs post challenge. AJ mice were challenge with 1x108 GFP- Bacillus anthracis spores and the lungs were analyzed at 5hrs post infection. The dot plots show percentage of CD11c+ lung cells that are positive for Bacillus anthracis.
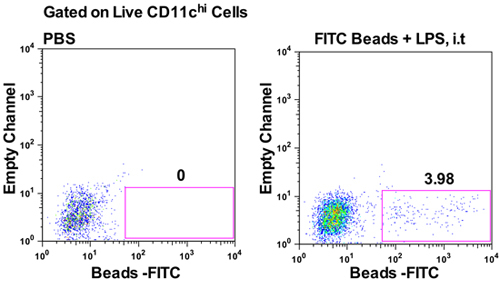 Figure 4. Detection of fluorescent beads (yellow/green) in the lung draining lymph nodes 24 hours following i.t injection. AJ mice were given either PBS (left panel) or 5x109 Fluoresbrite YG Microspheres (0.5μm) with 10μg LPS (right panel) i.t and LDLNs were analyzed 24hpi. Representative dot plots show percentage of CD11chi cells in the LDLNs that harbor fluorescent beads plotted against an empty channel.
Figure 4. Detection of fluorescent beads (yellow/green) in the lung draining lymph nodes 24 hours following i.t injection. AJ mice were given either PBS (left panel) or 5x109 Fluoresbrite YG Microspheres (0.5μm) with 10μg LPS (right panel) i.t and LDLNs were analyzed 24hpi. Representative dot plots show percentage of CD11chi cells in the LDLNs that harbor fluorescent beads plotted against an empty channel.
Discussion
We have utilized this protocol to study trafficking of Bacillus anthracis spores from the lungs to the LDLNs. For similar applications, the number of particles delivered to the lungs should be carefully chosen so that the injected material can be detected in the LDLNs by flow cytometry. We have also successfully used this method for adoptive transfer of cells and labeling of specific lung cell populations using fluorescently labeled antibodies. In addition, this method is routinely used to administer bleomycin (a chemical inducer of pulmonary fibrosis) and cytokines directly into the lungs10.
Although this method allows consistent delivery of test materials into the lungs, it requires extensive training and validation (achieved by instilling dye into the lungs). This method may not have the same physiological relevance as other routes of inhalation because it excludes the upper respiratory tract where important interactions and immune responses may be initiated. However, it is preferable where interpretation can be complicated by uptake of the administered agent by regional lymph nodes in the head and neck and by stimulation of the respiratory tract epithelium. As with all models, experimental factors such as dose, vehicle for delivery, distribution of the injected material, damage to the respiratory epithelium and effects of anesthesia should all be considered while designing experiments. Compared to the conventional procedures for i.t instillation, we believe that this method significantly reduces damage to the respiratory tract epithelium. Nonetheless, appropriate control groups should be used to account for injury since damage at the site of inoculation might affect the delivery of antigens to the lymph nodes11. We recommend using saline as a vehicle but if the test material requires specific media for effectiveness, vehicle controls should not be neglected. Distribution of the injected particulate material into the different lobes of the lungs may be an important parameter for certain experiments. In such cases, each of the lobes can be analyzed separately immediately following i.t injection. The distribution of materials into the different lobes of the lungs following i.t route of instillation has been described by Costa et al12.
We focus our analysis on the draining lymph nodes described above which are accessible in most wild type and knock out strains of mice used in our labs. However, there are additional draining lymph nodes in the thoracic region13,14 that might also be analyzed. If more lymph nodes are desirable for an experiment, we recommend initial identification using i.p injection of fluorescent beads as described in the protocol. Two of the lymph nodes that we recommend for analysis are in close proximity to the thymus therefore care should be taken during extraction.
As previously mentioned, this method can be adapted to expose lungs to a variety of infectious organisms, allergens or toxins. It provides a simple and consistent alternative to conventional routes of inhalation exposure and excludes the upper respiratory tract. It is particularly useful for experiments that focus on events that initiate in the lungs and the lung draining lymph nodes before any other site.
Disclosures
No conflicts of interest declared.
References
- Grayson MH. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1438. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–442. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- Bar-Haim E. Interrelationship between dendritic cell trafficking and Francisella tularensis dissemination following airway infection. PLoS Pathog. 2008;4:e1000211–e1000211. doi: 10.1371/journal.ppat.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204–e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakocevic N, Worbs T, Davalos-Misslitz A, Forster R. T cell-dendritic cell interaction dynamics during the induction of respiratory tolerance and immunity. J Immunol. 2010;184:1317–1317. doi: 10.4049/jimmunol.0902277. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. Influence of particle size on the pathology and efficacy of vaccination in a murine model of inhalational anthrax. J Med Microbiol. 2010 doi: 10.1099/jmm.0.024117-0. [DOI] [PubMed] [Google Scholar]
- Kiyono H, Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–699. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parungo CP. Lymphatic drainage of the peritoneal space: a pattern dependent on bowel lymphatics. Ann Surg Oncol. 2007;14:286–286. doi: 10.1245/s10434-006-9044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337:121–121. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DM. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J Immunol. 2008;180:4892–4892. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- Kool M. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–869. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Lehmann JR, Harold WM, Drew RT. Transoral tracheal intubation of rodents using a fiberoptic laryngoscope. Lab Anim Sci. 1986;36:256–256. [PubMed] [Google Scholar]
- Takahashi S, Patrick G. Patterns of lymphatic drainage to individual thoracic and cervical lymph nodes in the rat. Lab Anim. 1987;21:31–31. doi: 10.1258/002367787780740671. [DOI] [PubMed] [Google Scholar]
- Broeck WVanden, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–12. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]


