Abstract
Previous findings suggested the role of the prefrontal cortex, hippocampus, and cingulate gyrus in major depressive disorders (MDD), but the white matter microstructural abnormalities of the fibers connecting these brain structures are not known. The purpose of this study was to test the hypothesis that white matter abnormalities are present in association fibers of the uncinate fasciculus (UF) and cingulum bundle (CB) among MDD subjects. A total of 21 MDD subjects aged between 30 and 65 years and 21 age-matched healthy controls (HC) were recruited. All subjects were right-handed and without history of diabetes or other cardiac diseases. We extracted quantitative tract-specific measures based on diffusion tensor imaging tractography to examine both diffusivity and geometric properties of the UF and CB. Significantly decreased fractional anisotropy (FA) and increased radial diffusivity of the right UF were observed in MDD patients compared with HC (p<0.05), while their geometric characteristics remained relatively unchanged. Among MDD subjects, depression severity had a significant negative correlation with normalized number of fibers (NNF) in the right UF (r=−0.53, p=0.02). We also found significant age effect (old<young) in HC group and laterality effect (L>R) in both groups in the FA measure of the CB. Our study demonstrates novel findings of white matter microstructural abnormalities of the right UF in MDD. In the MDD group, the severity of depression is associated with reduced NNF in the right UF. These findings have implications for both clinical manifestations of depression as well as its pathophysiology.
Keywords: diffusion tensor imaging, tractography, major depression, uncinate, cingulum, fractional anisotropy
INTRODUCTION
Using magnetic resonance imaging (MRI) techniques, researchers studying major depressive disorder (MDD) have found a number of brain regions to be implicated in mood regulation, including prefrontal areas, limbic structures, and subcortical gray matter regions (Drevets et al, 1997; Blood et al, 2010; Seminowicz et al, 2004). Structural MRI studies have demonstrated that gray matter volume alterations within inferior prefrontal regions (including the orbitofrontal cortex), the anterior cingulate, and the hippocampus, are associated with depression (Ballmaier et al, 2004; Sheline et al, 2003; Bae et al, 2006; Bremner et al, 2002). In addition, deep white matter hyperintensities (WMH) have been linked to depression symptomatology, especially in late life depression (Kumar et al, 2002; Sassi et al, 2003).
Diffusion tensor imaging (DTI) is a MRI technique to study water diffusion in tissue, providing vital information on white matter microstructure, such as fiber connectivity and integrity in the healthy and diseased brain. Fractional anisotropy (FA) quantifies the directionally preferential water molecule diffusivity, and is usually used to assess overall white matter integrity. Using voxel- or region-based analyses, several DTI studies have revealed frontal and temporal white matter abnormalities in late-life depression (Nobuhara et al, 2006; Shimony et al, 2009), as well as in non-geriatric MDD (Wu et al, 2011; Ma et al, 2007; Zhu et al, 2011). For example, lower FA has been reported in frontal, temporal, and parietal regions for first-episode, treatment-naive younger adults with MDD (Ma et al, 2007). A more recent study acquired DTI data on middle-aged patients with MDD and controls and analyzed the images using the tract-based spatial statistics technique (Kieseppä et al, 2010). Suggestive areas of decreased FA were found in the right mid-cingulate cortex and posterior body of the corpus callosum in MDD patients compared with controls. In a somewhat related DTI study, altered white matter integrity in the anterior cingulate was found in patients with acute coronary syndrome who developed persistent depression when compared with patients who were not depressed (Rapp et al, 2010).
Results from treatment studies are mixed and at times contradictory (Taylor et al, 2008; Alexopoulos et al, 2002, 2008). For example, Taylor et al (2008) investigated DTI measures in subjects who underwent a 12-week open-label trial of sertraline, a selective serotonin reuptake inhibitor using a region-based approach. FA was measured in regions of interest placed in the white matter of the dorsolateral prefrontal cortex, anterior cingulate cortex, and corpus callosum. Subjects who did not remit with sertraline showed higher FA values in the superior frontal gyri and anterior cingulate cortices bilaterally. By contrast, in another treatment study, the authors reported lower FA values in multiple frontal limbic brain areas as well as select temporal and parietal regions associated with poor antidepressant response in late life depression (Alexopoulos et al, 2008). Although the precise relationship of FA alterations to treatment response is unclear, FA may represent the neuroanatomical substrates that are related to the pathophysiology of major depression.
On the basis of the evidence from the above volumetric and voxel-based DTI studies, suggesting the role of select regions of the prefrontal cortex, hippocampus, and cingulate gyrus in mood regulation and major depression, we hypothesize that microstructural white matter abnormalities may be present in two association fibers connecting these brain structures: the uncinate fasciculus (UF) and the cingulum bundle (CB). The UF is a ventral associative bundle that connects the anterior temporal lobe, including the amygdala and hippocampus, with the medial and lateral orbitofrontal cortex. The CB is a medial associative bundle that runs within the cingulate gyrus superior to the corpus callosum. It contains fibers of different lengths, the longest of which runs from the anterior temporal gyrus to the orbitofrontal cortex and the shortest (ie, U-fibers) connects the medial frontal, parietal, occipital, temporal lobes, and cingulate cortex. Both the UF and CB tracts, considered part of the limbic system, are thought to be involved in emotion processing, attention, and memory. Thus, a detailed examination of the tracts that connect these regions has implications for understanding the management and treatment of major depression.
As voxel-based DTI analyses may suffer from inter-subject mis-coregistration and lower spatial resolution because of smoothing in image pre-processing, we used a DTI-tractography technique to probe tract-specific white matter microstructural integrity of UF and CB in middle-aged major depression. By directly extracting and comparing tracts of interest, this DTI-tractography-based approach may provide high detection power in investigating white matter abnormalities. In addition to the standard diffusivity measures of white matter integrity, such as FA and mean diffusivity (MD), we explored tract-specific measures that quantify and summarize the geometric properties of the reconstructed tracts. We also computed two additional measures including the axial diffusivity (the degree of water diffusion along the direction parallel to the fiber bundles) and radial diffusivity (water diffusion perpendicular to the axonal wall). These two measures provide more information on the relative diffusivity of water molecules beyond standard FA, and have been utilized to differentiate among various white matter microstructural changes (Song et al, 2003; Metwalli et al, 2010; Bennett et al, 2010). We hypothesized that white matter abnormalities will be present in the UF and CB tracts of MDD subjects when compared with healthy controls (HC). Moreover, given previous reports of age effects on regional FA values that point toward compromised axonal morphology in aging (Sullivan et al, 2010) and studies of hemispheric asymmetries in brain structure (Gong et al, 2005), we assessed age and laterality effects for UF and CB tracts in a series of exploratory analyses using FA measures.
MATERIALS AND METHODS
Subjects
A total of 21 subjects with MDD and 21 age-matched HC were recruited from the greater Chicago area through flyers and local advertisements. This study was approved by the University of Illinois-Chicago Institutional Review Board, and written informed consent was obtained from each participant. The inclusion criteria for all subjects were right-handedness, age between 30 and 65 years, medication-naive or anti-depressant free for at least 2 weeks, and no history of diabetes, cardiac, or neurological diseases. The exclusion criteria included as folows: schizophrenia, bipolar or any psychotic disorders; current and past alcohol, or substance abuse; history of anxiety disorder outside of major depressive episodes; history of severe eating disorder and somatoform disorders.
All eligible subjects were assessed by a trained research assistant with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (First et al, 1997). The severity of depression was quantified by a board certified/board eligible psychiatrist (AK or OA) using the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1967). At the time of enrollment, MDD subjects met DSM-IV criteria for MDD and required a score of 15 or greater on the HAM-D. Subjects were also administered the Center for Epidemiologic Studies of Depression (CES-D) Scale as an independent measure of depression severity (Radloff, 1977). The CES-D was used for correlation analyses as the HAM-D was the measure used in the determination of subject eligibility.
MRI Data Acquisition
All brain MRI data were acquired using Philips 3.0T Achieva scanner (Philips Medical Systems, Best, The Netherlands) with 8-channel SENSE (sensitivity encoding) head-coil. Subjects were fitted with soft ear plugs, positioned comfortably in the coil, and instructed to remain still. Foam pads were used to minimize head motion. For each subject, DTI images were acquired using single-shot spin-echo echo-planar imaging sequence (field of view (FOV)=240 mm; voxel size=0.83 × 0.83 × 2.2 mm; TR/TE=6994/71 ms; flip angle=9°). In all, 67 contiguous axial slices aligned to the anterior commissure–posterior commissure line were collected along 32 gradient directions with b=700 s/mm2 and one minimally diffusion-weighted scan (the b0 image). Parallel imaging technique was utilized with factor at 2.5 to reduce scanning time to ∼4 min. High-resolution three-dimensional T1-weighted images were acquired with MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence (FOV=240 mm; 134 contiguous axial slices; TR/TE=8.4/3.9 ms; flip angle=8° voxel size=1.1 × 1.1 × 1.1 mm). In addition, a T2-weighted FLAIR (fluid-attenuated inversion recovery) scan was also acquired with turbo spin echo sequence (FOV=240 mm; 67 contiguous axial slices; TR/TI/TE=11 000/2800/68 ms; voxel size=0.83 × 0.83 × 2.2 mm).
Image Processing
T2 FLAIR images were visually inspected to rule out cases with serious WMH, especially in the regions containing the uncinate and cingulum tracts. Visual inspection of all DTI image data was also conducted to ensure quality, and data with serious artifacts from substantial movements were removed. All diffusion weighted images (32 gradient directions) were first coregistered onto the b0 images using the automatic image registration (AIR) algorithm with affine transformation to minimize eddy currents and remove any potential small bulk motions that occurred during the scans (Woods et al, 1998). The diffusion directions in the diffusion gradient table were transformed by the rotation matrix calculated by AIR, to correct changes in section angulation due to coregistration. Diffusion tensor calculation and fiber tracking were then carried out using the DtiStudio software (Jiang et al, 2006; Laboratory of Brain Anatomical MRI, Johns Hopkins Medical Institute, Baltimore, MD). At each voxel, the signals from the 32 diffusion-weighted images were fitted to obtain the six elements of the diffusion tensor. The diffusion tensors were then diagonalized to obtain three eigenvalues (λ1, λ2, and λ3) and three eigenvectors (v1, v2, and v3). The eigenvector (v1) associated with the largest eigenvalue (λL: axial diffusivity) was assumed to represent the local fiber direction. Radial diffusivity (λT) is the mean of two minor eigenvalues (λ2+λ3)/2. To quantify the relative degree of anisotropy in a voxel, FA is calculated by the square root of the sum of squares (SRSS) of the diffusivity differences, divided by the SRSS of the diffusivities:
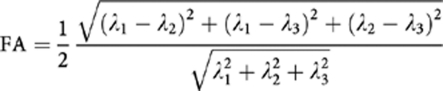 |
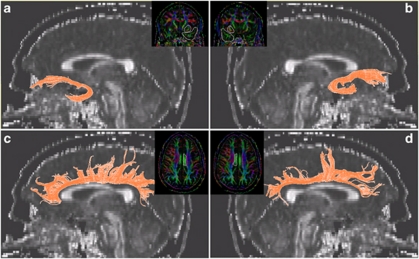
fiber tracking was performed with the fiber assignment by continuous tractography method (Jiang et al, 2006). For each subject, tractography was first performed with brute-force tracking on the whole brain by initiating tracts at each voxel. Fiber tracking was stopped when FA value falls <0.15 or a turning angle becomes >60°. To obtain a specific tract, a region of interest (ROI) was carefully traced and delineated. The extraction of UF followed the method described by (Wakana et al, 2007). We first identified and selected the most posterior coronal slice in which the temporal lobe is separated from the frontal lobe. A two-ROI approach was used with the first ROI including the entire temporal lobe and the second ROI the entire fiber projections in the frontal lobe. For the CB, we used a one-ROI approach, as recommended by (Catani and Thiebaut de Schotten, 2008). Because the majority of the fibers are short U-shaped fibers connecting adjacent gyri, the use of a two-ROI approach may exclude the majority in these short fibers from the analysis. We first examined the three axial slices above the corpus callosum and identified the slice with the largest continuous cingulum. The seeding ROI was then defined on the corresponding color-coded map with a single cigar-shaped region delineating the contour of the cingulum on this slice. Figure 1 demonstrates sample tract profiles superimposed on the corresponding FA map in a sagittal view. Inter-rater reliability on fiber extraction was performed by authors AZ and AL on a subset of five subjects (randomly selected from the entire sample of 42 subjects) and an intraclass correlation of >99.4% was reached in terms of the FA values for all the tracts. To ensure consistency in extracting fiber tracts, all reconstructed fibers were visually inspected for quality assurance by an experienced psychiatrist and brain imaging researcher (AL). None of the scans were rejected or considered unusable.
Figure 1.
Tract visualization superimposed on sagittal FA maps: (a) left uncinate fasciculus; (b) right uncinate fasciculus; (c) left cingulum bundle; and (d) right CB. The insert for each of the panel displays the regions of interest drawn on the color map.
Quantitative Tract-Specific Measures
After tracts are obtained, the corresponding fiber data were used to calculate tract-specific metrics with a wide range of measures to quantify white matter characteristics along the tracts. These tract-specific measures can be categorized into two types: the diffusivity measures and the fiber geometric metrics (Table 1).
Table 1. Quantitative Tract-Specific Measures in Two Categories: Diffusivity and Fiber Geometry.
| Type | Abbreviation | Definition |
|---|---|---|
| Diffusivity | FA | Mean fractional anisotropy |
| λL | Mean longitudinal (axial) diffusivity | |
| λT | Mean transverse (radial) diffusivity | |
| MD | Mean diffusivity | |
| Fiber geometry | NNF | Normalized number of fibers: the total number of fibers corrected for ICV |
| NFV | Normalized fiber volume: the total volume of fibers in tract-of-interest corrected for ICV | |
| NAFL | Normalized average fiber length: The total summed length of all fibers divided by number of fibers and then corrected for ICV | |
| NAFLFA | Normalized average FA-weighted fiber length: The total summed length of all fibers after weighting each fiber by FA and then divided by number of fibers and corrected for ICV |
Abbreviation: ICV, total intracranial volume.
Diffusivity measures included mean FA, axial (λL), and radial diffusivity (λT), MD. For each tract, these measures were extracted along the voxels that the fibers travel through and weighted by the number of reconstructed fibers in the particular voxel. All these values were then averaged to obtain a single number for each tract.
Fiber geometric measures included number of fibers (NNF), fiber volume (NFV), average fiber length (NAFL), and FA-weighted NAFL (NAFLFA). These geometric measures were normalized by the total intracranial volume, which was computed from the subject's high-resolution MPRAGE images using an automatic segmentation procedure in the FreeSurfer software (Fischl et al, 2004; Segonne et al, 2004; Jovicich et al, 2009; Martinos Center for Biomedical Imaging, Charlestown, Massachusetts).
Statistical Analyses
All statistical analyses were performed in the SPSS software, version 18.0 (SPSS, Somers, NY). Individual one-way analyses of covariance (ANCOVA) were used to assess group differences in all tract measures. Given the reported impact of age and sex on neuroanatomical measurements across various neuroimaging modalities (Barnes et al, 2010), these variables were used as covariates. We planned for post-hoc Pearson's product moment correlations (two-tailed) to further explore the relationship between depression severity scale CES-D and results of the aforementioned ANCOVAs in the MDD group. These analyses were also controlled for age and sex. We did not perform correlation with HAM-D as it was used in our inclusion criteria.
Exploratory analyses for age and laterality effect were performed for tract FA only. Pearson's product moment correlations (two-tailed) were conducted between tract FA and age. Analysis of laterality was performed on FA values of the left and right tract by paired-sample t-test. And then an asymmetry index (A) was computed which quantifies the differences between FA of the left and right tract, for both UF and CB using the following formula:
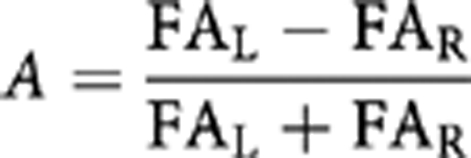 |
where FAL is the FA derived from the left UF or left CB and FAR is the FA of the right UF or right CB.
RESULTS
Demographic and Clinical Data
Table 2 summarizes the demographical and clinical characteristics of the MDD subjects and HC, including CES-D total score, Hamilton score, and duration of current symptoms in months. There were no significant differences between the MDD group and HC group in either age or sex. As expected, there were significant differences between the MDD group and HC across measures of depressive symptomatology (p<0.01).
Table 2. Demographic and Clinical Characteristics of Depressed (MDD) and Healthy Control Subjects.
| Characteristics |
MDD (N=21) |
Healthy control (N=21) |
Statistics |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | df | p | |
| Age (years) | 47.76 | 10.15 | 48.33 | 14.30 | 0.25 | 1 | 0.62 |
| Sex | 12M/9F | — | 13M/8F | — | 0.39 | 1 | 0.54 |
| CES-D total score | 32.15 | 8.84 | 3.40 | 3.60 | 181 | 1 | 0.00 |
| Hamilton score | 18.76 | 3.06 | 0.48 | 0.75 | 642 | 1 | 0.00 |
| Duration of current symptoms (months) | 20.39 | 28.39 | — | — | |||
Note: age and sex are not significantly different between groups.
Between-Group Comparison
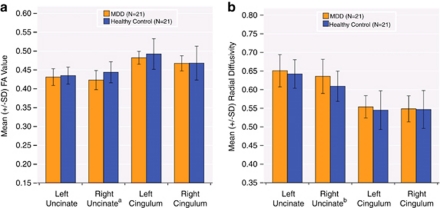
A significant FA reduction was observed for the right UF (F(1, 40)=6.86, p=0.01) in MDD subjects compared with HC. Within the right UF, radial diffusivity was significantly increased in the depressed group (F(1, 40)=4.46, p=0.04) whereas axial diffusivity remained relatively unchanged compared with HC. Figures 2a and b display FA and radial diffusivity respectively for all four tracts in both the MDD and HC groups. Post-hoc analyses revealed that FA and radial diffusivity in the right UF are negatively correlated (r=−0.735, p<0.001). Furthermore, the use of radial diffusivity as a covariate eliminated the group difference in FA for the right UF (F(1, 39)=2.27, p=0.140), suggesting a single physiological process responsible for FA and radial diffusivity changes. For MD, no significant between-group differences were detected for any of the four tracts. Similarly, the MDD and control groups did not differ on any tract geometric measure, including NNF, average length, and NFV for any of the four tracts.
Figure 2.
Mean values of: (a) FA; (b) radial diffusivity (λT) for MDD (N=21; in orange), and healthy control (HC) (N=21; in blue) groups. Error bars represent ±SD. aSignificant reduction in FA for the right UF (F(1, 40)=6.86, p=0.01). bSignificant increase in λT for the right UF (F(1, 40)=4.46, p=0.04).
Correlations of Tract Measures to Depression Severity
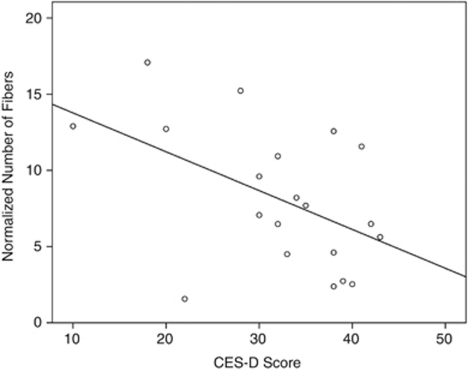
To further investigate the relationship between depression severity and tract-specific measures of the right UF in the MDD group, two-tailed partial Pearson's correlation controlled for age and sex were performed between CES-D total score and tract-specific diffusivity as well as geometric measures. Results revealed that in the right UF, the normalized NNF (r=−0.53, p=0.02) significantly correlated with CES-D total score; diffusivity measures (eg, FA), average length measures, and NFV did not. Figure 3 shows the scatter plot of CES-D vs NNF in the right UF. In addition, in our exploratory analysis, we did not find any significant correlation between HAM-D and any of the tract measures. It should be noted that any potential correlation with HAM-D could be attenuated by the fact that the HAM-D scores were artificially thresholded at 15 and higher (as 15 was the cutoff point for a depressed subject to be eligible for the study).
Figure 3.
Scatter plot of CES-D score vs NNF in the right UF.
Age Effect
A general trend of decreased FA was observed with aging, in all four tracts. Bilateral FA measures for the CB showed a significant negative correlation with age across the entire sample (left: r=−0.46, p=0.00; right: r=−0.49, p=0.00) and within the HC group only (left: r=−0.62, p=0.00; right: r=−0.58, p=0.00). Neither left nor right side of UF tract showed an association with age.
Laterality Effect
Paired-sample t-test on FA values showed that CB demonstrated significant laterality effect (L>R) in all three subject groups (MDD, HC, and the combined group; p<0.001). UF showed no significant laterality effect (L=R). Table 3 shows the asymmetry index values for the UF and the CB.
Table 3. Asymmetry Index Values for FA of Uncinate and Cingulum.
| Asymmetry index of FA |
MDD (N=21) |
Healthy control (N=21) |
MDD+healthy control (N=42) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Uncinate | −0.010 | 0.034 | 0.010 | 0.030 | −0.000 | 0.033 |
| Cingulum | 0.016 | 0.019 | 0.026 | 0.017 | 0.021 | 0.018 |
Note: significant hemisphere differences were found in the Cingulum for all three groups (L>R; p<0.001) but not in the uncinate (L=R), by paired-sample t-test.
DISCUSSION
For the first time, we have investigated tract-specific white matter alterations of UF and CB in non-geriatric MDD subjects utilizing DTI-tractography-based quantitative measures. The main finding of our study showed that white matter integrity of the right UF is significantly impaired in MDD subjects compared with HC as measured by FA. The decrease of FA in the right UF may be explained by the increase in the radial diffusivity, an index of myelin integrity, without significant alteration in the axial diffusivity. The geometric characteristics of the right UF did not contribute to the group differences in this tract. As concluded in the pioneering work of Song et al (2002, 2003), a decrease of axial diffusivity may reflect axonal injury whereas an increase of radial diffusivity may indicate a demyelination process. Thus, our results (unchanged axial but increased radial diffusivity) suggested that the white matter impairment of the right UF may be primarily demyelination in nature. This demyelination process could be due to neuronal loss, inflammation, or vascular damage as discussed in our previous work (Kumar and Cook, 2002). Lastly, newer reconstruction techniques based on high-angular-resolution-diffusion-weighted imaging are capable of disentangling the confound of variable degrees of sub-voxel fiber crossing, a variable previously known to alter axial and/or radial diffusivity (Tuch, 2004; Leow et al, 2009). Future studies may thus benefit from these novel techniques that allow us to differentiate among various neuropathological processes.
The UF connects the medial and lateral orbitofrontal cortex, the anterior cingulate cortex, and the anterior temporal lobe including the amygdala and hippocampus, all of which are structures known to be important in mood regulation. The FA reduction in the right UF implicates that the regions connected via UF could contribute to the impairment of the white matter integrity. As previously mentioned in the Introduction section, these regions have been previously investigated and shown to be altered in several imaging studies on mood disorders. For example, decreased radial diffusivity in the orbitomedial prefrontal cortex was reported in bipolar subjects relative to HC (Versace et al, 2008) and decreased FA in the anterior cingulate cortex was reported in subjects with acute coronary syndrome who developed persistent depression (Rapp et al, 2010). However, further studies are needed to directly examine which specific regions contribute to the FA reduction in the right UF.
Although the exact function of the UF in MDD is not well understood, a recent study of late-onset major depression found that depression severity significantly correlated with lower FA in white matter lesions of the right UF (Dalby et al, 2010). In contrast, Taylor et al (2007) found early-onset geriatric depressed subjects exhibited lower FA of the left UF compared with mid and late onset or HC subjects. Furthermore, converging lines of evidence implicated the possible involvement of UF in other neuropsychiatric illnesses that often overlap with MDD including anxiety disorders (Phan et al, 2009) and bipolar affective disorder (McIntosh et al, 2008; Versace et al, 2008). Compared with HC subjects, patients with anxiety disorder had significantly lower FA localized to the right UF white matter near the orbitofrontal cortex (Phan et al, 2009). Likewise, patients with bipolar disorder showed similar abnormalities in the UF (McIntosh et al, 2008; Versace et al, 2008; Versace et al, 2010). Although we did not find abnormalities in the left UF in our depressed subjects, a previous study demonstrated that bipolar patients exhibited divergent FA changes in the left vs the right UF (greater FA in the left UF and lower FA in the right UF) (Versace et al, 2008). Further evidence of the involvement of the right amygdala-orbitofrontal cortical functional connectivity was found using fMRI (Versace et al, 2010). Thus, alterations in the structural and functional connectivity of this circuit may represent a marker of mood disturbances in general. Overall, our findings pointing to the right UF involvement in mid-life MDD adds to the growing literature on the importance of this white matter tract to psychiatric illnesses involving mood and affective disturbances.
Although the severity of depression as measured by CES-D in MDD subjects did not correlate with FA, it did negatively correlate with the geometric measure of normalized NNF. To our knowledge, this is the first report of such findings in the literature. Here, we note that the NNF as a measure may be dependent on the protocols or procedures used, and thus can be problematic in multiple- or cross-site studies. Also, NNF summarizes one aspect of the mathematical representation of white matter tracts, rather than the actual count of fibers, and thus its real physiological meaning is not straightforward. Nevertheless, it remains a valid measure as in this paper we used the same procedure/protocol (eg, whole brain tractography by seeding at the center of each voxel with same stopping criteria of FA <0.15 and/or bending angle >60°), which was executed by two of the authors AZ and AL. As FA did not correlate with CES-D in the depressed group (but NNF did), our results support that some of the novel measures (such as NNF) introduced in this paper may be useful imaging metrics that provide complementary information on white matter microstructure when combined with other conventional DTI measures. Although further studies are needed to better understand the neuroanatomical nature of this correlation and its possible clinical implications, evidence from postmortem studies may provide some initial insight. Neuropathological analyses in depressed subjects have shown abnormal reduction in glial cells in the subgenual prefrontal cortex, orbital cortex, dorsal anterolateral prefrontal, and amygdala (Manji et al, 2001). Such reduction may negatively impact glial functions integral to structural plasticity in the brain resulting in a reduction of fiber number in MDD (Coyle and Schwarcz, 2000).
White matter provides the primary structural substrate for brain circuitry by connecting critical cortical and subcortical regions. The UF, in particular, connects the amgydala and hippocampus with the lateral and medial orbitofrontal cortex. Impaired connectivity between these important regions, reflected by decreased FA or reduced fiber number, may have a key role in the pathophysiology of MDD. Furthermore, functional imaging studies demonstrate the importance of intact white matter connectivity to successful mood regulation (Drevets, 2001). Abnormal functional connectivity in the subgenual cingulate and thalamus has been associated with major depression using resting-state functional MRI (Greicius et al, 2007). On the basis of similar functional imaging data, suggesting the over-activation of the cingulate region (Brodmann area 25) in MDD, Seminowicz et al (2004) found that the anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for depression supports the hypothesis that treatment efficacy is mediated via effects on a distributed network of frontal, limbic, and visceromotor brain regions (Johansen-Berg et al, 2008). Given that the UF is a key white matter tract coursing through the anterior cingulate cortex and amygdala, our findings may have implications for treatment studies as well.
Exploratory analyses of age and laterality effects revealed that bilateral FA values in the CB correlated with age in HC, and across each subject group the CB exhibited significant asymmetry (L>R). The fact that the age and FA correlation in the combined sample was driven by HC subjects suggests that the presence of depression may attenuate the effect of age on FA of the cingulum. In contrast to the attenuated age effect for the CB, the presence of depression did not alter the laterality properties of either tract. We theorize that greater FA found in the left CB may reflect a higher degree of axonal myelination or orientational/directional coherence of fibers in the left CB in comparison to the right side. Thus, the asymmetry of the CB may reflect the structural and functional differences between the two hemispheres, a finding that is consistent with the literature (Gong et al, 2005; Kubicki et al, 2003). More directed studies of age and laterality effects using DTI-derived measures of white matter integrity and geometry should be conducted.
Lastly, our study is limited by a relatively small sample size. Although previous diffusion imaging studies revealed similar results, the small sample size may limit the generalizability of these findings. In addition, with regards to the DTI acquisition, we applied the well-established protocol optimized for Philips 3T scanner, with a SENSE factor of 2.5 chosen to well balance the scan time and the signal-to-noise ratio (Reich et al, 2006). Note that the sequence did not use the dual spin echo technique (Reese et al, 2003) to reduce eddy current effects which in our study are less prominent with a low b-value of 700 s/mm2. Moreover, any residual effects were further minimized by the coregistation step in image post-processing before tensor fitting.
In summary, based on previous findings suggesting the role of select regions of the frontal cortex, hippocampus, and cingulate gyrus in mood regulation and major depression, we investigated the white matter microstructural integrity of two important association tracts connecting these regions: the UF and the CB in MDD using DTI-tractography. Our study makes the following novel contributions in clarifying the tract-specific abnormalities in major depression: (i) supports the role of the right UF in the pathology of MDD; (ii) demonstrates that radial (but not axial) diffusivity is altered in the right UF despite intact fiber geometry; and (iii) associates the severity of depression in MDD subjects with NNF in the right UF. We believe that this study points to the role of demyelination and reduced fiber number, highlighting the importance of abnormalities in major white matter association tracts in MDD.
Acknowledgments
This research was supported by the NIH grants 5R01MH63764-7, 5R01MH73989-5, and 5K23MH081175-02. We thank Ms Monya Meinel and Mr Piotr Daranowski for their assistance in coordinating this study.
The authors declare no conflict of interest.
Footnotes
Preliminary data were presented at the 40th Annual Meeting of the Society for Neuroscience (November 13–17, 2010; San Diego, CA, USA) entitled ‘Quantitative tract-specific measures of association fibers in major depression using diffusion tensor imaging: a preliminary study' (presentation number: 264.25).
References
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance. Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Iosifescu DV, Makris N, Perlis RH, Kennedy DN, Dougherty DD, et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Schwarcz R. Mind glue: implications of glial cell biology for psychiatry. Arch Gen Psychiatry. 2000;57:90–93. doi: 10.1001/archpsyc.57.1.90. [DOI] [PubMed] [Google Scholar]
- Dalby RB, Frandsen J, Chakravarty MM, Ahdidan J, Sørensen L, Rosenberg R, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010;184:38–48. doi: 10.1016/j.pscychresns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Drevets W. Neuroimaging and neuropathological studies of depression: implications for the cognitive emotional manifestations of mood disorders. Curr Op Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinical Version (SCID-CV) American Psychiatric Publishing: Washington, DC; 1997. [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23 (Suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, et al. Asymmetry analysis of cingulum based on scale-invariant parameterization by diffusion tensor imaging. Hum Brain Mapp. 2005;24:92–98. doi: 10.1002/hbm.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseppä T, Eerola M, Mäntylä R, Neuvonen T, Poutanen V, Luoma K, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120:240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin C, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Cook IA. White matter injury, neural connectivity and the pathophysiology of psychiatric disorders. Dev Neurosci. 2002;24:255–261. doi: 10.1159/000066746. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mintz J, Bilker W, Gottlieb G. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229–236. doi: 10.1016/S0893-133X(01)00331-1. [DOI] [PubMed] [Google Scholar]
- Leow AD, Zhu S, Zhan L, McMahon K, de Zubicaray GI, Meredith M, et al. The tensor distribution function. Magn Reson Med. 2009;61:205–214. doi: 10.1002/mrm.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Li L, Shu N, Liu J, Gong G, He Z, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res. 2010;1348:156–164. doi: 10.1016/j.brainres.2010.05.067. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Maniega SM, Lymer GK, McKirdy J, Hall J, Sussmann JE, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, et al. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rapp MA, Rieckmann N, Lessman DA, Tang CY, Paulino R, Burg MM, et al. Persistent depressive symptoms after acute coronary syndrome are associated with compromised white matter integrity in the anterior cingulate: a pilot study. Psychother Psychosom. 2010;79:149–155. doi: 10.1159/000286959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresia PA, et al. Quantitative characterization of the corticospinal tract at 3T. Am J Neuroradiol. 2006;27:2168–2178. [PMC free article] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. White matter hyperintensities in bipolar and unipolar patients with relatively mild-to-moderate illness severity. J Affect Disord. 2003;77:237–245. doi: 10.1016/s0165-0327(02)00170-2. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry. 2009;66:245–252. doi: 10.1016/j.biopsych.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2010;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Kuchibhatla M, Payne ME, MacFall JR, Sheline YI, Krishnan KR, et al. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One. 2008;3:e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3:669–674. [PMC free article] [PubMed] [Google Scholar]
- Tuch DS. Q-ball imaging. Magn Reson Imaging. 2004;52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JRC, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JRC, Hassel S, Klein CR, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state vs trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. general methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Wu F, Tang Y, Xu K, Kong L, Sun W, Wang F, et al. White matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191:80–83. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–229. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]