Abstract
In Parkinson's disease (PD) patients, the associated pathology follows a characteristic pattern involving inter alia the enteric nervous system (ENS) 1,2, the olfactory bulb (OB), the dorsal motor nucleus of the vagus (DMV)3, the intermediolateral nucleus of the spinal cord 4 and the substantia nigra, providing the basis for the neuropathological staging of the disease4,5. The ENS and the OB are the most exposed nervous structures and the first ones to be affected. Interestingly, PD has been related to pesticide exposure6-8. Here we show in detail two methods used in our previous study 9. In order to analyze the effects of rotenone acting locally on the ENS, we administered rotenone using a gavage to one-year old C57/BL6 mice. Rotenone is a widely used pesticide that strongly inhibits mitochondrial Complex I 10. It is highly lipophylic and poorly absorbed in the gastrointestinal tract 11. Our results showed that the administration of 5 mg/kg of rotenone did not inhibit mitochondrial Complex I activity in the muscle or the brain. Thus, suggesting that using our administration method rotenone did not cross the hepatoportal system and was acting solely on the ENS. Here we show a method to administer pesticides using a gavage and the image analysis protocol used to analyze the effects of the pesticide in alpha-synuclein accumulation in the ENS. The first part shows a method that allows intragastric administration of pesticides (rotenone) at a desired precise concentration. The second method shows a semi-automatic image analysis protocol to analyze alpha-synuclein accumulation in the ENS using an image analysis software.
Protocol
1) Intragastric Administration of Rotenone
Weigh the animal.
Calculate the total volume of rotenone/vehicle solution needed. In this case 0.01 ml per gram of the animal weight.
Charge a 1 mL syringe with the previously calculated volume of rotenone solution (0.625 mg/mL rotenone, 4% carboxymethylcellulose and 1.25% chloroform) or vehicle solution (4% carboxymethylcellulose and 1.25% chloroform). In this case it corresponds to a 5 mg/kg rotenone dose.
Pick up the mouse and, holding the mouse's tail with one hand, stretch the skin of the neck by pulling it with the first two fingers of the other hand.
Once the head is fixed between the two fingers, lean it against the palm of the hand and turn it upside-down. Fix the tail using the fourth and fifth fingers.
Keeping the head towards the back, press the palate to open the mouse's mouth. Gently introduce the gavage in the mouth. It is important to use a gavage needle and not a regular needle. Slowly, move the gavage in the direction of the stomach far enough. This will avoid material getting in the lungs.
Administer the rotenone solution or vehicle by gently pressing the embolus. When completed, slowly extract the gavage.
Place the animal back in another cage until all animals from one cage are done.
2) Immunostaining Procedures
Following treatment, mice are anesthetized using Ketamin 100 mg/kg und Xylazin 10 mg/kg i.p. and intracardially perfused with 4% Paraformaldehyde in PBS.
The guts are then extracted by dissection, and placed in 15% sucrose overnight. The next day, the tissue is transferred to 30% sucrose and incubated again overnight at 4°C.
The tissue is then placed in Tissue-tek and stored in a -80°C freezer until use.
Using a cryostat, 20 micron intestine cross-sections are transferred to Superfrost slides.
Blocking and immunostaining procedures using anti-βIII-tubulin and anti-alpha-synuclein antibodies are then performed as previously described 9.
3) Alpha-synuclein Inclusion Image Analysis
It is recommended that an external person unaware of images' origin performs the analysis. Therefore, the data should be codified. Image analysis was performed using FIJI software.
Open the confocal image file. Then, split the channels so that it is possible to work on each channel independently.
Close those channels that are not going to be use and select Analyse>Set Scale and set the Pixel size to 1. Set the known distance to 0.13 (this will depend on the objective and the resolution used) and click on global. The size of the image will now be shown in microns.
Select alpha-synuclein channel and select ganglion, make sure that the selection encloses the ganglion in all slices.
Press Image>Duplicate to duplicate the image. With the ganglion selected, only an image the size of the ganglion will be duplicated.
Press Edit>Clear Outside. The part of the image outside the selected region will become black.
Duplicate the image again to determine in later steps how well the procedure went. Images that have a high signal to noise to background ratio should not be used for image analysis.
Select Image>Adjust>Threshold and then select MaxEntropy. Then move over the slices in the cropped image and the original one to ensure that the procedure went well. The entropic threshold selects an intensity signal level based on the image histogram and only those pixels with intensities higher than this threshold will be displayed in a white over black image.
Select Process, then click on Smooth. This step transforms the pixeled edges of the images in smooth edges.
Select Process, then click on Binary, and choose Make Binary. The image will be displayed as a black over white image.
Duplicate image to perform next step on the duplicated image.
Click on Analyze>Analyze particles. Then select Show:Outlines and click on Summarize. The rest of settings should be leaved in default. This function recognizes the total number of pixels with a signal and those that are grouped together. The total surface of pixels with signal plus the number of groups of pixels and the mean size are reported.
Compare and select the largest slice that adjusts well. The slice will be marked.
Copy the data table with the total alpha-synuclein surface, the number of inclusions and the mean particle size. Paste the data into an excel spreadsheet or annotated elsewhere.
Return to FIJI and find the previously selected slice in the β-tubulin channel, and select it.
Select ganglion area and click on Analyze then select Measure. Another table will appear showing the total surface of the ganglion.
Extract the data from the table to the excel sheet near the alpha-synuclein measurement or copy it down elsewhere.
Using excel, divide the different parameters obtained in the alpha-synuclein measurement by the ganglion surface to obtain total alpha-synuclein surface and inclusion number normalized by ganglion size.
4) Representative Results
When gavage administration protocol is performed correctly the inconveniences for the animal are minimal. Treatment using this concentration of rotenone enables a local effect of rotenone on the ENS without rotenone levels in blood and no inhibition of muscle or brain mitochondrial Complex I even after 1.5 months of treatment (see Figure 1). If image analysis is performed correctly a rigorous analysis of alpha-synuclein pattern should be possible. It gives reliable data on the total amount of alpha-synuclein (total surface), alpha-synuclein pattern in the cell (number of inclusions) and the presence of alpha-synuclein inclusions (inclusion size) (Figure 2).
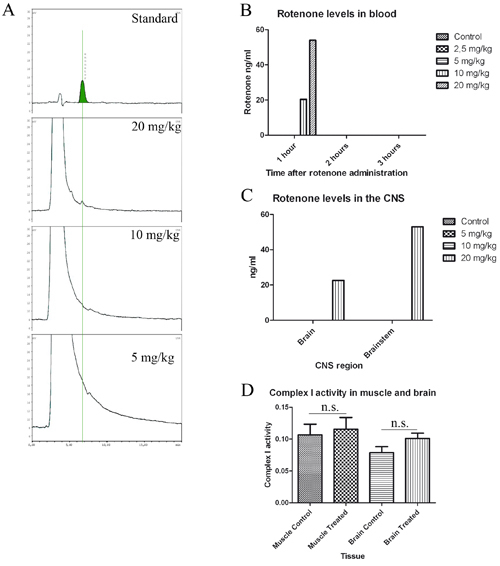 Figure 1. Motor dysfunction in rotenone treated mice without detection of rotenone in blood or CNS. A, standard (50 ng/mL) and chromatogram from brain samples of 20, 10 and 5 mg/kg treated mice. B and C, quantification of rotenone levels in blood (B) and CNS (C). B, blood levels 1, 2 and 3 hours after treatment. Mice were divided in three groups (n=3) and treated with 2.5, 5, 10 and 20 mg/kg rotenone (n=3), 300 μL of blood was extracted 1, 2 and 3 hours after rotenone administration and pooled together for HPLC analysis. C, mice were treated for one week once a day with 5 (n=3), 10 (n=3) and 20 (n=1) mg/kg rotenone, brain and brainstem were extracted 1 and 2 hours after last administration and prepared for HPLC analysis. D, mitochondrial Complex I activity in muscle and brain samples of 1.5 month treated mice.
Figure 1. Motor dysfunction in rotenone treated mice without detection of rotenone in blood or CNS. A, standard (50 ng/mL) and chromatogram from brain samples of 20, 10 and 5 mg/kg treated mice. B and C, quantification of rotenone levels in blood (B) and CNS (C). B, blood levels 1, 2 and 3 hours after treatment. Mice were divided in three groups (n=3) and treated with 2.5, 5, 10 and 20 mg/kg rotenone (n=3), 300 μL of blood was extracted 1, 2 and 3 hours after rotenone administration and pooled together for HPLC analysis. C, mice were treated for one week once a day with 5 (n=3), 10 (n=3) and 20 (n=1) mg/kg rotenone, brain and brainstem were extracted 1 and 2 hours after last administration and prepared for HPLC analysis. D, mitochondrial Complex I activity in muscle and brain samples of 1.5 month treated mice.
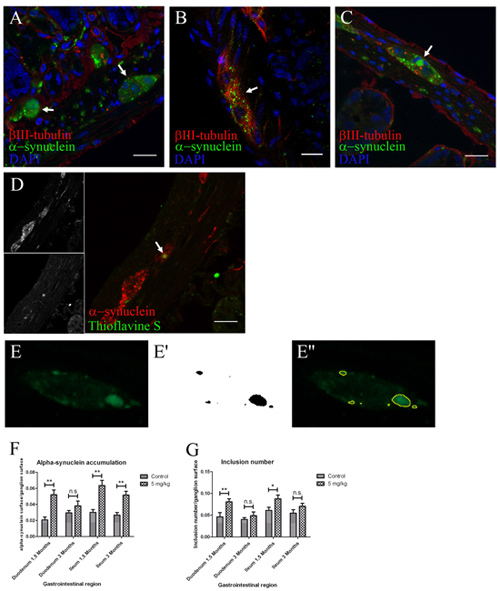 Figure 2. Locally administered rotenone induces alpha-synuclein phosphorylation, accumulation and aggregation with gliosis in ENS ganglia. (scale bars 20 μm). A, B, C, anti βIII-tubulin, alpha-synuclein and DAPI staining in duodenum (B) and ileum (A,C) sections. Arrow in B, 1.5 months treatment induced an increased alpha-synuclein punctate pattern inside enteric nervous system ganglia when compared to 3 months controls (A). Arrow in C, 3 months treatment induced formation of larger alpha-synuclein inclusions (ø>6 μm). D, immunofluorescence staining using anti-alpha-synuclein, Thioflavine S and DAPI. Arrow in D, only 3 month treated mice showed aggregation of these larger alpha-synuclein accumulations. E, E', E'', image analysis quantification steps. E, example of image used in quantification showing an ENS ganglion immunostained against alpha synuclein showing different inclusions. E', example of image obtained after image analysis protocol. E'', overlap between some of the original inclusions (green) and the representation obtained after performing the protocol (yellow lines). F-G, quantification of the experiment shown in A-C was made using automatic segmentation and entropy-based thresholding methods. Single-asterisk, P<0.05, and double-asterisk, P<0.01. F, each column represents total alpha-synuclein surface/ganglion surface. G, each column represents total number of alpha-synuclein inclusions/ganglion surface. All graphs show mean ± s.e.m.
Figure 2. Locally administered rotenone induces alpha-synuclein phosphorylation, accumulation and aggregation with gliosis in ENS ganglia. (scale bars 20 μm). A, B, C, anti βIII-tubulin, alpha-synuclein and DAPI staining in duodenum (B) and ileum (A,C) sections. Arrow in B, 1.5 months treatment induced an increased alpha-synuclein punctate pattern inside enteric nervous system ganglia when compared to 3 months controls (A). Arrow in C, 3 months treatment induced formation of larger alpha-synuclein inclusions (ø>6 μm). D, immunofluorescence staining using anti-alpha-synuclein, Thioflavine S and DAPI. Arrow in D, only 3 month treated mice showed aggregation of these larger alpha-synuclein accumulations. E, E', E'', image analysis quantification steps. E, example of image used in quantification showing an ENS ganglion immunostained against alpha synuclein showing different inclusions. E', example of image obtained after image analysis protocol. E'', overlap between some of the original inclusions (green) and the representation obtained after performing the protocol (yellow lines). F-G, quantification of the experiment shown in A-C was made using automatic segmentation and entropy-based thresholding methods. Single-asterisk, P<0.05, and double-asterisk, P<0.01. F, each column represents total alpha-synuclein surface/ganglion surface. G, each column represents total number of alpha-synuclein inclusions/ganglion surface. All graphs show mean ± s.e.m.
Discussion
Intragastric administration has been previously performed 12. However, according to Inden's manuscript, rotenone was administered dissolved in 0.5% carboxymethylcellulose sodium salt (CMSS) alone. Rotenone is a high liphophylic substance. Therefore, rotenone cannot be dissolved in 0.5% CMSS alone and will precipitate if done so. The use of chloroform creates an evenly distributed rotenone suspension avoiding precipitation. Moreover, due to the higher concentrations of the pesticide in the CMSS, the final volume of administered solution is also significantly decreased. Thus, reducing complications in the administration.
We recommend the use of straight gavages. The esophagus of the mouse is straight, ending directly on one side of the stomach. Therefore, straight gavages are more adequate for this task.
Image analysis of confocal images was done using FIJI software. The plug-ins for this software can be downloaded online. The immunofluorescent staining for image analysis was performed as previously described 9. Images were acquired using a LSM 510 Zeiss Confocal Microscope. It is important that images are acquired using the same signal/noise ratio. This can be achieved by adjusting gain and offset before acquisition.
Disclosures
Francisco Pan-Montojo has a patent application pending for this animal model (Application number PCT/EP 2009/005688).
Acknowledgments
The Pedro Barrie de la Maza Foundation supported this work.
References
- Braak H, de Vos RA, Bohl J, Del Tredici K, K Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K. Restricted occurrence of Lewy bodies in the dorsal vagal nucleus in a patient with late-onset parkinsonism. J Neurol Sci. 1999;165:188–191. doi: 10.1016/s0022-510x(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113:421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Butterfield PG, Valanis BG, Spencer PS, Lindeman CA, Nutt JG. Environmental antecedents of young-onset Parkinson's disease. Neurology. 1993;43:1150–1158. doi: 10.1212/wnl.43.6.1150. [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5:e8762–e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inden M. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem. 2007;101:1491–1504. doi: 10.1111/j.1471-4159.2006.04440.x. [DOI] [PubMed] [Google Scholar]


