Abstract
Nicotine has both unconditioned and conditioned stimulus properties. Conditioned stimulus properties of nicotine may contribute to the tenacity of nicotine addiction. The purpose of this experiment was to use neurohistochemical analysis of rapidly developing c-Fos protein to elucidate neurobiological loci involved in the processing of nicotine as an interoceptive conditioned stimulus (CS). Rats were injected (SC) in an intermixed fashion with saline or nicotine (16 sessions of each) and placed in conditioning chambers where they were given one of the three conditions depending on group assignment: (a) nicotine paired 100% of the time with intermittent access to sucrose (nicotine-CS condition), (b) nicotine and saline each paired 50% of the time with sucrose (chamber-CS condition), or (c) no sucrose US control (CS-alone condition). Rats in the nicotine-CS condition acquired the discrimination as evidenced by goal-tracking (ie, increased dipper entries before initial sucrose delivery) only on nicotine sessions. The chamber-CS condition showed goal-tracking on all sessions; no goal-tracking was seen in the CS-alone condition. On the test day, rats in each condition were challenged with saline or nicotine and later assessed for c-Fos immunoreactivity. In concordance with previous reports, nicotine induced c-Fos expression in the majority of areas tested; however, learning-dependent expression was specific to dorsomedial and ventromedial regions of caudate-putamen (dmCPu, vmCPu). Only rats in the nicotine-CS condition, when challenged with nicotine, had higher c-Fos expression in the dmCPu and vmCPu. These results suggest that medial areas of CPu involved in excitatory conditioning with an appetitive nicotine CS.
Keywords: nicotine, appetitive stimuli, c-Fos, medial CPu, drug discrimination, Pavlovian conditioning
INTRODUCTION
Consumption of tobacco products is a leading cause of preventable deaths both globally and in the United States. Although rates of tobacco consumption are more prevalent in the developing countries (70% of all tobacco users), developed countries such as the United States suffer considerable financial ($193 billion a year in health care expenditures and lost productivity) and personal (443 000 people per year) loss (CDC, 2008). Chronic tobacco use is a debilitating habit in which a small fraction are able to quit without intervention and others are in need of pharmacological and/or cognitive-behavioral therapies to increase chances of long-term abstinence from tobacco.
Preclinical animal research in this area has focused heavily on understanding the acute and repeated behavioral and neurobiological effects of nicotine (Azizian et al, 2009; Balfour, 2009; Barik and Wonnacott, 2009). A subset of this research has focused on Pavlovian conditioning processes where nicotine serves as an unconditioned stimulus (US). This research has led to a better understanding of nicotine dependence, and the translation of this work could improve current treatment approaches such as cue-exposure therapy (Conklin and Tiffany, 2002). Nicotine also has conditioned-stimulus (CS) effect that may contribute to the persistence of the smoking habit (Bevins and Murray, 2011; Bevins and Palmatier, 2004). These interoceptive-stimulus effects of nicotine serving as a CS have not been as well studied. As a CS, the perceptible interoceptive effects of nicotine enter into learned association with other appetitive stimuli or USs that co-occur in the environment (eg, peer interaction/acceptance, alcohol, food, work breaks). These acquired appetitive properties of nicotine may evoke conditioned response (CR) that contributes to the tenacity of the addiction (Alessi et al, 2002; Bevins and Murray, 2011).
Animal studies have been especially useful in examining nicotine in its role as a CS (Besheer et al, 2004; Murray and Bevins, 2011; Struthers et al, 2009). In this animal model, rats received nicotine (the CS) paired with intermittent access to sucrose (the US); on intermixed saline days sucrose is not available. Across sessions, differential goal-tracking (anticipatory approach to location of the US; Farwell and Ayres, 1979) develops in response to the nicotine CS (Besheer et al, 2004; Murray and Bevins, 2007a; Palmatier et al, 2005). Behaviorally, this conditioning follows many of the postulates of Pavlovian conditioning and could possibly simulate learning processes in human smokers (Murray and Bevins, 2011; Murray et al, 2009). Previous studies with this model have focused on the behavioral or neuropharmacological mechanisms involving the nicotine CS. However, to date there are no studies focused on elucidating the neural substrates involved in the expression of CR evoked by the nicotine CS. One technique that is widely used to assess neuronal activity is immunostaining for the immediate early gene c-Fos (Curran and Morgan, 1995; Kovács, 1998). The purpose of the present experiment was to use neurohistochemical analysis of rapidly developing c-Fos protein to elucidate the potential neurobiological loci involved in the processing of nicotine as an interoceptive CS.
MATERIALS AND METHODS
Animals
Subjects used were 72 male Sprague-Dawley rats purchased from Harlan as young adults (275–290g; Indianapolis, IN, USA). Rats were housed individually in clear polycarbonate cages (48.3 × 26.7 × 20.3 cm) lined with wood shavings. The temperature- and humidity-controlled colony room was kept on a 12-h light/dark schedule. All experimental manipulations were conducted during the light cycle. Water access was freely available in the home cage and access to chow (Harlan Teklad Rodent Diet; Harlan, KY, USA) was restricted to maintain rats at 85% of their free-feeding body weight. This 85% target weight was increased by 2 g after 4 weeks into the study. Protocols were approved by the University of Nebraska–Lincoln Animal Care and Use Committee, and followed the ‘Guide for the Care and Use of Laboratory Animals' (Institute of Laboratory Animal Resources (U.S.), 1996).
Apparatus
Behavioral testing was conducted in commercially available chambers (ENV-008CT; Med Associates; St Albans, VT, USA) measuring 30.5 × 24.1 × 21.0 cm (l × w × h) enclosed in a sound and light attenuating cubicle equipped with an exhaust fan. Each conditioning chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. A recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) was centered on one of the sidewalls. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose solution in the receptacle. Access to the dipper was monitored by an infrared beam mounted 1.2 cm into the receptacle and 3 cm above the chamber floor. A second infrared beam that monitored general chamber activity was located 4 cm above the floor and 14.5 cm from the sidewall-containing receptacle. Beam breaks for dipper entries and chamber crosses were monitored using Med Associates interface and software (Med-PC for Windows, version IV; St Albans, VT, USA).
Drugs
(−)-Nicotine hydrogen tartrate and sodium pentobarbital (Sigma; St Louis, MO, USA) were dissolved in 0.9% saline. Nicotine pH was adjusted to 7.0±0.2 with a dilute NaOH solution. Nicotine dose (reported as base) and the 5-min injection-to-placement interval were selected on the basis of previous research (Murray and Bevins, 2007a, 2007b).
Behavioral Procedures
Training. Rats were handled for a minimum of 2 min on each of 3 consecutive days before the start of the experiment. Rats were treated with 0.4 mg/kg nicotine (SC) for 3 consecutive days before training to attenuate initial locomotor-suppressant effects of nicotine (Besheer et al, 2004; Bevins et al, 2001). For each 32 daily training sessions, all rats were injected (SC) with either nicotine (0.4 mg/kg) or saline 5 min before placement in the conditioning chamber for a 20-min session. Rats were randomly assigned to one of three learning conditions: nicotine CS, chamber CS, and CS alone (n=23–24 per condition). Rats in each condition received a unique pseudorandom order of 16 nicotine and 16 saline sessions with the condition that no more than two of the same session type (nicotine vs saline) occurred in a row. In the nicotine-CS condition, nicotine was paired with intermittent access to sucrose. Access to sucrose was initiated between 124 and 152 s from the start of the session with four possible times randomized throughout the training phase. There were 36 separate 4-sec deliveries of sucrose per nicotine session. Time between sucrose deliveries ranged from 4 to 80 s (mean=25 s) and was randomized for each session. For intermixed saline sessions, sucrose was withheld. The chamber-CS condition differed from nicotine-CS condition only in that nicotine and saline were each pseudorandomly paired 50% of the time with sucrose. That is, half of the saline sessions throughout training phase had 36 intermittent sucrose deliveries, whereas the remaining half was without sucrose; the same was true for nicotine sessions. The CS-alone condition differed from nicotine-CS condition only in that neither nicotine nor saline included sucrose deliveries (ie, no access to sucrose throughout experiment). Because our standardized testing session for assessing stimulus control without an influence of sucrose delivery is significantly shorter (ie, 4 min) than a training session (ie, 20 min), this change of protocol on the final test day could serve as a stressor contributing to the undesirable non-specific c-Fos expression (Cullinan et al, 1995; Senba and Ueyama, 1997). In order to minimize this possibility, four mock-test sessions (two nicotine and two saline) were interspersed within the training schedule to familiarize rats with this testing protocol. All rats experienced either nicotine or saline mock test following days 8, 12, 20, and 24 of training.
Testing. On a final test day, half of the rats from each condition (nicotine-CS, chamber-CS, CS-alone) were injected SC with nicotine 5 min before the start of the session; the remaining rats were injected with saline (3 × 2 design; 6 total groups; n=11–12 per group). For that test session, rats were placed in the conditioning chambers for 4 min. Dipper entries and chamber crosses were recorded, but sucrose was withheld.
c-Fos Immunohistochemistry
All rats were anesthetized and transcardially perfused (0.9%. saline, 4% paraformaldehyde) 90 min after the final test injection (detailed in Supplementary Materials and Methods). Brains were rapidly removed and processed for the c-Fos immunoreactivity as previously described by Zhao and Li (2010) and detailed in Supplementary Methods.
Fos-Immunoreactive (Fos-I) Cell Counting
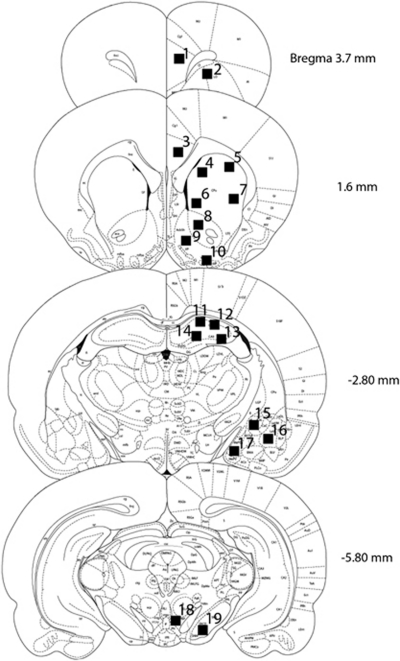
Two digital images ( × 10 magnification; 340 μm2) from each area of interest were taken bilaterally from anatomically matched sections using an Olympus CX41RF microscope (Japan) fitted with digital camera (Infinity lite, Canada). Positively labeled nuclei (thresholded against background) were identified and automatically counted using NIH ImageJ software (Abramoff et al, 2004). The number of Fos-I cells was averaged between the two bilateral images from any given brain region and used as a unit of measurement for statistical analyses (Shram et al, 2007). Nuclei selected for the assessment (Figure 1) represented brain regions implicated in the rewarding and/or incentive motivational effects of drugs of abuse (+1.60 and −5.80 from bregma; eg, caudate-putamen (dmCPu, dlCPu, vmCPu, vlCPu), nucleus accumbens (AcbC, AcbSh), ventral pallidum (VP), ventral tegmental area (VTA), substantia nigra (SNR)) (Pagliusi et al, 1996; Robinson and Berridge, 2003; Smith et al, 2009), learning and memory (−2.80 from bregma; eg, hippocampus (CA1, CA2, CA3, DG), amygdala (CeM, BLA, MeAD)) (Everitt and Wolf, 2002; Squire, 1992), and executive and cognitive functions (3.7 and 1.6 from bregma; eg, prelimbic cortex (PrL), orbitofrontal cortex (OFC), anterior cingulate cortex (Cg2)) (Balleine and Dickinson, 1998; Coutureau and Killcross, 2003; Dalley et al, 2004; Killcross and Coutureau, 2003; Robbins, 2005).
Figure 1.
Schematic representation (adapted from Paxinos and Watson, 2007) of areas sampled for c-Fos immunoreactivity assessment. All areas were located within anatomical levels represented on four coronal sections (+3.7, +1.6, −2.80, −5.80). Black squares corresponding to adjacent numbers represent approximate locations where digital images were acquired from: (1) prelimbic cortex (PrL); (2) orbitofrontal cortex (OFC); (3) Cg2 area of anterior cingulate cortex; (4) dorsomedial caudate-putamen (dmCPu); (5) dorsolateral caudate-putamen (dlCPu); (6) ventromedial caudate-putamen (vmCPu); (7) ventrolateral caudate-putamen (vlCPu); (8) nucleus accumbens core (AcbC); (9) nucleus accumbens shell (AcbSh); (10) ventral pallidum (VP); (11) CA1 area of hippocampus; (12) CA2 area of hippocampus; (13) CA3 area of hippocampus; (14) dentate gyrus (DG); (15) centromedial amygdaloid nucleus (CeM); (16) basolateral amygdaloid nucleus (BLA); (17) medial amygdaloid nucleus (MeAD); (18) ventral tegmental area (VTA); (19) substantia nigra (SNR).
Statistical Analyses
Statistical analyses were performed using the freely available statistical package R (http://www.r-project.org). An omnibus analysis of variance (ANOVA) preceded all planned comparisons. Higher-order interactions (detailed in the Supplementary Materials and Methods) were further analyzed by two-way ANOVAs and followed, if necessary, by Tukey's HSD post-hoc tests (p<0.05). Although every effort was taken to minimize variation between different batches of immunohistochemical assays, subtle variation is often expected (Rhodes et al, 2005). Thus to examine the effect of training on Fos-I expression, a 3 × 2 (condition × drug) randomized block design between-subject ANOVA was performed with batch, containing equal number of representatives from each group, as a blocking factor. Data from two rats were excluded from all analyses because of an injection error on the final test day.
RESULTS
Training
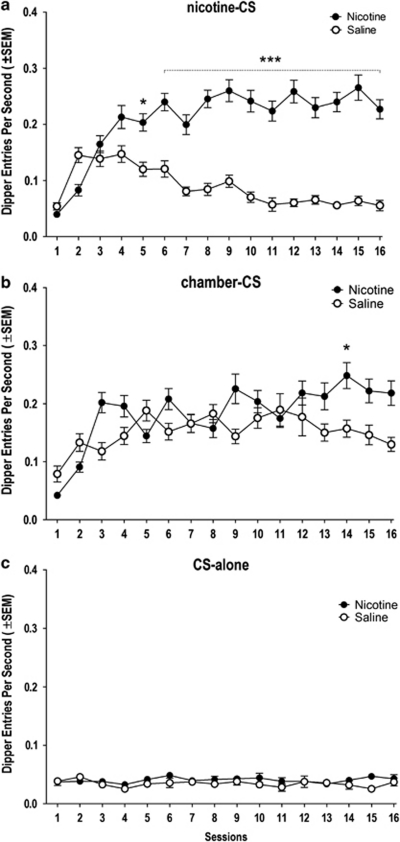
An omnibus ANOVA revealed that there were significant main effects of training condition (ie, nicotine-CS, chamber-CS, CS-alone; F(2, 2142)=772.61, p<0.0001), drug (nicotine vs saline; F(1, 2142)=330.85, p<0.0001), and their interaction over time (condition × drug × session; F(30, 2142)=5.87, p<0.0001). Overall, responding on nicotine sessions was significantly higher than on saline sessions in the nicotine-CS and chamber-CS conditions, but not in the CS-alone condition (condition × drug interaction; F(2, 2077)=175.94, p<0.0001; Tukey HSD tests; see Figure 2). In the nicotine-CS condition, conditioned responding evoked by the nicotine CS was higher than saline from session 5 through 16 (separate two-way ANOVA; drug × session interaction; F(15, 724)=18.15, p<0.0001; Tukey HSD tests; Figure 2a). There was weak differential responding across session type in the chamber-CS condition; post-hoc comparisons revealed greater responding in the nicotine than saline session only on session 14 (separate two-way ANOVA; drug × session interaction; F(15, 675)=4.43, p<0.0001; Tukey HSD tests; Figure 2b).
Figure 2.
Dipper entry rates (±SEM) from rats in (a) nicotine-CS, (b) chamber-CS, and (c) CS-alone conditions of a training phase. *Significant from saline session(s) (*P<0.05, ***P<0.001).
Testing
In the 4-min test, there was a main effect of condition (F(2, 63)=46.59, p<0.0001) and a main effect of test drug (F(1, 63)=48.47, p<0.0001) on rate of dipper entries. Further, the condition × drug interaction was significant on this test; F(2, 63)=11.78, p<0.0001. Unlike rats that underwent CS-alone training, rats in the nicotine-CS and chamber-CS conditions had significantly higher dipper entries when challenged with nicotine relative to saline (Tukey HSD tests; Figure 3). Among nicotine-challenged rats, dipper entries in the nicotine-CS and chamber-CS condition did not differ from each other, but were significantly higher than CS-alone rats (Tukey HSD tests). Among rats pretreated with saline, only rats in the chamber-CS condition differed significantly from CS-alone rats (Tukey HSD tests). Notably, there was no main effect of training condition or test drug, nor was there an interaction for general chamber activity (F(2, 63)=0.61, p>0.05; data not shown).
Figure 3.
Dipper entry rates (±SEM) on the final 4-min test. **,***Significant from saline treatment. aSignificantly different from bnicotine-treated CS-alone control (**P<0.01, ***P<0.001).
Fos Immunoreactivity
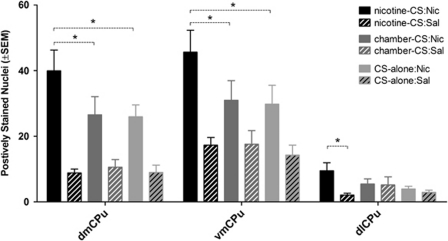
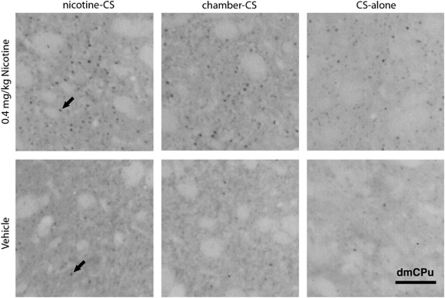
Table 1 summarizes main effects (condition, drug) and interactions with a number of positively identified Fos-I cells as the dependent measure. On the test day, rats in nicotine-CS condition had significantly higher expression of Fos-I cells in the AcbC and vmCPu when compared with the CS-alone condition (for all means (±SEM) see Table 2). In concordance with previous reports, nicotine challenge on the test day significantly increased c-Fos expression in a majority of the areas assessed (main effect of drug; PrL, OFC, Cg2, dmCPu, dlCPu, vmCPu, vlCPu, AcbC, AcbSh, CA1, CeM, BLA, MeaD, VP, VTA). Among rats challenged with nicotine, rats in the nicotine-CS condition (ie, those expressing a nicotine-evoked CR) had significantly higher c-Fos expression in dmCPu and vmCPu when compared with chamber-CS and CS-alone conditions (condition × drug interaction; Tukey HSD tests; Figure 4). Rats challenged with nicotine in the nicotine-CS condition had significantly higher c-Fos expression in the dlCPu when compared with saline (condition × drug interaction; Tukey HSD tests; Figure 4). Figure 5 illustrates representative sample of c-Fos immunoreactivity in the dmCPu.
Table 1. ANOVA (Main Effects and Interaction) Results of c-Fos Expression by Regions Tested.
|
Condition ME |
Drug ME |
Condition × Drug |
||||
|---|---|---|---|---|---|---|
| F (2,53) | P | F (1,53) | P | F (2,53) | P | |
| Prefrontal Cortex | ||||||
| PRL | 0.09 | 0.97 | 19.41 | *** | 0.60 | 0.55 |
| ORF | 0.91 | 0.40 | 13.60 | *** | 0.54 | 0.58 |
| CG2 | 1.48 | 0.23 | 27.94 | *** | 1.27 | 0.29 |
| Striatum | ||||||
| dmCPu | 2.28 | 0.11 | 63.13 | *** | 3.81 | * |
| dlCPu | 1.42 | 0.25 | 6.11 | * | 4.09 | * |
| vmCPu | 4.07 | * | 49.46 | *** | 3.51 | * |
| vlCPu | 0.86 | 0.42 | 6.02 | * | 2.08 | 0.13 |
| ACBc | 3.76 | * | 48.17 | *** | 0.92 | 0.4 |
| ACBsh | 1.02 | 0.36 | 35.02 | *** | 1.98 | 0.14 |
| Hippocampus | ||||||
| CA1 | 2.13 | 0.12 | 10.00 | ** | 0.16 | 0.85 |
| CA2 | 1.54 | 0.22 | 1.57 | 0.21 | 0.99 | 0.37 |
| CA3 | 1.83 | 0.17 | 0.89 | 0.34 | 0.98 | 0.38 |
| DG | 2.44 | 0.09 | 0.02 | 0.88 | 0.85 | 0.43 |
| Amygdala | ||||||
| CEM | 0.32 | 0.72 | 11.87 | ** | 0.09 | 0.90 |
| BLA | 1.10 | 0.33 | 27.25 | *** | 1.04 | 0.35 |
| MEAD | 0.09 | 0.90 | 9.76 | ** | 2.65 | 0.08 |
| Other areas | ||||||
| VP | 2.29 | 0.11 | 27.76 | *** | 1.09 | 0.34 |
| VTA | 2.23 | 0.11 | 10.45 | ** | 1.29 | 0.28 |
| SNR | 0.01 | 0.98 | 1.12 | 0.29 | 2.79 | 0.07 |
*P<0.05, **P<0.01, ***P<0.001.
Table 2. Number (±SEM) of c-Fos Positively Labeled Nuclei by Groups and Regions Tested.
|
0.4 mg/kg Nicotine |
Vehicle |
|||||
|---|---|---|---|---|---|---|
| nicotine-CS | chamber-CS | CS-alone | nicotine-CS | chamber-CS | CS-alone | |
| Prefrontal Cortex | ||||||
| PrL | 39.63±6.39 | 35.67±8.62 | 36.69±6.42 | 19.58±3.21 | 23.41±4.46 | 23.91±3.24 |
| OFC | 106.56±15.92 | 91.81±18.41 | 100.54±9.20 | 78.38±12.60 | 69.25±14.59 | 57.09±14.02 |
| Cg2 | 39.00±6.45 | 31.21±8.51 | 25.83±3.67 | 16.00±2.84 | 12.59±3.97 | 15.77±3.04 |
| Striatum | ||||||
| dmCPu | 39.96±6.32 | 26.54±5.54 | 25.96±3.61 | 8.83±1.17 | 10.57±2.37 | 9.00±2.22 |
| dlCPu | 9.50±2.44 | 5.48±1.54 | 3.94±0.86 | 2.10±0.54 | 5.18±2.46 | 2.93±0.64 |
| vmCPu | 45.63±6.68 | 31.00±5.94 | 29.81±5.72 | 17.31±2.31 | 17.59±4.16 | 14.25±3.07 |
| vlCPu | 9.56±2.32 | 6.90±2.15 | 4.77±1.07 | 3.19±1.08 | 4.89±2.46 | 4.11±1.05 |
| AcbC | 41.63±6.12 | 38.48±6.80 | 30.02±4.78 | 21.44±3.33 | 20.55±3.94 | 16.68±2.94 |
| AcbSh | 23.31±4.19 | 18.19±4.64 | 17.38±2.86 | 9.77±1.46 | 9.84±1.76 | 10.20±1.78 |
| Hippocampus | ||||||
| CA1 | 8.31±1.53 | 7.27±1.70 | 9.04±1.66 | 5.35±1.10 | 4.82±1.56 | 7.02±1.63 |
| CA2 | 8.77±1.83 | 7.58±1.79 | 7.25±1.47 | 8.15±1.89 | 5.23±1.78 | 6.98±2.07 |
| CA3 | 9.08±1.93 | 6.71±1.25 | 6.35±1.48 | 7.21±1.07 | 5.23±1.55 | 7.09±2.52 |
| DG | 17.70±5.02 | 15.27±3.03 | 12.68±3.41 | 12.20±3.50 | 8.07±1.96 | 11.26±3.41 |
| Amygdala | ||||||
| CeM | 12.81±4.03 | 11.46±1.45 | 11.04±3.09 | 7.08±1.49 | 5.11±0.69 | 6.39±1.49 |
| BLA | 10.44±2.18 | 13.15±2.48 | 9.13±2.13 | 5.10±1.06 | 4.70±0.59 | 4.70±0.78 |
| MeAD | 14.94±2.31 | 15.29±2.41 | 12.33±1.51 | 9.79±1.97 | 10.20±1.47 | 12.30±2.10 |
| Other areas | ||||||
| VP | 17.05±2.50 | 18.96±4.94 | 12.15±1.27 | 8.63±2.52 | 7.11±1.37 | 6.05±0.96 |
| VTA | 26.02±3.55 | 22.04±3.48 | 18.96±2.77 | 18.17±2.45 | 10.91±1.97 | 16.48±3.61 |
| SNR | 9.69±1.95 | 6.77±1.46 | 5.50±1.39 | 3.33±1.18 | 6.64±3.00 | 7.36±3.11 |
Figure 4.
Means (±SEM) of positively labeled c-Fos nuclei. *Significant difference indicated by dashed brackets (P<0.05).
Figure 5.
Photomicrographs ( × 10 magnification) of c-Fos immunoreactivity in the dmCPu of rats from all groups challenged by nicotine or saline on a final test day. Black arrows are indicating positively labeled nuclei. Scale bar=100 μm.
DISCUSSION
This is the first study to identify neurobiological loci (dmCPu, vmCPu) involved in the expression of CR evoked by the interoceptive CS effects of nicotine. The magnitude of expression of rapidly developing c-Fos protein oncogene was used as a measure of neuronal activity within brain regions carefully selected for their possible involvement in excitatory conditioning processes involving the nicotine stimulus. Specificity of neuronal activity for this conditioning was achieved by comparing the nicotine-CS condition to two control conditions. In the first control condition (chamber-CS), exposure to nicotine and sucrose reward was identical to the nicotine-CS condition except that nicotine was not reliably paired with sucrose. That is, across training, half of the nicotine sessions were paired with the sucrose US and half of the saline sessions were paired with sucrose. Thus, the conditioning chamber cues, and not the presence or absence of nicotine, were the most reliable stimuli associated with sucrose (50% of all sessions with chamber stimuli reinforced). In the second control condition (CS-alone), all rats had identical exposure to nicotine, when compared with nicotine-CS and chamber-CS conditions, but sucrose was never available. Therefore, this condition served to establish levels of neuronal activation following repeated nicotine-alone treatment in a pattern like the nicotine-CS condition.
With these controls in mind, nicotine-induced c-Fos expression in the medial caudate-putamen (dmCPu, vmCPu) was dependent on the learning history. Rats in the nicotine-CS condition, when challenged with nicotine on the test day, had significantly higher c-Fos expression in the medial regions of caudate-putamen (dmCPu, vmCPu) in comparison with controls. In addition, nicotine CS was the only condition that exhibited differential (nicotine vs saline) c-Fos expression in the dlCPu. Previous investigations into the functioning of dorsal CPu may provide a better understanding of a role of this area as it relates to our experimental conditions. For example, Schultz (1998, 2006) has shown that dopaminergic neurons within the caudate-putamen can be activated by just presenting a stimulus (ie, CS) that had been reliably paired with reward. Furthermore, it appears that the dorsal CPu and not NAc mediates cue-activated drug-seeking in rats with chronic cocaine self-administration history (Vanderschuren et al, 2005a; Vanderschuren and Everitt, 2005b). During cocaine-seeking behavior contingent upon presentation of a light stimuli previously paired with cocaine (no cocaine available), dopamine levels are elevated in the dorsal CPu, but not in the AcbC or AcbSh (Ito et al, 2000, 2002; Neisewander et al, 1996). Moreover, dopamine receptor blockade in the dorsal CPu, but not in the AcbC, dose-dependently attenuates cocaine-seeking (Vanderschuren et al, 2005a). These findings lend support to the hypothesis that as drug use progresses from the initial stages to the dependence state, the behavior depends less on NAc and progressively more on dorsal CPu. Because this transition could be indicative of dorsal CPu's involvement in habitual stimulus–response processes (Berke and Hyman, 2000; Everitt and Robbins, 2005; Tiffany, 1990; Vanderschuren et al, 2005a), finding of the present study may in part reflect effects of habitual learning with nicotine as an appetitive (associated with reward) CS.
Although no previous studies have investigated anatomical regions involved in learning with nicotine as the appetitive CS, chronic nicotine administration has been shown to induce c-Fos immunoreactivity in a number of cortical and mesolimbic areas (Marttila et al, 2006; Pagliusi et al, 1996; Salminen et al, 1999). Our findings corroborate these previous reports. Receiving a nicotine challenge on the test day induced c-Fos immunoreactivity in 15 out of 19 areas examined. The exceptions were CA2, CA3, and DG of the hippocampus and SNR. These areas of hippocampus and SNR are not typically assessed for the c-Fos activity associated with nicotine treatments, however, at least one study (Pagliusi et al, 1996) also found little to no (eg, <3 positive cells in the case of DG) immunoreactive c-Fos nuclei following chronic nicotine self-administration in these areas.
The mesolimbic system is central to reward- and drug-dependence processes. The mesolimbic system also is critically involved in mediating direct reinforcing effects of nicotine (Corrigall, 1999; Corrigall and Coen, 1989; Corrigall et al, 1992, 1994; Di Chiara, 2000). However, our results indicate that c-Fos expression in both VTA (mesolimbic dopamine production) and ACBc (recipient of dopamine signal from the VTA) was not affected by the appetitive learning history. This possible neuroanatomical difference between loci of the primary appetitive or reinforcing effects and the acquired appetitive properties is a potentially important dissociation that deserves future empirical attention.
The behavioral pattern for the three learning conditions in this experiment was somewhat predictable on the basis of the previous investigations into nicotine as an interoceptive CS. As noted earlier, the interoceptive-stimulus effects of nicotine readily acquired the ability to evoke a food-seeking CR when nicotine was consistently paired with sucrose (ie, nicotine-CS condition) (Bevins, 2009). In comparison, rats that were trained with nicotine alone (ie, no sucrose throughout entire experiment; CS-alone condition) had very low dipper entry rates on either session type (nicotine or saline) compared with nicotine-CS or chamber-CS conditions. Thus, mere exposure to nicotine and its psychomotor effects was not sufficient to significantly increase dipper entries. As detailed earlier, the chamber cues serve as the best predictor for sucrose access in the chamber-CS condition. As the chamber was the most reliable stimulus associated with sucrose, the chamber stimuli evoked goal-tracking on nicotine and saline sessions. By the end of training, goal-tracking in the chamber-CS condition on nicotine sessions was higher when compared with saline sessions. Although significance was only observed at one time point (session 14), this difference seemed to be stabilizing towards the end of training; the difference continued on the tests session.
Although the increased responding in the later nicotine sessions for the rats in the chamber-CS condition was not anticipated, there are possible explanations for this effect. One possibility is that nicotine enhanced the salience of the chamber-associated stimuli. This explanation is based on work by Olausson, Jentsch, and Taylor (2004). In that study, they found that nicotine enhanced responding for the compound CS (light+tone) previously associated with water (see also Caggiula et al, 2001; Chaudhri et al, 2006). Accordingly, perhaps the acquired appetitive properties of the chamber stimuli, evidenced by the goal-tracking in the saline state, were enhanced by the nicotine. Such enhancement would augment the CR evoked by the chamber CS. Additional support for this account is found in the nicotine self-administration literature. For example, responding for a light+tone compound stimulus that had been previously paired with sucrose as a means to increase its mildly rewarding effect, was enhanced by either contingent or non-contingent nicotine infusions (IV) when compared with saline controls (Chaudhri et al, 2006).
Another possibility is that under this intermittent schedule where nicotine is partially paired with sucrose, the nicotine stimulus slowly acquires some control over goal-tracking—with the other features (elements) of the chamber still controlling a majority of the responding, given the richer reinforcement density. Albeit possible, we have published data that suggests that this possibility is unlikely (Bevins et al, 2007; Murray et al, 2011). In these studies, the nicotine stimulus, along with all the chamber stimuli, was paired with sucrose on every session. There were no saline sessions, thus 100% of the nicotine+chamber sessions were reinforced. This schedule of reinforcement is much richer than the present work (50% of nicotine sessions, with 50% on no-nicotine sessions also being reinforced). According to this account, the nicotine stimulus in the 100% scenario should control some of the goal-tracking. To test this possibility, rats were treated with saline rather than nicotine for the first time. Removal of the nicotine stimulus did not significantly disrupt goal-tracking behavior (Bevins et al, 2007; Murray et al, 2011), suggesting that nicotine in these studies was not a sufficiently salient stimulus element to acquire control of behavior when the external chamber cues were as good of a predictor. In the present study, these chamber cues were even a better predictor than nicotine. Regardless, even if nicotine was an element with some control over the goal-tracking behavior, the magnitude of nicotine's control was not sufficient to drive activation of medial caudate-putamen.
Finally, one might suggest that the locomotor stimulant effects of nicotine contributed to the increase in goal-tracking on nicotine sessions for the chamber-CS condition. This possibility also seems unlikely. First, there was no difference in general chamber activity among the groups tested with either nicotine or saline on the final test day; ie, no stimulant effect of nicotine detected in that brief 4-min test. Second, nicotine administration did not enhance dipper entries (goal-tracking) during the training or testing phase in the CS-alone condition. This lack of effect suggests that there needs to be some learning regarding access to sucrose to occur for nicotine to enhance the goal-tracking response (see previous paragraph).
Although nicotine enhanced goal-tracking behavior evoked by chamber stimuli in the chamber-CS condition, this difference was not manifested in differential expression of c-Fos in the areas examined in this study. Perhaps we were not able to find a neurobiological substrate for this effect because of the limited sensitivity of the immunohistochemical assay, limited number of regions tested, or because this effect was also a minor contributing factor in the nicotine-CS condition. Nevertheless, in light of these findings, chamber-CS training protocol provides new evidence for the nicotine enhancement effect of the acquired incentive salience of other stimuli (for recent review see Bevins and Caggiula, 2009) and could possibly serve as a new model to further elucidate this phenomenon.
In sum, nicotine is capable of serving as a CS for an appetitive reward, such as sucrose. This effect was evidenced here by nicotine acquiring differential control of a goal-tracking CR in the nicotine-CS condition. The present research suggests that this appetitive conditioning involves dorsomedial and ventromedial regions of CPu. Our findings present a first account of specific regional neural activation by nicotine as a CS. Understanding neurobiological mechanisms underlying nicotine's function as an appetitive CS could provide a foundation for more comprehensive paradigms deployed to elucidate what seem to be complex and multifaceted mechanisms underlying nicotine dependence.
Acknowledgments
This work was supported by NIH research grant DA018114 and DA023951. We thank Catalin V. Buhusi for his thoughtful comments on an earlier version of this manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with imageJ. Biophoton Int. 2004;11:36–42. [Google Scholar]
- Alessi SM, Roll JM, Reilly MP, Johanson CE.2002Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice Exp Clin Psychopharmacol 1077–83.discussion 101–103. [PubMed] [Google Scholar]
- Azizian A, Monterosso J, O'neill J, London ED. Magnetic resonance imaging studies of cigarette smoking. Handb Exp Pharmacol. 2009;192:113–143. doi: 10.1007/978-3-540-69248-5_5. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol. 2009;192:209–233. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Molecular and cellular mechanisms of action of nicotine in the Cns. Handb Exp Pharmacol. 2009;192:173–207. doi: 10.1007/978-3-540-69248-5_7. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier M, Metschke D, Bevins R. Nicotine as a signal for the presence or absence of sucrose reward: a pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins R, Besheer J, Pickett K. Nicotine-conditioned locomotor activity in rats: dopaminergic and gabaergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Altering the motivational function of nicotine through conditioning processes. Nebr Symp Motiv. 2009;55:111–129. doi: 10.1007/978-0-387-78748-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Caggiula AR. Nicotine, tobacco use, and the 55th Nebraska symposium on motivation. Nebr Symp Motiv. 2009;55:1–3. doi: 10.1007/978-0-387-78748-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Murray JE.2011Internal stimuli generated by abused substances: role of pavlovian conditioning and its implications for drug addictionIn: Schachtman T, Reilly S (Eds).Associative Learning and Conditioning: Human and Non-Human Applications Oxford University Press: New York, Ny; 270–289. [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a pavlovian appetitive goal tracking task with rats. Behav Brain Res. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- CDC 2008Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004 Morbidity and Mortality Weekly ReportVol 2010. [PubMed]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Cue-exposure treatment: time for change. Addiction. 2002;97:1219–1221. doi: 10.1046/j.1360-0443.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- Corrigall W. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall W, Coen K. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall W, Coen K, Adamson K. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall W, Franklin K, Coen K, Clarke P. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘Goal Tracking') in rats. Learn Motiv. 1979;10:295–312. [Google Scholar]
- Institute Of Laboratory Animal Resources (U.S.) 1996Guide for the Care and Use of Laboratory Animals7th edn.National Academy Press: Washington, D.C.xii, 125 P.Pp. [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kovács KJ. c-Fos as a transcription factor: a stressful (Re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Marttila K, Raattamaa H, Ahtee L. Effects of chronic nicotine administration and its withdrawal on striatal Fosb/Deltafosb and c-Fos expression in rats and mice. Neuropharmacology. 2006;51:44–51. doi: 10.1016/j.neuropharm.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007a;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol. 2007b;18:707–716. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Excitatory conditioning to the interoceptive nicotine stimulus blocks subsequent conditioning to an exteroceptive light stimulus. Behav Brain Res. 2011;221:314–319. doi: 10.1016/j.bbr.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Penrod RD, Bevins RA. Nicotine-evoked conditioned responding is dependent on concentration of sucrose unconditioned stimulus. Behav Process. 2009;81:136–139. doi: 10.1016/j.beproc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Walker AW, Polewan RJ, Bevins RA. An examination of nmda receptor contribution to conditioned responding evoked by the conditional stimulus effects of nicotine. Psychopharmacology (Berl) 2011;213:131–141. doi: 10.1007/s00213-010-2022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O'dell LE, Tran-Nguyen LT, Castañeda E, Fuchs RA. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Pagliusi SR, Tessari M, Devevey S, Chiamulera C, Pich EM. The reinforcing properties of nicotine are associated with a specific patterning of c-Fos expression in the rat brain. Eur J Neurosci. 1996;8:2247–2256. doi: 10.1111/j.1460-9568.1996.tb01188.x. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.2007The Rat Brain in Stereotaxic Coordinates6th edn.Academic Press: San Diego, CA, USA [Google Scholar]
- Rhodes JS, Ryabinin AE, Crabbe JC. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Salminen O, Seppä T, Gäddnäs H, Ahtee L. The effects of acute nicotine on the metabolism of dopamine and the expression of fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci. 1999;19:8145–8151. doi: 10.1523/JNEUROSCI.19-18-08145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Acute nicotine enhances c-Fos Mrna expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav. 2009;94:319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005a;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol. 2005b;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Zhao C, Li M. c-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience. 2010;166:1043–1055. doi: 10.1016/j.neuroscience.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.