Abstract
Based on the glutamatergic hypothesis of schizophrenia we assessed the effects of a novel mGlu5 positive allosteric modulator, LSN2463359 [N-(1-methylethyl)-5-(pyridin-4-ylethynyl)pyridine-2-carboxamide] on deficits in cognitive flexibility in two distinct rodent models of schizophrenia, the neurodevelopmental MAM E17 model and the acute PCP model. Cognitive flexibility was measured with the intra-dimensional and extra-dimensional set-shifting and reversal learning digging paradigm. Regional effects of MAM on the expression of parvalbumin-positive cells (PV) and mGlu5 receptors were also examined, to further characterize the model. Results showed that LSN2463359 selectively attenuated reversal learning deficits in the MAM but not acute PCP model. Whilst both models led to deficits in reversal learning and extra-dimensional set-shifting, the reversal impairments were qualitatively distinct, with MAM increasing perseverative responding, whereas the PCP deficit was mainly due to the inability of rats to maintain reinforced choice behavior. Reduction of PV and mGlu5 expression was found in the MAM model in several regions of importance in schizophrenia, such as the orbitofrontal and medial prefrontal cortex, which also mediate reversal learning and extra-dimensional set-shifting. The present findings confirm that the positive modulation of mGlu5 receptors may have beneficial effects in the treatment of certain aspects of cognitive impairment associated with schizophrenia. This study also illustrates the importance of studying putative cognitive enhancing drug effects in a number of models which may have implications for the future development of the compound.
Keywords: metabotropic glutamate receptor 5, animal models of schizophrenia, NMDA glutamate receptor, phencyclidine, methylazoxymethanol acetate, reversal learning
INTRODUCTION
Cognitive dysfunction represents a major target for remediation in schizophrenia, and is viewed by many as being at least as important, if not more so, than the effective treatment of positive and negative symptoms (Green, 2006). This recognition has motivated recent attempts to produce a regulatory framework (MATRICS) (Nuechterlein et al, 2008) and consensus translational neuropsychological test battery (CNTRICS) (Barch et al, 2009) to be used in evaluation of putative cognitive pharmacotherapies. Several pharmacological strategies for cognitive enhancement in schizophrenia have already been proposed, including agents active at dopamine, nicotine, GABA and glutamate receptors, as well as phosphodiesterease inhibitors (Barch, 2010). Given that key hypotheses of the molecular pathology of schizophrenia implicate abnormalities in glutamatergic dysfunction, efforts to target this biology have been substantial and have included candidate mechanisms directed towards modulation of function of both mGlu2/3 and mGlu5 receptors (Coyle, 1996; Tsai and Coyle, 2002). However, in order to test the viability of these strategies pre-clinically it is necessary to employ animal models of schizophrenia that not only result in cognitive deficits relevant to the disease, but ideally do so in a plausible neurobiological manner. Currently, several classes of model are used, from neurodevelopmental models that over time produce neural deficits in hippocampal and cortico-striato-thalamic circuitry, to pharmacological models of NMDA receptor antagonism or dopaminergic hyperactivity, and transgenic models focusing on the behavioral sequelae of manipulating specific genes implicated in schizophrenia (Lipska and Weinberger, 2000; Robbins, 2011). To date there has been little direct comparison of such models in the evaluation of drug candidates for cognitive enhancement. Ultimately, such comparisons will be vital for the purposes of model validation by back-translation (Moore, 2010).
In the present study, the effects of a novel mGlu5 positive allosteric modulator (PAM), LSN2463359 [N-(1-methylethyl)-5-(pyridin-4-ylethynyl)pyridine-2-carboxamide] on deficits in cognitive flexibility were examined in two distinct rodent models of schizophrenia, the MAM E17 neurodevelopmental model (Moore et al, 2006) and the acute phencyclidine (PCP) model (Jentsch and Roth, 1999). The MAM E17 model is based on the injection of the mitotic neurotoxin methylazoxymethanol acetate into pregnant female rats at the E17 stage of development. Subsequently born pups are found to exhibit characteristic neuroanatomical (Moore et al, 2006), electrophysiological (Lodge and Grace, 2007; Sanderson et al, 2011) and behavioral changes, including impairments in cognitive flexibility (Moore et al, 2006; Featherstone et al, 2007). The effects of LSN2463359 in the MAM E17 model were compared to those in the acute PCP model in adult rats, a commonly used method for inducing cognitive (Egerton et al, 2005) and also neural deficits (Amitai et al, 2011) that may have relevance to glutamatergic theories of schizophrenia. Cognitive flexibility in both models was assessed using the rodent attentional set-shifting ‘digging' paradigm (Birrell and Brown, 2000). The use of the digging task was justified (i) as it is a test of ‘reasoning and problem-solving' that conforms to one of the seven cognitive domains of impairment as described in the MATRICS battery (Nuechterlein et al, 2008); (ii) by its recent adoption as a valid cognitive test paradigm by the CNTRICS initiative, assaying the construct of ‘rule-generation and selection' (Barch et al, 2009); and (iii) by considerable evidence showing impairments in cognitive flexibility in schizophrenia, using homologous human neuropsychological tests with established translational validity (Leeson et al, 2009). The rodent digging task is particularly informative because as well as measuring the ability to shift attentional set, it also incorporates three tests of reversal learning and a test of compound discrimination learning. Each of these behavioral processes may tax different neuropsychological substrates related to cognitive flexibility, hopefully offering a more complete picture of potential recovery of function within this domain. The neural substrates of set shifting are fairly well defined, where deficits have been linked to neurocircuitry including the lateral prefrontal cortex in human and non-human primates (Dias et al, 1996; Hampshire and Owen, 2006) and the medial prefrontal cortex in both rats and mice (Birrell and Brown, 2000; Bissonette et al, 2008). Somewhat distinctly, reversal learning impairments observed in schizophrenia are dependent on a network involving orbitofrontal, amygdaloid and striatal circuitry (see Barnett et al, 2010).
Finally, because an inherent value of the MAM E17 model may lie in its ability to relate specific patterns of neuroanatomical disruption to behavioral deficits relevant to schizophrenia, the opportunity was taken to assess regional effects of MAM on the expression of mGlu5 receptors using immunohistochemical techniques, as well as the expression of parvalbumin (PV)-containing GABAergic interneurons known to be implicated in the neuropathology of schizophrenia (Lewis, 2000).
MATERIALS AND METHODS
Animals
All rats were housed in standard housing conditions (two to four per cage, 0700–1900 h light phase, controlled temperature and humidity, ad libitum water). Animals were at least 3 months old and were kept for a minimum of seven days before any behavioral training commenced. During this time, rats were acclimated to food restriction regimens (ie, maintained at no less than 85% of their free-feeding weight) and were handled during weighing and general husbandry procedures. All experiments were conducted in accordance with the regulations laid down in the United Kingdom Animals (Scientific Procedures) Act 1986 and the ethical policies of Eli Lilly. Facilities were also AAALAC accredited.
MAM
Time-mated Sprague Dawley dams (Charles River, UK) were obtained at day 10 of pregnancy (embryonic day 10: E10). At E17, dams were dosed intraperitoneally (ip) with MAM (22 mg/ml/kg) or vehicle (experiment 1: MAM injection=7, saline=7; experiment 2: MAM=6, saline =6; experiment 3: MAM=3, saline=3). Litters were weaned 28 days after birth and re-housed with non-littermates from the same treatment group. Only male offspring were used (experiment 1: MAM offspring=10, saline=10; experiment 2: MAM=24, saline=24; experiment 3: MAM=6, saline =6), with each experimental group consisting of at least one animal from each litter.
PCP
Sixty male Sprague Dawley rats (Charles River, UK) were used in this study (experiment 1: PCP=10, saline=10; experiment 2: PCP=20, saline=20). They were dosed subcutaneously (sc) with either PCP (2.5 mg/ml/kg) or vehicle and were tested 120 min post-administration.
Drugs
Methylazoxymethanol acetate (MAM) (Midwest Research Institute, Kansas City, Missouri) was dissolved in a 0.9%. (w/v) saline solution and administered ip at a dose of 22 mg/ml/kg (expressed as free base weight). MAM is light sensitive and was stored, formulated and maintained in light-restricting bottles. Phencyclidine hydrochloride (PCP) (Sigma-Aldrich, UK) was formulated in a 5% (w/v) glucose solution and administered via the sc route at a concentration of 2.5 mg/ml/kg, 120 min before behavioral testing. LSN2463359 [N-(1-methylethyl)-5-(pyridin-4-ylethynyl)pyridine-2-carboxamide; synthesized in-house] was formulated as a suspension in a 1% (w/v) carboxymethylcellulose, 0.25% Tween 80, 0.05% antifoam vehicle and administered orally at a dose of 2.5 mg/ml/kg, 30 min before behavioral testing. Dose, pretreatment time and route of administration of each compound were chosen on the basis of both previous studies (Moore et al, 2006; Egerton et al, 2005) and dose-response studies done in house (Malik et al, 2009; Gastambide et al, 2010). Experimenters were blinded to treatment conditions.
Behavioral Studies
Intra-dimensional/extra-dimensional (ided) attentional set-shifting task
IDED testing was conducted in an opaque white test box (L70 × W40 × H30 cm3) where a Plexiglas panel was used to divide one-third of the box length into two equal sections, forming the choice chambers to which access could be blocked via removable doors. During behavioral testing, one ceramic bowl (diameter of 7 cm and depth of 4 cm) was placed in each choice section. Food rewards were one-third pieces of Honey Nut Loops (Kellogg, UK), which were placed in one bowl per trial and covered with digging media. The media varied by odor and/or texture to provide two different stimulus dimensions to guide choice behavior. Testing was performed according to a modified version of the protocol described by Birrell and Brown (2000). Rats were habituated to the testing box and then initially trained to dig in bowls filled with cage bedding to retrieve food rewards. Once habituated, rats were trained on two simple discriminations (SDs): one based on odor (thyme vs cloves) and one based on texture (large vs small clay pieces). SD order and to-be reinforced stimuli were pseudo randomly chosen per rat, but counterbalanced across the squad. These odor and texture stimuli were not used again in later phases of the experiment. The purpose of this preliminary phase was to acquaint rats with the basic discrimination learning process, as well as to encourage attention to the two different dimensions of the digging media that could be relevant for subsequent stages of discrimination learning. The following day, rats were given a series of seven discriminations; a single discrimination (SD); a compound discrimination (CD) in which digging media differed according to both odor and texture but with correct and incorrect exemplars remaining similar to the preceding SD; a reversal (Rev1), where the reward contingency of the CD exemplars is reversed; an intra-dimensional shift (IDS) in which a novel discrimination is learnt with new stimuli, the new correct exemplar being of the same dimension as before; a second reversal (Rev2); and an extra-dimensional shift (EDS), in which another discrimination with new stimuli is learned, but in this case the correct exemplar is now from the other previously irrelevant dimension; and finally a third reversal (Rev3). For each discrimination stage, testing continued until rats reached a criterion level of six correct consecutive trials. The procedure was the same for each stage: a trial was initiated by raising the removable doors to give rats access to the two digging bowls, only one of which was baited. The first four trials of each discrimination stage were deemed ‘discovery' trials, where rats were permitted to dig in both bowls if they chose the incorrect bowl first. An error was recorded if rats dug first in the unbaited bowl. On subsequent trials, if rats started to dig in the unbaited bowl, an error was recorded, and the trial was terminated. If rats did not dig at all in either bowl within 3 min, the trial was aborted, recorded as an omission and reinitiated. The number of errors made to reach criterion was recorded per rat for each stage of the test. Additional measures were analyzed on each reversal learning stage to determine whether model and/or drug treatment selectively altered perseverative (ie, continuing to choose the previously correct stimulus) or regressive behavior (ie, inability to maintain a choice after initially reversing away from the previously correct one). Perseveration was defined as digging in the incorrect bowl for 3 or more trials in consecutive blocks of 4 trials each. Once rats made less than 3 errors per block, those and all subsequent errors were counted as regressive errors. All data were analyzed for statistical significance using two-way repeated-measures ANOVA, with discrimination stage or error type as a within-subjects factor, and model and/or treatment as between-subjects factors. This was followed by planned comparisons where appropriate. In all cases, statistical significance was defined as p<0.05.
Neuropathological Studies
Immunohistochemistry
At 3 months old, 6 MAM and 6 saline animals were transcardially perfused with PBS followed by 4% paraformaldehyde, the brains removed and post-fixed in buffered formalin. For quantification of parvalbumin (PV)-positive interneurons and the evaluation of the mGlu5 immunoreactivity, these brains were embedded in paraffin and 6 μm-thick sections were cut on a microtome. Sections were sampled from orbitofrontal cortex (from bregma: AP +2.52 to +5.64), medial prefrontal cortex (from bregma: AP +2.52 to +5.16), striatum (from Bregma: AP +0.84 to +2.52), amygdala (from Bregma: AP −3.96 to −1.80) and hippocampus (from Bregma: AP −2.40 to −5.40). Every 15th slice was used for PV immunostaining, while every 25th slice was used for mGlu5 immunostaining. After antigen retrieval in 100 °C citrate buffer, endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide solution for 30 min. Non-specific binding was blocked by incubating slides for one hour at room temperature in bovine serum albumin and normal serum solution. Slides were then incubated overnight at 4 °C with the anti-PV primary antibody (1 : 5000 dilution, Swant, Bellinzona, Switzerland) or one hour at room temperature with the anti-mGlu5 antibody (2.5 μg/ml, Millipore, Billerica, MA, USA). Slices were rinsed, and incubated successively in a secondary antibody solution and an avidin-biotin-horseradish peroxidase complex (VECTASTAIN Elite ABC Kit Mouse IgG, Vector Labs, Burlingame, CA, USA). The immunostain was revealed using 3,3′-diaminobenzidine as a chromogen (DAB peroxidase substrate kit, Vector Labs, Burlingame, CA, USA). Slices were then dehydrated in ethanol and xylene and coverslipped using xylene-based Shandon Consul-Mount (Thermo Fisher Scientific, Waltham, MA, USA).
Semi-stereological quantification and relative staining intensity
Immuno-stained slices were scanned with ScanScope XT and digital images were stored in a virtual space provided by Spectrum v10.2.2.2317. The brain areas of interest were measured and the number of PV-positive neurons and mGlu5 receptors contained in each delineated domain were determined using ImageScope software v10.2.2.2319 (Aperio Technologies, Vista, CA, USA). The total number of PV-positive cortical neurons was estimated by the following formula: PV-positive neurons=[sum (PV-positive neurons counted per slice)]*15. For the quantification of mGlu5 reactivity, the immunopositive signal was evaluated using a positive pixel count function of ImageScope. The total relative staining intensity for each area of interest was calculated as follows: [Average (positive pixels)]/Total area investigated (mm2). All data were analyzed for statistical significance using Student's t-tests in GraphPad Prism v5 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Experiment 1: Qualitative Distinction Between MAM- and PCP-Induced Deficits in Reversal Learning and Extra-Dimensional Set-Shifting
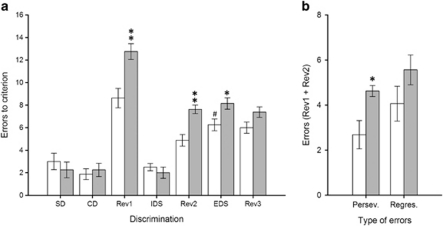
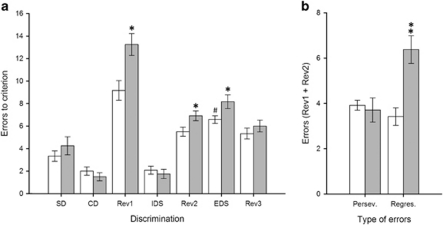
As illustrated in Figures 1 and 2, saline-treated animals made significantly more errors to reach the criterion level of six consecutive correct trials in the EDS than in the IDS (p<0.001), demonstrating that they had formed an attentional set towards the relevant dimension before the EDS stage (Birrell and Brown, 2000; Garner et al, 2006). In addition, the reinforced dimension (ie, odor or texture) on which animals were trained prior to the EDS stage did not differentially affect their learning performance across discrimination stages (all p-values >0.05; data not shown). Animals treated with either MAM (Figure 1) or PCP (Figure 2) were impaired in their ability to perform both reversal learning and an extra-dimensional shift. Indeed, MAM and PCP significantly increased the number of errors to criterion in the Rev1 (MAM: p<0.01; PCP: p<0.01), Rev2 (MAM: p<0.01; PCP: p<0.05) and EDS (MAM: p<0.05; PCP: p<0.05) stages of the task, with no significant effects on any other stage of discrimination learning occurring during the test session. Whilst both MAM and PCP models led to superficially similar deficits in reversal learning and extra-dimensional set-shifting, additional measures of error type made suggested that the reversal learning impairments were qualitatively distinct. The MAM impairment resulted from a significant increase in maladaptive perseverative responding (Figure 1b; p<0.05), whereas the PCP deficit was caused by a significant increase in the number of regressive errors (Figure 2b; p<0.001).
Figure 1.
Performance of both saline- (white bars) and MAM-treated rats (gray bars) in the attentional set-shifting task (N=8 per group). Graph bars represent the mean±SEM of the number of errors made to reach the criterion of six correct consecutive trials in each test discrimination (a) and the average number of perseverative vs regressive errors made within the first two reversal learning discriminations (b). Symbols: #p<0.001 vs the saline-treated group at the IDS discrimination; *p<0.05 and **p<0.01 vs the saline-treated group within the same discrimination (a) or error type category (b).
Figure 2.
Performance of both saline- (white bars) and PCP-treated rats (gray bars) in the attentional set-shifting task (N=12 per group). Graph bars represent the mean±SEM of the number of errors made to reach the criterion of six correct consecutive trials in each test discrimination (a) and the average number of perseverative vs regressive errors made within the first two reversal learning discriminations (b). Symbols: #p<0.001 vs the saline-treated group at the IDS discrimination; *p<0.05 and **p<0.01 vs the saline-treated group within the same discrimination (a) or error type category (b).
Experiment 2: Selective Remediation of Reversal Learning Deficits in the MAM but not PCP Model by LSN2463359
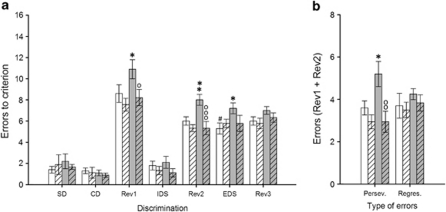
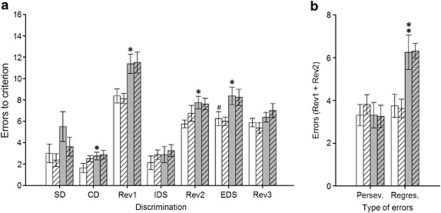
Animals treated with MAM (Figure 3) and PCP (Figure 4) were again significantly impaired in the Rev1 (MAM: p<0.05;. PCP: p<0.05), Rev2 (MAM: p<0.01; PCP p<0.05) and EDS (MAM: p<0.05; PCP: p<0.05) stages of the task, attesting to the reproducibility and reliability of these deficits. In comparison to experiment 1, rats treated with PCP were also impaired in discrimination learning when the irrelevant stimulus dimension was added in the CD stage (p<0.05). Analyses of the type of errors made in each reversal learning stage confirmed the MAM-induced reversal learning deficit derived from a significant increase in perseverative responding (p<0.05), whereas the PCP deficit was again selectively related to a significant increase in the number of regressive errors (p<0.01). The administration of the mGlu5 PAM LSN2463359 selectively reversed MAM-induced deficits. Indeed, MAM rats treated with LSN2463359 made significantly fewer errors to reach the criterion during the first two reversal learning stages (Rev1: p<0.05; Rev2: p<0.001) as compared with vehicle-treated MAM rats; only a trend towards improvement in the extra-dimensional shift was observed (EDS: p=0.08). Interestingly, the administration of LSN2463359 did not reverse any of those deficits in rats treated with PCP (p-values >0.05). This selective remediation of MAM- but not PCP-induced reversal learning deficits may be partly explained by the nature of the reversal deficit. Indeed, LSN2463359 significantly decreased MAM-induced perseverative responding (p<0.01) but not the increase in regressive errors induced by PCP (p>0.05). Finally, the administration of LSN2463359 to both saline-treated control groups did not produce any effect across any of the discrimination phases of the task (all p-values >0.05), suggesting the effect of this mGlu5 PAM was specific for MAM-induced deficits.
Figure 3.
Performance of both saline- (white bars) and MAM-treated rats (gray bars) in the attentional set-shifting task after an administration of either vehicle (blank bars) or LSN2463359 (striped bars) (N=9–10 per group). Graph bars represent the mean±SEM of the number of errors made to reach the criterion in each test discrimination (a) and the average number of perseverative vs regressive errors made within the first two reversal learning discriminations (b). Symbols: #p<0.001 vs the saline/veh-treated group at the IDS discrimination; *p<0.05 and **p<0.01 vs the saline/veh-treated group within the same discrimination (a) or error type category (b); °p<0.05, °°p<0.01, °°°p<0.001 vs the MAM/veh-treated group within the same discrimination (a) or error type category (b).
Figure 4.
Performance of both saline- (white bars) and PCP-treated rats (gray bars) in the attentional set-shifting task after an administration of either vehicle (blank bars) or LSN2463359 (striped bars) (N=8 per group). Graph bars represent the mean±SEM of the number of errors made to reach the criterion in each test discrimination (a) and the average number of perseverative vs regressive errors made within the first two reversal learning discriminations (b). Symbols: #p<0.001 vs the saline/veh-treated group at the IDS discrimination; *p<0.05 and **p<0.01 vs the saline/veh-treated group within the same discrimination (a) or error type category (b).
Experiment 3: Consistent Reductions in mGlu5 Receptors and Parvalbumin-Positive Cells in Specific Brain Regions of MAM Rats
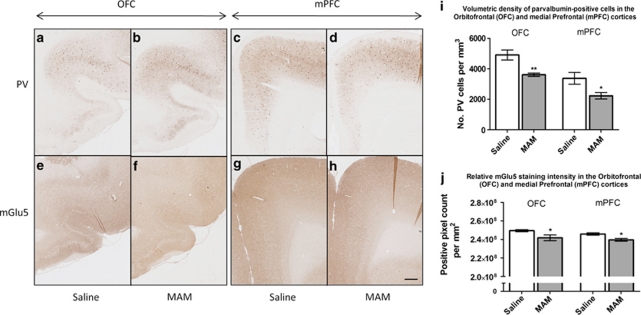
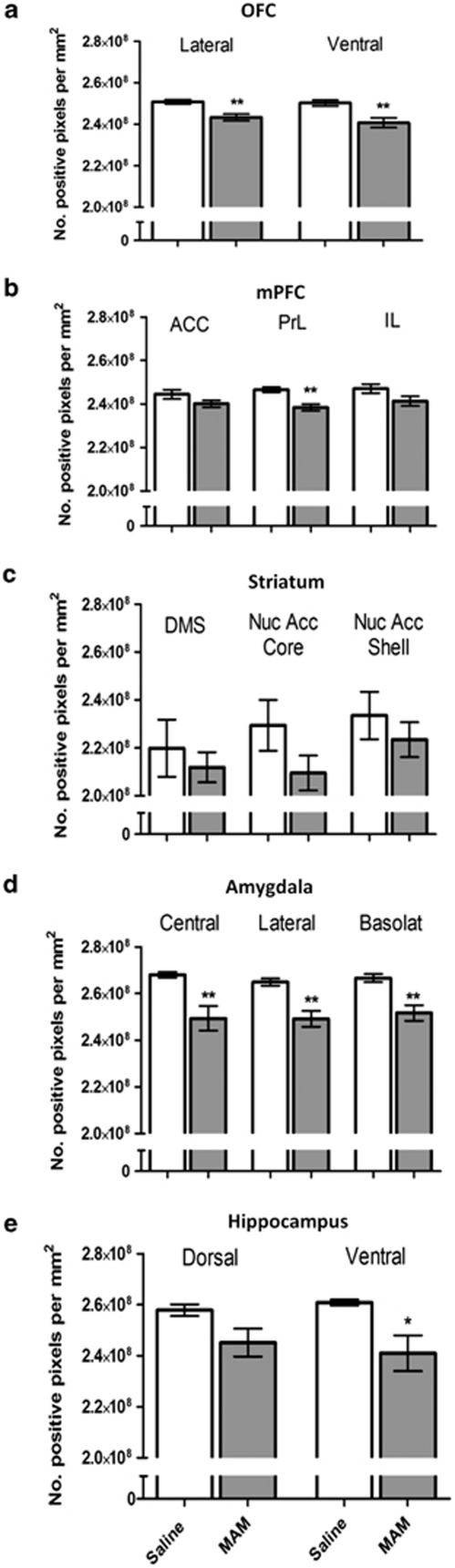
The quantification of fast-spiking PV-positive interneurons in the orbitofrontal cortex (OFC) and the medial prefrontal cortex (mPFC) (Figure 5i) revealed a significant difference in volumetric densities between the two groups of animals, with MAM rats exhibiting respectively a 26.45%. (p<0.01) and 33.84% (p<0.05) decrease compared to saline rats. Regarding the mGlu5 immunoreactivity in the two same regions, the difference between saline and MAM animals was smaller but significant with a decrease both in the OFC (p<0.05) and the mPFC (p<0.05) of MAM rats (Figure 5j). More precisely, the OFC (Figure 6a) showed a significant reduction of mGlu5 expression in the lateral (p<0.01) and the ventral (p<0.01) parts. Specific changes were found in the subregions of the mPFC (Figure 6b), with a significant reduction of mGlu5 expression in the prelimbic cortex (PrL) (p<0.01). Neither the anterior cingulate cortex (ACC) nor the infralimbic cortex (IL) were significantly altered (p>0.05). Regarding the subcortical regions (Figure 6c-e), neither the dorsomedial striatum (DMS), the nucleus accumbens (Nuc Acc Core and Shell) nor the dorsal hippocampus showed any change (all p-values >0.05). However, mGlu5 levels were significantly decreased in the ventral hippocampus (p<0.05) and all of the amygdalar nuclei investigated, central (p<0.01), lateral (p<0.01), and basolateral nuclei (p<0.01).
Figure 5.
: (a-h) Photomicrographs of parvalbumin (PV) (a-d)- and mGlu5 (e-h)- immunostained sections from saline (a, c, e, g)- and MAM (b, d, f, h)-treated animals, aged 3 month-old. Scale bar =500 μm. (i-j) Graph bars express the mean±SEM of PV-positive neurons per mm3 (i) and mGlu5-immunoreactivity per mm2 (j) measured in the orbitofrontal cortex (OFC) and the medial prefrontal cortex (mPFC) of saline- (white bars) and MAM-treated animals (gray bars). *p<0.05; **p<0.01 vs the saline-treated controls.
Figure 6.
Graph bars express the mean±SEM of the number of mGlu5-positive pixels expressed by area measure units within cortical (a, b) and sub-cortical (c-e) regions of saline- (white bars) and MAM-treated animals (gray bars). *p<0.05; **p<0.01 vs the saline-treated controls.
DISCUSSION
The present study demonstrates that the novel mGlu5 PAM LSN2463359 selectively remediates reversal learning deficits in the MAM E17 model but not the acute PCP model of schizophrenia, as measured by the rodent attentional set-shifting and reversal learning ‘digging' task. Whilst both neurodevelopmental and pharmacological models led to deficits in reversal learning and extra-dimensional set-shifting, the reversal impairments were shown to be qualitatively distinct, with the MAM impairment deriving from maladaptive perseverative responding, whereas the PCP deficit was mostly related to non-perseverative or regressive errors. Overall, these findings are noteworthy for their demonstration of a cognitive enhancing effect of LSN2463359 in a prominent test of fronto-executive function in an animal model of schizophrenia. However, they also demonstrate the importance of testing candidate cognitive enhancers in different animal models of schizophrenia within the same study—discrepant findings like this across two different models clearly have potential implications for clinical translation, back-translation and subsequent model validation.
Reversal learning is known to be controlled by a neural network including several regions of importance in schizophrenia, such as the hippocampus, amygdala, medial striatum, orbitofrontal (OFC) and medial prefrontal cortex (mPFC). This network has been defined through lesion and electrophysiological studies in rats and monkeys (Becker et al, 1981; Dias et al, 1996; Murray et al, 1998; Chudasama and Robbins, 2003; Stalnaker et al, 2007; Clarke et al, 2008; Kimchi and Laubach, 2009), and there is also some functional neuroimaging data in humans consistent with this anatomical organization of reversal learning (eg Hampshire and Owen, 2006). Impaired reversal learning has recently been highlighted to occur reliably in schizophrenia. Indeed, Leeson et al (2009) found in a large group of first episode schizophrenic patients that although they were impaired on set-shifting, they also exhibited small but consistent and highly significant deficits in reversal learning. These deficits in reversal learning, unlike those of set-shifting, were unrelated to general intelligence and correlated significantly with the disorganization syndrome, especially of positive formal thought disorder, which presumably interferes with cognitive function and has been shown to be related to reductions in OFC volume (Nakamura et al, 2008). Similarly, reversal learning impairments have been observed in schizophrenics in the context of changes in fronto-striatal activation (Murray et al, 2008). Such evidence supports the notion that reversal learning may be a viable translational element of schizophrenic cognitive deficits that can be studied in both rodents and humans.
MAM treatment at E17 reproduces many of the neural and neurochemical changes observed in schizophrenia, including changes in hippocampal and prefrontal volume (Flagstad et al, 2004; Moore et al, 2006), prefrontal cortex organization (Moore et al, 2006) and up-regulation of striatal dopamine (Flagstad et al, 2004; see Lodge and Grace, 2009). Earlier studies had reported PV changes in the ventral hippocampus and medial prefrontal cortex (Penschuck et al, 2006; Lodge et al, 2009), while the present study extends these findings to both the OFC and the mPFC, mirroring histological changes in schizophrenia (Lewis, 2000). Also, down-regulation of mGlu5 receptors in the OFC might be consistent with the reversal learning deficit in the MAM model being mediated by orbitofrontal dysfunction. It is possible that these effects were mediated at other sites within the neural network associated with reversal learning, such as the amygdala and hippocampus in which mGlu5 receptors were also down-regulated. However, mGlu5 receptors in the striatum were not affected by the MAM treatment and so this candidate site perhaps is less plausible. In behavioral terms, the MAM model has been shown previously to produce a wide variety of cognitive impairments, including in reversal learning and attentional set-shifting (Moore et al, 2006; Featherstone et al, 2007). Our data confirm reversal learning as being a consistent indicator of MAM-induced deficits, but show in addition a specific form of reversal learning deficit, namely in perseverative responding that was not observed following acute PCP administration in the alternative animal model. Interestingly, perseverative behavior is widely observed in patients with schizophrenia (Perry and Braff, 1998; see Crider, 1997). This comparison between animal models is therefore important in demonstrating the need for detailed analysis of behavioral changes which may superficially be similar, but in fact arise from distinct neural and behavioral mechanisms. It is also of interest to note the variability between two cohorts of PCP-treated animals during discrimination learning with an added distractor, or irrelevant stimulus (CD stage). This finding strongly suggests the need for assessing the reliability and robustness of cognitive deficits induced by such pharmacological and neurodevelopmental manipulations in rodents.
mGlu5 receptors are expressed in key brain regions for cognition, including hippocampus, amygdala, striatum and prefrontal cortex (Shigemoto et al, 1993; Romano et al, 1995). Accordingly, modulation of mGlu5 can alter a wide range of cognitive functions in rodents (Kinney et al, 2003; Homayoun et al, 2004; Manahan-Vaughan and Braunewell, 2005; Semenova and Markou, 2007; Ayala et al, 2009; Xu et al, 2009; see Homayoun and Moghaddam, 2010). In addition, studies with the mGlu5 PAM ADX47273 have reported both antipsychotic and procognitive effects, such as increased novel object recognition and reduced impulsivity in the five-choice serial reaction time test in rats (Liu et al, 2008), but no studies are yet reported in neurodevelopmental rodent models of schizophrenia. The present findings demonstrate that the administration of the mGlu5 PAM LSN2463359 can improve performance of MAM-treated rats in reversal learning. However, the precise mechanism underlying this remediation remains unclear. mGlu5 receptors are a key postsynaptic signaling partners of NMDA receptors (see Marino and Conn, 2002). Activation of mGlu5 may therefore potentiate the function of NMDA receptors within the reversal learning ‘network' and as a result, restore plasticity mechanisms which have been reported to be disrupted in this MAM model (Moore et al, 2006; Lodge et al, 2009; Sanderson et al, 2011). While there are no published reports of down-regulation of mGlu5 receptors in schizophrenia to date, such a finding would be consistent with a hypoglutamatergic account of the cognitive deficit. Molecular mechanisms have been described that could explain how specific down regulation of mGlu5 expression might occur, for example by decreases in neuronal Shank3 expression (Verpelli et al, 2011).
Apart from the improved reversal learning performance, the mGlu5 PAM did not significantly affect performance of the extra-dimensional shift, although the deficit there was comparable to that of reversal learning. There was however a trend for improvement consistent with the down-regulation of mGlu5 receptors observed in the mPFC, which is more implicated in set-shifting performance than the OFC (Birrell and Brown, 2000; McAlonan and Brown, 2003). Finally, there were no effects of LSN2463359 on basic discrimination learning, on learning with an added distractor (CD stage) or intra-dimensional set learning, indicating a degree of behavioral specificity to the effects, which we presume are unrelated to such general factors as perception or motivation. Interestingly, the mGlu5 PAM LSN2463359 did not overcome the PCP-induced reversal learning deficit. A simple caveat here might be that only a single dose of LSN2463359 was tested and that, it might have been possible to reverse the PCP deficit with another higher or lower dose. However, doses of LSN2463359 up to 30 mg/kg have little effect on PCP-induced hyperlocomotion and given the number of optimal dose-response studies done in-house, we think this unlikely. It is possible that this apparent discrepancy in the effects of the mGlu5 PAM results from the non-competitive nature of the channel blocking action of PCP at the NMDA receptor, as previously observed by Schlumberger et al (2010). However, a number of other mGlu5 PAMs have been shown to antagonize behavioral effects of non-competitive NMDA receptor antagonists in other assays (Stefani and Moghaddam, 2010; Rosenbrock et al, 2010; Fowler et al, 2011). Alternatively, the inability to attenuate the PCP-induced reversal learning deficit could arise from the fact that it is behaviorally distinct between the two models, and might therefore engage different aspects of the reversal learning network, and thus probably distinct glutamatergic substrates.
Overall, the present results confirm that positive modulation of mGlu5 receptors may have beneficial effects in the treatment of certain cognitive impairments associated with schizophrenia. Further studies are needed to test the generality of the mGlu5 effects in other memory and executive functions, such as working memory. This study also illustrates the importance of studying effects of putative cognitive enhancers in a number of models which may have implications for the future development of the compound. A drug that induces performance improvements across a range of models will generate greater confidence of seeing effects in man, perhaps against a broader range of possible clinical applications. If a drug is effective in only a limited number of models, this may speak to the specificity of its actions, but it also obviously remains possible that such a profile is likely to generate possible ‘false alarms', which can only be resolved by clinical trials in the selected patient group. In turn this would serve to test the validity of the model by ‘back-translation'. In this sense, the present findings offer an excellent opportunity to test the predictive validity of the MAM E17 and acute PCP models for remediating specific cognitive deficits in schizophrenia.
Acknowledgments
TWR acknowledges MRC and Wellcome Trust for their co-funding of the BCNI.
FG, M-CC, GG, MJON and MDT are employees of Eli Lilly and Co. Ltd. TWR discloses his consultancy with Cambridge Cognition, Lilly, Lundbeck and GlaxoSmithKline, plus research grants with Lilly, Lundbeck and GSK.
References
- Amitai N, Kuczenski R, Behrens MM, Markou A.2011Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats Neuropharmacology(in press). [DOI] [PMC free article] [PubMed]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. Pharmacological strategies for enhancing cognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:43–96. doi: 10.1007/7854_2010_39. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35:109–114. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Becker JT, Olton DS, Anderson CA, Breitinger ER. Cognitive mapping in rats: the role of the hippocampal and frontal system in retention and reversal. Behav Brain Res. 1981;3:1–22. doi: 10.1016/0166-4328(81)90025-5. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology. 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, Fletcher PJ. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: parallels to schizophrenia. Neuropsychopharmacology. 2007;32:483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van BJ, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR, et al. Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem. 2011;95:73–79. doi: 10.1016/j.nlm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Smith JW, Gilmour G, Foss J, Lloyd K, Loomis S, et al. The acute administration of ketamine and phencyclidine in rats induces distinct deficits in attention, working memory and cognitive flexibility that increase in specificity with time after injection. 40th Annual Meeting of the Society for Neuroscience. 2010.
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 (Suppl 9:3–8. [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol. 2010;639:33–39. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kimchi EY, Laubach M. Dynamic encoding of action selection by the medial striatum. J Neurosci. 2009;29:3148–3159. doi: 10.1523/JNEUROSCI.5206-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, et al. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, et al. ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204:306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N, Tricklebank M, Robbins TW, Marsden C, Fone KCF, Smith JW. Behavioural profiling of the MAM model of schizophrenia: Comparison of rat strain, age and gender. 39th Annual Meeting of the Society for Neuroscience. 2009.
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Moore H. The role of rodent models in the discovery of new treatments for schizophrenia: updating our strategy. Schizophr Bull. 2010;36:1066–1072. doi: 10.1093/schbul/sbq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Perry W, Braff DL. A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr Res. 1998;33:69–77. doi: 10.1016/s0920-9964(98)00061-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW.2011Animal models of schizophrenia revisitedIn: David AS, Kapur S, McGuffin P (eds).Schizophrenia: The Final Frontier - A Festschrift for Robin M. Murray (Maudsley Series) Psychology Press: Wiley, New York; 115–128. [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Kramer G, Hobson S, Koros E, Grundl M, Grauert M, et al. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol. 2010;639:40–46. doi: 10.1016/j.ejphar.2010.02.057. [DOI] [PubMed] [Google Scholar]
- Sanderson TM, Cotel MC, O'Neill MJ, Tricklebank MD, Collingridge GL, Sher E.2011Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia Neuropharmacology(in press). [DOI] [PubMed]
- Schlumberger C, Pietraszek M, Gravius A, Danysz W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol Biochem Behav. 2010;95:23–30. doi: 10.1016/j.pbb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats' performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, et al. Importance of shank3 in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286:34839–34850. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]