Abstract
Antidepressant–placebo response-differences (RDs) in controlled trials have been declining, potentially confounding comparisons among older and newer drugs. For clinically employed antidepressants, we carried out a meta-analytic review of placebo-controlled trials in acute, unipolar, major depressive episodes reported over the past three decades to compare efficacy (drug–placebo RDs) of individual antidepressants and classes, and to consider factors associated with year-of-reporting by bivariate and multivariate regression modeling. Observed drug–placebo differences were moderate and generally similar among specific drugs, but larger among older antidepressants, notably tricyclics, than most newer agents. This outcome parallels selective increases in placebo-associated responses as trial-size has increased in recent years. Study findings generally support moderate efficacy of clinically employed antidepressants for acute major depression, but underscore limitations of meta-analyses of controlled trials for ranking drugs by efficacy. We suggest that efficiency and drug–placebo differences may be improved with fewer sites and subjects, and better quality-control of diagnostic and clinical assessments.
Keywords: antidepressants, evidence-based medicine, depression, meta-analysis, controlled trials, secular changes
INTRODUCTION
Efficacy of antidepressant drugs for treatment of acute, unipolar, major depressive episodes continues to be a research and clinical topic of considerable interest. There have been major changes in clinical practice involving antidepressants since the early 1990s (Healy, 1997; Baldessarini, 2005; Ghaemi, 2008). Currently favored drugs include the serotonin reuptake inhibitors (SRIs) introduced since the late 1980s, and a series of additional modern, ‘second-generation' antidepressants. These include agents with mixed inhibitory actions on the neuronal-uptake and inactivation of serotonin and norepinephrine (SNRIs, including desvenlafaxine, duloxetine, milnacipran, venlafaxine, and others), and ‘atypical' agents with other actions (such as bupropion, nefazodone, mirtazapine, and vilazodone). These modern or ‘second-generation' antidepressants have largely displaced older antidepressants including tricyclics (TCAs) and monoamine oxidase (MAO) inhibitors (Baldessarini, 2005, 2012).
The superiority of most clinically employed antidepressants over placebos in controlled trials has been modest in adult patients diagnosed with major depression, even lower in juvenile depressed patients, and probably has declined in recent years (Walsh et al, 2002; Baldessarini, 2005; Cipriani et al, 2007; Papakostas et al, 2007; Gartlehner et al, 2008; Kirsch et al, 2008; Tsapakis et al, 2008; Bridge et al, 2009; Wooley et al, 2009; Masi et al, 2010; Pigott et al, 2010; Khin et al, 2011). Evident decline in superiority of drugs over placebos has occurred despite evidence of selective reporting of positive findings of potential commercial interest from therapeutic trials (Ioannidis, 2008; Turner et al, 2008).
Moreover, there is little evidence that one antidepressant or pharmacological class of antidepressants is clearly and convincingly more effective than others (Anderson, 2001; Baldessarini, 2005, 2012; Cipriani et al, 2007; Papakostas et al, 2007; Gartlehner et al, 2008; Kirsch et al, 2008; Khin et al, 2011). In part, this lack of clear differentiation may arise from the modest drug–placebo differences in many controlled trials of antidepressants, which, in turn, may reflect broad clinical heterogeneity arising from the current broad concept of ‘major depression' (Healy, 1997; Ghaemi, 2008). Possible differentiation of efficacy among antidepressants was a lively question soon after introduction of the SRIs and SNRIs (Healy, 1997; Baldessarini, 2005, 2012). However, the popularity of most modern antidepressants owes far more to their perceived safety, relative ease of use, and broad clinical utility rather than to well-demonstrated superior efficacy in major depressive disorder compared with older agents (Baldessarini, 2005, 2012; Cipriani et al, 2007; Papakostas et al, 2007; Wooley et al, 2009; Pigott et al, 2010; Khin et al, 2011).
Given current uncertainties regarding the relative efficacy of specific drugs and pharmacological classes of antidepressants, we carried out a systematic, meta-analytic review of peer-reviewed, placebo-controlled trials, reported since 1980, limiting inclusion to drugs with regulatory approval for major depression that are currently employed clinically in the United States. Specific aims were to: (a) compare the efficacy of older and modern antidepressants compared with placebo; (b) further test the apparently widely held assumption that modern agents are at least equivalent in efficacy to older antidepressants (specifically TCAs); and (c) examine factors associated with anticipated declining differences in drug- vs placebo-associated responses in randomized, placebo-controlled trials of antidepressants.
MATERIALS AND METHODS
Search Strategy
We conducted a computerized literature review using Medline, CINAH Library, Cochrane Library, and PsycINFO literature databases using the following search-terms: ‘antidepressant, amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine, depression (or major depression), desmethylvenlafaxine, duloxetine, escitalopram, fluoxetine, imipramine, isocarboxazid, mirtazapine, maprotiline, monoamine oxidase (or MAO) inhibitors, nortriptyline, phenelzine, paroxetine, S-citalopram, selegiline, sertraline, tranylcypromine, trazodone, tricyclic antidepressants, trimipramine, and venlafaxine,' alone and in various combinations. Also, reference lists of articles and reviews on antidepressant efficacy were hand-searched for relevant reports. The search was limited to peer-reviewed, published, randomized, placebo-controlled trials (RCTs) in acute episodes of adult major depressive disorder diagnosed by standardized criteria, and reported from 1980 through August 2011.
Eligibility Criteria
Included were reports of randomized, double-blind, placebo-controlled trials in adults in an acute, apparently unipolar, major depressive episode (or with ⩽10% identified cases of bipolar depression or diagnoses other that major depression) based on DSM-III, III-R, or -IV, ICD-9 or -10, or RDC diagnostic criteria, and with at least 20 subjects per arm. We excluded trials of drugs that are not US FDA-approved and indicated in the United States for treatment of acute episodes of major depressive disorder, as well as reports involving special populations, such as juvenile or geriatric patients, treatment-resistant depression or depression associated with major neuromedical or other psychiatric disorders. Only monotherapy trials were included; antidepressant doses could be fixed or flexible, with or without low-doses (below the approximate equivalent (Baldessarini, 2005) of 2 mg/day of lorazepam) of supplemental sedative or hypnotic agents. For 35 trials with three randomized treatment conditions involving two active agents and a placebo arm, we compared each drug–placebo pair separately; in some three-arm trials involving an experimental agent and a standard comparator, we considered only a marketed agent vs placebo. When an active agent was used in different doses in the same trial, we calculated mean doses and outcome measures, all considered as a single drug-arm. Total daily drug doses (mg/day) were converted to approximate imipramine-equivalents (IMI-eq), based on the median of the range of clinical doses recommended by the manufacturers as summarized elsewhere (Baldessarini et al, 2010), so as to permit comparisons of agents of dissimilar potency.
Outcome Measures
The primary outcome measure was categorical ‘response,' usually defined as ⩾50% reduction in initial depression rating-scale scores. Most often, ratings were based on the Hamilton (HDRS) or Montgomery–Åsberg (MADRS) Depression Rating Scales (Hamilton, 1960; Montgomery and Åsberg, 1979), or Clinical Global Impression (CGI) ratings (Guy, 1976) when these measures were not available. Scores employed for analyses were standardized as the percentage of maximum attainable scores on each rating scale (eg, 48 for 17-item HDRS, 60 for the MADRS, and 61 for 21-item HDRS). When the number of items in the HDRS was not specified by the investigators, we considered it to be the most commonly employed 17-item version. When more than one depression rating scale was employed, we gave priority to results obtained with the HDRS for greater comparability. All measures of initial depression severity and its change by end-point were standardized by use of percentages of observed ratings to the maximum attainable score with each rating scale employed. Continuous measures of change in depression ratings with drug vs placebo were considered as secondary measures, because lack of variance measures in most trials precluded formal meta-analysis. We considered factors that might influence outcomes, including numbers of subjects and collaborating sites, percentage women, initial depression ratings, IMI-eq daily drug doses, trial-duration, dropout rates, specific drugs and types, and year of reporting. As manufacturers of the drugs involved sponsored almost all trials, sources of support were not further considered.
Data Analysis
Averaged data are means with SD, unless stated otherwise. Meta-analyses based on Stata metan programs, used random-effects modeling to limit effects of inter-trial variance; responder rates for each drug–placebo pair yielded pooled rate ratios (RRs) and rate differences (RDs) with their computed 95% confidence intervals (CIs) (Tsapakis et al, 2008; Yildiz et al, 2011a, 2011b). Percentage-improvement in depression for drug–placebo pairs was compared by paired-t testing and averaged to provide overall estimates of response differences (RDs). We also carried out bivariate and multiple linear regression modeling from these analyses to evaluate associations of selected covariates with reporting year. Correlations employed nonparametric Spearman rank methods (rs) to avoid effects of non-normally distributed data and potential nonlinear relationships. The primary study-hypothesis was that all marketed antidepressants would be statistically more effective than placebo, on average, with only minor differences among specific drugs or types. Analyses were based on standard commercial software (Stata.8; StataCorp, College Station, TX; Statview.5; SAS Institute, Cary, NC).
RESULTS
Trials Characteristics
Initially, we screened >2000 potentially relevant reports appearing between 1980 and 2011. Based on reviewing abstracts, 179 reports appeared to meet selection criteria and not to include multiple reports of the same trials. Exclusions (71/179) were as follow: (a) 17 studies involved <20 patients per arm; (b) another 17 included >10% of subjects with diagnoses other than major depressive episode; (c) 11 studies involved special populations; (d) 4 trials did not include a placebo control arm; (e) another 22 reports were excluded for various other reasons, including outcomes that were not quantified or did not include responder rates or improvement in depression ratings, represented subpopulations of larger trials already considered, or involved unapproved drugs. Detailed review of entire reports led to inclusion of 107; they involved 142 drug–placebo comparisons (Table 1), owing to 35 trials arising from studies with three randomized arms (for which a total of 3677 placebo-treated subjects were considered twice). There were 27 127 non-duplicated adult subjects (17 059 randomized to an antidepressant, 9925 to placebo), of average age 40 years (62.0±9.9% women). Antidepressants tested (n=19) ranked by trial-count as: imipramine (23 trials), fluoxetine (17), venlafaxine (15), paroxetine (14), amitriptyline (12), duloxetine (10), bupropion (9), desvenlafaxine (8), sertraline (8), R,S-citalopram (7), S-citalopram (5), mirtazapine (4), selegiline (3), desipramine (2), clomipramine (1), nortriptyline (1), phenelzine (1), tranylcypromine (1), and trazodone (1). Types of antidepressants ranked: SRIs (52 trials (36.6%)), TCAs (38 (26.8%)), SNRIs (33 (23.2%)), atypical agents (bupropion, mirtazapine, trazodone; 14 (9.9%)), and MAO-inhibitors (5 (3.5%)). Subjects per trial ranked: SNRIs (288±118) >SRIs (230±146) > atypical agents (224±144) >MAO-inhibitors (181±98) > TCAs (139±101); there were far more sites per trial since the median reporting-year of 1998 (range 1983–2010): 22.7±16.8 vs 7.22±5.98, as well as more subjects per trial: 270±114 vs 181±122, indicating a major secular trend toward increasing trial-size.
Table 1. Characteristics of Placebo-Controlled Trials of Antidepressants in Major Depression.
| Trial (reference) | Drug | mg/day | IMI-eq | Weeks | Total N | Sites | % Women | Response | N Rx | % Resp Rx | N Pbo | % Resp Pbo | RR Resp | Ratings | Initial Dep Rx | Change Rx (%) | Initial Dep Pbo | Change Pbo (%) | RD % Change | Dropout Rx (%) | Dropout Pbo (%) | ITT | Washout |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Claghorn et al (1983) | AMI | 180 | 180 | 4 | 172 | 3 | 56 | HDRS≥50% | 85 | 62.4 | 87 | 42.5 | 1.47 | HDRS21 | 42.6 | — | 45.9 | — | — | 58 | 58 | Yes | Yes |
| Feighner et al (1983) | IMI | 163 | 163 | 6 | 487 | 5 | 71 | CGI | 244 | 57.8 | 243 | 32.5 | 1.78 | HDRS21 | 42.6 | — | 42.6 | — | — | 24 | 40 | Yes | Yes |
| Itil et al (1983) | IMI | 127 | 127 | 4 | 47 | — | 44 | CGI | 25 | 44 | 22 | 22.7 | 1.94 | HDRS16 | 47.8 | 53 | 43.5 | 18 | 34.8 | 48 | 50 | No | Yes |
| Pitts et al (1983) | BUP | 525 | 262 | 4 | 59 | — | 34 | HDRS≥50% | 34 | — | 25 | — | — | HDRS21 | 50.8 | 48 | 50.8 | 29 | 19.3 | — | — | No | Yes |
| White et al (1984) | NRT | 109 | 136 | 4 | 120 | 1 | 45 | CGI | 61 | 41 | 59 | 32.2 | 1.27 | HDRS | 52.1 | 54 | 56.2 | 37 | 16.6 | 34 | 24 | Yes | Yes |
| White et al (1984) | TCP | 44 | 145 | 4 | 122 | 1 | 45 | CGI | 63 | 39.7 | 59 | 32.2 | 1.23 | HDRS | 56.3 | 45 | 56.2 | 37 | 8.1 | 41 | 24 | Yes | Yes |
| Cohn and Wilcox (1985) | FLX | 70 | 350 | 6 | 112 | — | 62 | HDRS≥50% | 54 | 74.1 | 58 | 20.7 | 3.58 | HDRS21 | 42.6 | 55 | 41 | 16 | 39.3 | 35 | 72 | Yes | Yes |
| Cohn and Wilcox (1985) | IMI | 152 | 152 | 6 | 112 | — | 53 | HDRS≥50% | 54 | 40.7 | 58 | 20.7 | 1.97 | HDRS21 | 42.6 | 34 | 41 | 16 | 17.9 | 63 | 72 | Yes | Yes |
| Rickels et al (1985) | AMI | 148 | 148 | 6 | 254 | — | 66 | HDRS≥50% | 124 | 53.2 | 130 | 26.9 | 1.98 | HDRS21 | 41 | 42 | 42.6 | 28 | 13.6 | 27 | 45 | No | Yes |
| Mendels and Schless (1986) | IMI | 167 | 167 | 6 | 68 | — | 46 | HDRS≥50% | 34 | 38.2 | 34 | 17.6 | 2.17 | HDRS17 | 50 | 40 | 50 | 23 | 16.7 | 52 | 52 | Yes | Yes |
| Rickels et al (1987) | IMI | 143 | 143 | 6 | 124 | — | 62 | HDRS≥50% | 63 | 69.8 | 61 | 37.7 | 1.85 | HDRS21 | 39.3 | 42 | 41 | 20 | 21.2 | 41 | 39 | No | Yes |
| Wernicke et al (1987) | FLX | 40 | 200 | 6 | 240 | 10 | 57 | HDRS≥50% | 207 | 54.1 | 33 | — | — | — | — | — | 0 | — | — | 44 | 44 | No | Yes |
| Hollyman et al (1988) | AMI | 110 | 119 | 6 | 178 | — | 83 | CGI | 90 | 58.9 | 88 | 44.3 | 1.33 | HDRS | 31.3 | 62 | 31.2 | 41 | 21.3 | 26 | 16 | No | No |
| Wernicke et al (1988) | FLX | 22 | 110 | — | 363 | — | 61 | HDRS≥50% | 285 | 46.3 | 78 | 23.1 | 2 | HDRS21 | 41 | 43 | 42.6 | 27 | 15.7 | 37 | 46 | Yes | Yes |
| Feighner et al (1989a) | FLX | 80 | 400 | 6 | 99 | — | 75 | CGI | 51 | — | 48 | — | — | HDRS21 | 42.6 | 31 | 42.6 | 22 | 8.5 | 51 | 68 | No | Yes |
| Feighner et al (1989a) | IMI | 150 | 159 | 6 | 55 | — | 74 | CGI | 36 | — | 19 | — | — | HDRS21 | 42.6 | 38 | 42.6 | 22 | 2 | 48 | 68 | No | Yes |
| Feighner et al (1989b) | IMI | 159 | 150 | 6 | 94 | — | 89 | HDRS≥50% | 46 | — | 48 | — | — | HDRS21 | 44.3 | 39 | 41 | 37 | 15.9 | — | — | No | Yes |
| Larsen et al (1989) | CMI | 150 | 165 | 6 | 38 | — | 66 | HDRS<9 | 20 | 55 | 18 | 22.2 | 2.48 | HDRS17 | 37.5 | 58 | 37.5 | 32 | 26.2 | 15 | 28 | Yes | No |
| Miller et al (1989) | PRX | 30 | 150 | 4 | 47 | — | 71 | CGI | 22 | 45.5 | 25 | 36 | 1.26 | HDRS21 | 39.3 | 25 | 39.3 | 26 | –0.50 | 45 | 20 | Yes | Yes |
| Quitkin et al (1989) | IMI | 150 | 150 | 6 | 54 | 2 | 55.9 | HDRS≥50% | 27 | 51.9 | 27 | 18.5 | 2.81 | HDRS17 | 30.2 | 65 | 30.2 | 26 | 39.3 | 26 | 30 | Yes | Yes |
| Quitkin et al (1989) | PNZ | 75 | 150 | 6 | 53 | 2 | 55.9 | HDRS≥50% | 26 | 57.7 | 27 | 18.5 | 3.12 | HDRS17 | 30.2 | 50 | 30.2 | 26 | 24.8 | 26 | 27 | Yes | Yes |
| Gelenberg et al (1990) | IMI | 175 | 175 | 4 | 43 | — | 32 | HDRS≥50% | 22 | — | 21 | x | x | HDRS27 | 33.3 | 51 | 34 | 35 | 15.9 | 36 | 23 | No | Yes |
| Lineberry et al (1990) | BUP | 287 | 144 | 6 | 219 | 5 | 65 | HDRS≥50% | 110 | 50.9 | 109 | 33.9 | 1.5 | HDRS21 | — | — | 44.3 | 38 | — | 23 | 29 | Yes | Yes |
| Reimherr et al (1990) | AMI | 104 | 104 | 8 | 299 | 8 | 54 | HDRS≥50% | 149 | 57.7 | 150 | 32.7 | 1.77 | HDRS18 | 44.2 | 61 | 44.2 | 37 | 23.5 | 42 | — | — | — |
| Reimherr et al (1990) | SRT | 145 | 181 | 8 | 299 | 8 | 54 | HDRS≥50% | 149 | 51.7 | 150 | 32.7 | 1.58 | HDRS18 | 44.2 | 53 | 44.2 | 37 | 15.9 | 41 | 37 | Yes | Yes |
| Roth et al (1990) | DMI | 224 | 168 | 6 | 53 | 2 | 59 | CGI | 24 | 62.5 | 29 | 37.9 | 1.65 | HDRS17 | 62.5 | 38 | 60.4 | 29 | 8.6 | — | — | No | Yes |
| Smith et al (1990) | AMI | 111 | 111 | 6 | 100 | — | 57 | HDRS≥50% | 50 | 56 | 50 | 30 | 1.87 | HDRS17 | 50 | 54 | 47.9 | 29 | 24.8 | 30 | — | Yes | — |
| Smith et al (1990) | MTZ | 18 | 77.4 | 6 | 100 | — | 57 | HDRS≥50% | 50 | 54 | 50 | 30 | 1.8 | HDRS17 | 47.9 | 47 | 47.9 | 29 | 17.8 | 40 | 50 | Yes | Yes |
| Carman et al (1991) | AMI | 200 | 200 | 6 | 150 | — | — | HDRS≥50% | — | — | — | — | — | HDRS21 | 45.9 | 51 | 44.3 | 26 | 24.5 | 4 | 4 | No | Yes |
| Khan et al (1991) | VNX | 74 | 66.6 | 6 | 93 | — | 60 | HDRS≥50% | 67 | — | 26 | — | — | HDRS21 | 41 | 56 | 42.6 | 31 | 25.7 | 21 | 15 | — | Yes |
| Bakish et al (1992) | AMI | 112 | 112 | 7 | 112 | 5 | 43 | HDRS≥50% | 57 | 50.9 | 55 | 34.5 | 1.47 | HDRS17 | 47.9 | — | 47.9 | — | — | 32 | 49 | Yes | Yes |
| Claghorn et al (1992) | PRX | 38 | 190 | 6 | 337 | 4 | 52 | HDRS<10 | 168 | 38.1 | 169 | 24.3 | 1.57 | HDRS21 | 44.3 | 48 | 42.6 | 33 | 15.2 | 35 | 44 | Yes | Yes |
| Cohn and Wilcox (1992) | PRX | 37 | 175 | 6 | 67 | — | 58 | HDRS≥50% | 31 | — | 36 | — | — | HDRS17 | 52.1 | 34 | 54.2 | 20 | 20.9 | 31 | 67 | Yes | Yes |
| Cohn and Wilcox (1992) | IMI | 175 | 185 | 6 | 71 | — | 54 | HDRS≥50% | 35 | — | 36 | — | — | HDRS17 | 52.1 | 41 | 54.2 | 20 | 14.2 | 26 | 67 | Yes | Yes |
| Fabre (1992) | IMI | 135 | 135 | 6 | 80 | 1 | 62 | HDRS≥50% | 40 | — | 40 | — | — | HDRS21 | — | — | — | — | — | 21 | 53 | No | — |
| Fabre (1992) | PRX | 29 | 145 | 6 | 80 | 1 | 62 | HDRS % | 40 | — | 40 | — | — | HDRS21 | — | — | — | — | — | 21 | 53 | No | Yes |
| Feighner (1992) | IMI | 113 | 113 | 6 | 79 | — | — | HDRS≥50% | 40 | 50 | 39 | 12.8 | 3.9 | HDRS21 | — | — | — | — | — | 41 | 54 | Yes | Yes |
| Feighner (1992) | PRX | 26 | 130 | 6 | 78 | — | — | HDRS≥50% | 39 | 28.2 | 39 | 12.8 | 2.2 | HDRS21 | — | — | — | — | — | 60 | 54 | Yes | X |
| Kiev (1992) | PRX | 31 | 155 | 6 | 78 | — | 45 | HDRS≥50% | 34 | 55.9 | 44 | 25 | 2.24 | HDRS17 | 60.4 | 45 | 58.3 | 24 | 20.7 | 38 | 44 | No | Yes |
| Rickels et al (1992) | PRX | 32 | 160 | 6 | 111 | — | 64 | HDRS≥50% | 55 | 40 | 56 | 19.6 | 2.04 | HDRS21 | — | — | 42.6 | — | — | 29 | 21 | No | Yes |
| Feighner et al (1993) | IMI | 140 | 140 | 6 | 477 | 6 | 49 | HDRS<10 | 237 | 26.6 | 240 | 12.9 | 2.06 | HDRS21 | 42.6 | 35 | 44.3 | 22 | 13.3 | 54 | 53 | Yes | Yes |
| Feighner et al (1993) | PRX | 30 | 150 | 6 | 480 | 6 | 51 | HDRS<10 | 240 | 24.6 | 240 | 12.9 | 1.9 | HDRS21 | 42.6 | 38 | 44.3 | 22 | 16.1 | 42 | 53 | Yes | Yes |
| Cunningham et al (1994) | TZD | 297 | 346 | 6 | 153 | 6 | 66 | CGI | 77 | 59.7 | 76 | 55.3 | 1.08 | HDRS21 | 41 | 43 | 39.3 | 36 | 7.3 | 36 | 36 | Yes | Yes |
| Cunningham et al (1994) | VNX | 158 | 142 | 6 | 148 | 6 | 66 | CGI | 72 | 72.2 | 76 | 55.3 | 1.31 | HDRS21 | 41 | 48 | 39.3 | 36 | 11.1 | 29 | 36 | Yes | Yes |
| Doogan and Langdon (1994) | SRT | 75 | 93.8 | 6 | 200 | — | 68 | MADRS≥50% | 99 | 50.5 | 101 | 39.6 | 1.28 | MADRS | 46.6 | 55 | 44.5 | 45 | 10.4 | 19 | 10 | Yes | Yes |
| Fontaine et al (1994) | IMI | 214 | 214 | 6 | 90 | 1 | 58 | HDRS≥50% | 45 | 48.9 | 45 | 31.1 | 1.57 | HDRS17 | 54.2 | 42 | 54.2 | 26 | 15.6 | 42 | 47 | Yes | Yes |
| Rickels et al (1994) | IMI | 191 | 191 | 8 | 187 | 12 | 63 | HDRS≥50% | 95 | 65.3 | 92 | 44.6 | 1.46 | HDRS17 | 50 | — | 50 | — | — | 49 | 37 | No | Yes |
| Schweizer et al (1994) | IMI | 176 | 176 | 6 | 151 | 2 | 66 | HDRS≥50% | 73 | 61.6 | 78 | 47.4 | 1.3 | HDRS21 | 39.3 | 43 | 41 | 38 | 5.2 | 45 | 27 | No | — |
| Schweizer et al (1994) | VNX | 182 | 164 | 6 | 151 | 2 | 66 | HDRS≥50% | 73 | 76.7 | 78 | 47.4 | 1.62 | HDRS21 | 41 | 55 | 41 | 38 | 16.5 | 36 | 27 | No | Yes |
| Silverstone (1994) | IMI | 150 | 150 | 6 | 135 | 13 | 55 | HDRS≥50% | 66 | 50 | 69 | 50.7 | 0.99 | HDRS17 | 52.1 | 48 | 50 | 43 | 4.4 | 40 | 35 | No | No |
| Vartiainen and Leinonen (1994) | MTZ | 32.5 | 140 | 6 | 114 | 8 | 54 | HDRS≥50% | 59 | — | 55 | — | — | HDRS21 | 42.6 | 59 | 42.6 | 48 | 10.7 | 37 | 44 | Yes | Yes |
| Wilcox et al (1994) | AMI | 122 | 122 | 6 | 99 | 2 | 47 | HDRS≥50% | 50 | 56 | 49 | 24.5 | 2.29 | HDRS21 | 42.6 | 59 | 42.6 | 45 | 14.2 | 44 | 55 | Yes | Yes |
| Bremner (1995) | AMI | 186 | 186 | 6 | 100 | — | 68 | HDRS≥50% | 50 | 58 | 50 | 34 | 1.71 | HDRS17 | 56.3 | — | 56.2 | — | — | 20 | 24 | Yes | Yes |
| Bremner (1995) | CTP | 30 | 129 | 6 | 100 | — | 68 | HDRS≥50% | 50 | 70 | 50 | 34 | 2.06 | HDRS17 | 58.3 | — | 56.2 | — | — | 18 | 24 | Yes | Yes |
| Claghorn and Lesem (1995) | MTZ | 16 | 68.8 | 6 | 90 | — | 44 | HDRS≥50% | 42 | 50 | 48 | 27.1 | 1.85 | HDRS17 | 45.8 | — | 47.9 | — | — | 40 | 58 | No | Yes |
| Fabre et al (1995) | SRT | 171 | 214 | 6 | 369 | 8 | 53 | CGI | 278 | 60.1 | 91 | 41.8 | 1.44 | HDRS17 | 52.1 | 47 | 52.1 | 34 | 12.3 | 23 | 49 | Yes | Yes |
| Guelfi et al (1995) | VNX | 350 | 315 | 4 | 93 | 6 | 85 | HDRS≥50% | 46 | 52.2 | 47 | 31.9 | 1.63 | HDRS17 | — | — | 60.4 | 17 | — | 24 | 57 | Yes | Yes |
| Khan (1995) | MTZ | 36 | 155 | 6 | 54 | 1 | 67 | HDRS≥50% | 27 | 55.6 | 27 | 37 | 1.5 | HDRS17 | 47.9 | 53 | 45.8 | 29 | 24.7 | 33 | 41 | Yes | Yes |
| Laakman et al (1995) | AMI | 102 | 102 | 6 | 146 | — | 71 | HDRS≥50% | 72 | 73.6 | 74 | 21.6 | 3.4 | HDRS17 | 41.7 | 60 | 39.6 | 25 | 34.9 | 5 | 12 | No | Yes |
| Mynors-Wallis et al (1995) | AMI | 139 | 139 | 12 | 61 | 15 | 74 | HDRS≤7 | 31 | 51.6 | 30 | 26.7 | 1.94 | HDRS17 | — | — | 37.5 | 36 | — | 19 | 60 | No | No |
| Cassano et al (1996) | IMI | 150 | 150 | 6 | 123 | 18 | 52.8 | MADRS≥50% | 64 | — | 59 | — | — | MADRS | 51.8 | 41 | 51.7 | 28 | 13.1 | 27 | 39 | Yes | Yes |
| Claghorn et al (1996) | IMI | 136 | 136 | 6 | 89 | — | 64 | CGI | 44 | 45.5 | 45 | 26.7 | 1.7 | HDRS21 | 42.6 | 40 | 42.6 | 25 | 15.2 | 58 | 60 | Yes | Yes |
| Cohn et al (1996) | IMI | 126 | 126 | 8 | 80 | — | 70 | HDRS≥50% | 38 | 60.5 | 42 | 35.7 | 1.69 | HDRS17 | — | — | 47.9 | 39 | — | 39 | 26 | Yes | Yes |
| Feiger (1996) | IMI | 224 | 224 | 8 | 81 | 8 | 68 | CGI | 41 | 61 | 40 | 30 | 2.03 | HDRS17 | 50 | 46 | 50 | 29 | 16.6 | 33 | 55 | Yes | Yes |
| Cunningham (1997) | VNX | 128 | 115 | 12 | 278 | — | 63 | HDRS≥50% | 179 | 57.5 | 99 | 30.3 | 1.9 | HDRS21 | 39.3 | 55 | 40.8 | 36 | 18.8 | 34 | 41 | Yes | Yes |
| Lecrubier et al (1997) | IMI | 114 | 114 | 10 | 151 | 24 | 66 | MADRS≥50% | 75 | 62.7 | 76 | 59.2 | 1.06 | MADRS | 40 | 57 | 40 | 54 | 3.7 | 31 | 25 | Yes | Yes |
| Lecrubier et al (1997) | VNX | 125 | 112 | 10 | 154 | 24 | 69 | MADRS≥50% | 78 | 82.1 | 76 | 59.2 | 1.39 | MADRS | 41.7 | 64 | 40 | 54 | 10.6 | 29 | 25 | Yes | Yes |
| Lydiard et al (1997) | AMI | 91 | 91 | 8 | 260 | 15 | 67 | HDRS≥50% | 131 | 55.7 | 129 | 37.2 | 1.5 | HDRS17 | 45.8 | 58 | 45.8 | 40 | 18.1 | 38 | 29 | Yes | Yes |
| Lydiard et al (1997) | SRT | 91 | 114 | 8 | 261 | 15 | 67 | HDRS≥50% | 132 | 54.6 | 129 | 37.2 | 1.47 | HDRS17 | 45.8 | 52 | 45.8 | 40 | 11.8 | 27 | 29 | Yes | Yes |
| Thase (1997) | VNX | 150 | 135 | 8 | 197 | 12 | 61 | HDRS≥50% | 95 | 57.9 | 102 | 29.4 | 1.98 | HDRS21 | 39.3 | 48 | 39.3 | 30 | 18.2 | 27 | 40 | Yes | Yes |
| Ban et al (1998) | DMI | 150 | 112 | 4 | 174 | 6 | 62 | HDRS≥50% | 89 | 48.3 | 85 | 35.3 | 1.37 | HDRS17 | — | — | — | — | — | 10 | 10 | Yes | Yes |
| Fava et al (1998) | FLX | 50 | 250 | 12 | 73 | 5 | 51 | HDRS≥50% | 54 | 57.4 | 19 | 52.6 | 1.09 | HDRS21 | 39.3 | 45 | 39.3 | 48 | –3.30 | 31 | 21 | Yes | Yes |
| Fava et al (1998) | PRX | 35 | 175 | 12 | 74 | 5 | 51 | HDRS≥50% | 55 | 58.2 | 19 | 52.6 | 1.11 | HDRS21 | 37.7 | 48 | 39.3 | 48 | –0.50 | 29 | 21 | Yes | Yes |
| Khan et al (1998) | VNX | 142 | 128 | 12 | 382 | 12 | 64 | HDRS≥50% | 286 | — | 96 | — | — | HDRS21 | 41 | 45 | 41 | 30 | 15 | — | — | Yes | Yes |
| Massana (1998) | FLX | 30 | 150 | 8 | 255 | — | — | HDRS≥50% | 127 | 55.9 | 128 | 34.4 | 1.63 | HDRS21 | — | — | — | — | — | 24 | 41 | — | — |
| Reimherr et al (1998) | BUP | 218 | 109 | 8 | 362 | — | 68 | HDRS % | 241 | — | 121 | — | — | HDRS17 | — | — | — | — | — | 44 | 50 | Yes | Yes |
| Rudolph et al (1998) | VNX | 204 | 184 | 6 | 323 | — | 33 | HDRS≥50% | 231 | 48.9 | 92 | 29.3 | 1.67 | HDRS21 | — | — | — | — | — | 53 | 41 | Yes | Yes |
| Coleman et al (1999) | BUP | 290 | 145 | 8 | 235 | 9 | 57 | HDRS≥50% | 118 | 66.1 | 117 | 56.4 | 1.17 | HDRS31 | — | 59 | — | 50 | 9.4 | 22 | 32 | Yes | Yes |
| Coleman et al (1999) | SRT | 106 | 132 | 8 | 226 | 9 | 57 | HDRS≥50% | 109 | 60.5 | 117 | 56.4 | 1.07 | HDRS31 | — | 57 | — | 50 | 6.9 | 36 | 32 | Yes | Yes |
| Croft et al (1999) | BUP | 293 | 146 | 8 | 232 | 8 | 51 | HDRS≥50% | 116 | 66.4 | 116 | 47.4 | 1.4 | HDRS31 | — | — | — | — | — | 30 | 34 | Yes | Yes |
| Croft et al (1999) | SRT | 121 | 151 | 8 | 232 | 8 | 50 | HDRS≥50% | 116 | 68.1 | 116 | 47.4 | 1.44 | HDRS31 | — | — | — | — | — | 33 | 34 | Yes | Yes |
| Feighner and Overo (1999) | CTP | 33 | 142 | 6 | 650 | — | 60 | MADRS≥50% | 521 | — | 129 | — | — | HDRS21 | 41 | 46 | 40.8 | 38 | 7.7 | — | — | Yes | Yes |
| Mendels et al (1999) | CTP | 52 | 224 | 4 | 180 | 3 | 62 | HDRS≥50% | 89 | 80.9 | 91 | 47.3 | 1.71 | HDRS17 | 50 | 39 | 50 | 29 | 10.7 | 48 | 44 | Yes | Yes |
| Philipp et al (1999) | IMI | 100 | 100 | 8 | 151 | 18 | 78 | HDRS≥50% | 105 | 62.9 | 46 | 47.8 | 1.31 | HDRS17 | 45.8 | 64 | 47.9 | 53 | 10.7 | — | — | Yes | Yes |
| Rudolph and Feiger (1999) | FLX | 47 | 235 | 8 | 200 | 12 | 70 | HDRS≥50% | 103 | 50.5 | 97 | 42.3 | 1.19 | HDRS21 | 42.6 | 45 | 41 | 41 | 4.6 | 27 | 21 | — | — |
| Rudolph and Feiger (1999) | VNX | 175 | 158 | 8 | 192 | 12 | 70 | HDRS≥50% | 95 | 56.8 | 97 | 42.3 | 1.34 | HDRS21 | 41 | 50 | 41 | 41 | 9.2 | 19 | 21 | Yes | Yes |
| Silverstone and Ravindran (1999) | FLX | 40 | 200 | 12 | 237 | — | 60 | HDRS≥50% | 119 | 63 | 118 | 43.2 | 1.46 | HDRS17 | 56.3 | 56 | 56.2 | 41 | 15.1 | 26 | 40 | Yes | — |
| Silverstone and Ravindran (1999) | VNX | 141 | 127 | 12 | 240 | — | 60 | HDRS≥50% | 122 | 64.8 | 118 | 43.2 | 1.5 | HDRS17 | 56.3 | 58 | 56.2 | 41 | 17.7 | 29 | 40 | Yes | Yes |
| Corrigan et al (2000) | FLX | 20 | 100 | 8 | 70 | 8 | — | HDRS≥50% | 35 | 48.6 | 35 | 25.7 | 1.89 | HDRS17 | 45.8 | — | 43.8 | — | — | 14 | 34 | Yes | Yes |
| Stahl (2000) | CTP | 57 | 245 | 8 | 215 | 8 | 60 | HDRS≥50% | 107 | 55.1 | 108 | 39.8 | 1.38 | HDRS17 | 54.2 | 58 | 54.2 | 46 | 11.5 | 20 | 22 | Yes | Yes |
| Stahl (2000) | SRT | 143 | 179 | 8 | 216 | 8 | 60 | HDRS≥50% | 108 | 54.6 | 108 | 39.8 | 1.37 | HDRS17 | 56.3 | 55 | 54.2 | 46 | 9.1 | 26 | — | Yes | Yes |
| Coleman et al (2001) | BUP | 335 | 168 | 8 | 302 | 15 | 62 | HDRS≥50% | 150 | 56 | 152 | 50 | 1.12 | HDRS21 | 41 | 65 | 39.3 | 55 | 10.5 | 37 | 33 | Yes | Yes |
| Coleman et al (2001) | FLX | 29 | 145 | 8 | 306 | 15 | 63 | HDRS≥50% | 154 | 57.1 | 152 | 50 | 1.14 | HDRS21 | 41 | 63 | 39.3 | 55 | 8.4 | 37 | 33 | Yes | Yes |
| Andreoli et al (2002) | FLX | 40 | 200 | 8 | 255 | 33 | 60 | HDRS≥50% | 127 | 55.9 | 128 | 33.6 | 1.66 | HDRS21 | 44.3 | — | 44.3 | — | — | 8 | 12 | Yes | Yes |
| Bodkin and Amsterdam (2002) | SLG | 6 | 100 | 6 | 176 | 6 | 60 | HDRS≥50% | 88 | 37.5 | 88 | 22.7 | 1.65 | HDRS17 | 47.7 | 38 | 48.5 | 26 | 11.8 | 11 | 17 | Yes | Yes |
| Burke et al (2002) | CTP | 40 | 172 | 8 | 244 | 35 | 61 | MADRS≥50% | 125 | 45.6 | 119 | 27.7 | 1.65 | HDRS21 | 42.6 | 38 | 42.6 | 29 | 8.8 | — | — | Yes | Yes |
| Burke et al (2002) | S-CTP | 15 | 128 | 8 | 360 | 35 | 66 | MADRS≥50% | 241 | 50.6 | 119 | 27.7 | 1.83 | HDRS21 | 41 | 44 | 42.6 | 29 | 14.3 | — | — | Yes | Yes |
| Davidson (2002) | SRT | 75 | 93.8 | 8 | 225 | 12 | 66 | HDRS≥50% | 109 | 48.6 | 116 | 43.1 | 1.13 | HDRS17 | 47.9 | 46 | 47.9 | 40 | 5.8 | 28 | 28 | Yes | Yes |
| Detke et al (2002a) | DLX | 60 | 108 | 9 | 267 | 18 | 54 | HDRS≥50% | 139 | 44.7 | 128 | 23 | 1.95 | HDRS17 | 43.8 | 52 | 43.8 | 29 | –11.2 | — | — | Yes | Yes |
| Detke et al (2002b) | DLX | 60 | 108 | 9 | 245 | 21 | 99 | HDRS≥50% | 123 | 50.4 | 122 | 35.2 | 1.43 | HDRS17 | 41.7 | 40 | 41.7 | 52 | 23.1 | 39 | 35 | Yes | Yes |
| Golden et al (2002) | PRX | 43.2 | 216 | 12 | 622 | 40 | 65 | HDRS≥50% | 417 | 58 | 205 | 47.8 | 1.21 | HDRS17 | 50 | 53 | 50 | 46 | 7.1 | — | — | Yes | Yes |
| Goldstein et al (2002) | DLX | 107 | 193 | 8 | 140 | 8 | 64 | HDRS≥50% | 70 | 64.3 | 70 | 48.6 | 1.32 | HDRS17 | 37.5 | 53 | 39.6 | 34 | 18.9 | 34 | 34 | Yes | — |
| Goldstein et al (2002) | FLX | 20 | 100 | 8 | 103 | 8 | 64 | HDRS≥50% | 33 | 51.5 | 70 | 48.6 | 1.06 | HDRS17 | 37.5 | 44 | 39.6 | 34 | 9.2 | 36 | 34 | Yes | — |
| Wade et al (2002) | S-CTP | 10 | 85 | 8 | 380 | 40 | 76 | MADRS≥50% | 191 | 55 | 189 | 41.8 | 1.32 | MADRS | 48.3 | 51 | 48.3 | 42 | 9.2 | 16 | 15 | Yes | Yes |
| Amsterdam (2003) | SLG | 6 | 100 | 8 | 289 | 16 | 64 | HDRS≥50% | 145 | 32.4 | 144 | 27.8 | 1.17 | HDRS17 | 47.5 | 36 | 47.9 | 29 | 6.7 | 28 | 28 | Yes | Yes |
| Lepola et al (2003) | CTP | 28 | 120 | 8 | 313 | 69 | 72 | MADRS≥50% | 159 | 52.8 | 154 | 48.1 | 1.1 | MADRS | — | — | 48.3 | 42 | — | 5 | 10 | Yes | Yes |
| Lepola et al (2003) | S-CTP | 14 | 119 | 8 | 309 | 69 | 72 | MADRS≥50% | 155 | 63.9 | 154 | 48.1 | 1.33 | MADRS | — | — | 48.3 | 42 | — | 6 | — | — | — |
| Detke et al (2004) | DLX | 100 | 180 | 8 | 281 | — | 70 | HDRS≥50% | 188 | 68.1 | 93 | 44.1 | 1.54 | HDRS17 | 41.7 | 58 | 41.7 | 44 | 13.8 | 11 | 19 | Yes | Yes |
| Detke et al (2004) | PRX | 20 | 100 | 8 | 179 | — | 71 | HDRS≥50% | 86 | 74.4 | 93 | 44.1 | 1.69 | HDRS17 | 41.7 | 58 | 41.7 | 44 | 14.5 | 12 | 19 | Yes | Yes |
| Goldstein et al (2004) | DLX | 60 | 108 | 8 | 266 | 19 | 60 | HDRS≥50% | 177 | 47.5 | 89 | 31.5 | 1.51 | HDRS17 | 37.5 | 44 | 35.4 | 29 | 15 | 39 | 42 | Yes | Yes |
| Goldstein et al (2004) | PRX | 20 | 100 | 8 | 176 | 19 | 64 | HDRS≥50% | 87 | 40.2 | 89 | 31.5 | 1.28 | HDRS17 | 37.5 | 36 | 35.4 | 29 | 6.7 | 44 | 42 | Yes | Yes |
| Trivedi et al (2004) | PRX | 19 | 95 | 8 | 447 | 40 | 58 | HDRS≥50% | 301 | — | 146 | — | — | HDRS17 | 47.9 | 52 | 50 | 42 | 9.5 | 21 | 23 | Yes | Yes |
| Bjerkenstedt et al (2005) | FLX | 20 | 100 | 4 | 109 | 15 | 79 | HDRS≥50% | 54 | 37 | 55 | — | — | HDRS21 | 39.3 | 37 | 41 | 38 | –1.10 | 11 | 5 | Yes | Yes |
| Brannan et al (2005) | DLX | 60 | 108 | 7 | 280 | 25 | 65 | HDRS≥50% | 141 | 42 | 141 | 39.7 | 1.06 | HDRS17 | 47.9 | 46 | 45.8 | 46 | — | — | — | Yes | Yes |
| Fava et al (2005) | FLX | 20 | 100 | 12 | 90 | 2 | 59 | HDRS<8 | 47 | 29.8 | 43 | 20.9 | 1.42 | HDRS17 | 41.7 | 32 | 41.7 | 37 | –4.60 | 49 | 51 | Yes | Yes |
| Clayton et al (2006) | S-CTP | 13 | 158 | 8 | 549 | — | 59 | HDRS≥50% | 276 | 61.2 | 273 | 48.7 | 1.26 | HDRS17 | 47.9 | 58 | 47.9 | 52 | 4.4 | 25 | 24 | Yes | Yes |
| Clayton et al (2006) | BUP | 316 | 110 | 8 | 554 | — | 59 | HDRS≥50% | 281 | 59.1 | 273 | 48.7 | 1.21 | HDRS17 | 50 | 56 | 47.9 | 52 | 6.9 | 25 | 24 | Yes | Yes |
| Feiger et al (2006) | SLG | 9 | 150 | 8 | 265 | 3 | 57 | HDRS≥50% | 132 | 40.2 | 133 | 30.1 | 1.34 | HDRS17 | 48.8 | 37 | 49.4 | 32 | 5.6 | 24 | 20 | Yes | Yes |
| Gastpar et al (2006) | CTP | 20 | 86 | 6 | 257 | 21 | 69 | HDRS≥50% | 127 | 55.9 | 130 | 39.2 | 1.43 | HDRS17 | 45.8 | 53 | 45.8 | 41 | 11.9 | 18 | 19 | Yes | — |
| Jefferson et al (2006) | BUP | 352 | 176 | 8 | 274 | 24 | 68 | CGI | 135 | 53.3 | 139 | 38.1 | 1.4 | IDSIVR30 | 54.8 | 46 | 54.8 | 38 | 8.1 | 24 | 21 | Yes | No |
| Moreno et al (2006) | FLX | 20 | 100 | 8 | 46 | 1 | 83 | HDRS≥50% | 20 | 55 | 26 | 42.3 | 1.3 | HDRS21 | 24.6 | 53 | 26.2 | 31 | 22.1 | 20 | 27 | Yes | Yes |
| Perahia et al (2006) | DLX | 100 | 180 | 8 | 295 | 22 | 70 | HDRS≥50% | 196 | 66.3 | 99 | 51.5 | 1.29 | HDRS17 | 43.8 | 59 | 43.8 | 52 | 6.2 | 12 | 10 | Yes | No |
| Perahia et al (2006) | PRX | 20 | 100 | 8 | 196 | 22 | 70 | HDRS≥50% | 97 | 60.8 | 99 | 51.5 | 1.18 | HDRS17 | 43.8 | 57 | 43.8 | 52 | 4.3 | 9 | 10 | Yes | — |
| DeMartinis et al (2007) | dVNX | 233 | 291 | 8 | 470 | 25 | 60 | HDRS≥50% | 350 | — | 120 | — | — | HDRS17 | 47.9 | 45 | 47.9 | 33 | 11.7 | 25 | 18 | Yes | Yes |
| Liebowitz et al (2007) | dVNX | 187 | 234 | 8 | 238 | — | 60 | HDRS≥50% | 121 | 43 | 117 | 34.2 | 1.26 | HDRS17 | 50 | 40 | 50 | 36 | 4.2 | 18 | 25 | Yes | Yes |
| Nemeroff and Thase (2007) | FLX | 41 | 205 | 6 | 206 | 13 | 62 | HDRS≥50% | 104 | 45.2 | 102 | 37.3 | 1.21 | HDRS21 | 39.3 | — | — | — | — | 17 | 24 | Yes | — |
| Nemeroff and Thase (2007) | VNX | 142 | 128 | 6 | 204 | 13 | 62 | HDRS≥50% | 102 | 52.9 | 102 | 37.3 | 1.42 | HDRS21 | 39.3 | — | — | — | — | 24 | 24 | Yes | — |
| Nierenberg et al (2007) | S-CTP | 10 | 108 | 8 | 410 | 36 | 66 | HDRS≥50% | 273 | 45.3 | 137 | 37.2 | 1.22 | HDRS17 | 37.5 | 41 | 37.5 | 34 | 9.5 | 24 | 29 | Yes | Yes |
| Nierenberg et al (2007) | DLX | 60 | 85 | 8 | 411 | 36 | 63 | HDRS≥50% | 274 | 48.7 | 137 | 37.2 | 1.31 | HDRS17 | 37.5 | 43 | 37.5 | 34 | 6.9 | 31 | 29 | Yes | Yes |
| Septien-Velez et al (2007) | dVNX | 300 | 375 | 8 | 369 | 35 | 66 | HDRS≥50% | 245 | 58 | 124 | 37.9 | 1.53 | HDRS17 | 52.1 | 49 | 52.1 | 37 | 12.6 | 27 | 22 | Yes | No |
| Boyer et al (2008) | dVNX | 75 | 93.8 | 8 | 485 | 44 | 70 | HDRS≥50% | 324 | 63.9 | 161 | 50.3 | 1.27 | HDRS17 | 50 | 56 | 50 | 45 | 11.4 | 15 | 9 | Yes | Yes |
| Lieberman et al (2008) | VNX | 162 | 399 | 8 | 471 | — | 68 | HDRS≥50% | 226 | 60.3 | 245 | 46.9 | 1.29 | HDRS17 | 54.2 | 57 | 54.2 | 47 | 9.2 | 18 | 14 | Yes | Yes |
| Lieberman et al (2008) | dVNX | 319 | 146 | 8 | 487 | — | 66 | HDRS≥50% | 242 | 54.9 | 245 | 46.9 | 1.17 | HDRS17 | 52.1 | 56 | 54.2 | 47 | 10 | 26 | 14 | Yes | Yes |
| Liebowitz et al (2008) | dVNX | 75 | 93.8 | 8 | 447 | 25 | 60 | HDRS≥50% | 297 | 32.7 | 150 | 24 | 1.36 | HDRS17 | 47.9 | 49 | 47.9 | 41 | 7.6 | 21 | 16 | Yes | Yes |
| Cutler et al (2009) | DLX | 60 | 108 | 6 | 308 | 38 | 60 | MADRS≥50% | 151 | 49.7 | 157 | 36.3 | 1.37 | HDRS17 | 52.1 | 49 | 52.1 | 40 | 8.3 | 30 | 21 | Yes | Yes |
| Feiger et al (2009) | dVNX | 349 | 436 | 8 | 230 | 12 | 65 | HDRS≥50% | 117 | 41.9 | 118 | 29.7 | 1.41 | HDRS17 | 47.9 | 46 | 47.9 | 32 | 14.1 | 25 | 13 | Yes | Yes |
| Sheehan et al (2009) | FLX | 58 | 290 | 6 | 194 | 22 | 66 | HDRS≥50% | 99 | 35.3 | 95 | 36.7 | 0.96 | HDRS21 | 49.2 | 39 | 47.5 | 37 | 1.2 | 23 | 29 | Yes | No |
| Sheehan et al (2009) | VNX | 235 | 212 | 6 | 186 | 22 | 56 | HDRS≥50% | 91 | 51.6 | 95 | 36.7 | 1.41 | HDRS21 | 49.2 | 48 | 47.5 | 37 | 10.4 | 29 | 29 | Yes | No |
| Tourian et al (2009) | dVNX | 75 | 108 | 8 | 317 | 21 | 64 | HDRS≥50% | 157 | 44 | 160 | 38.1 | 1.15 | HDRS17 | 47.9 | 44 | 50 | 36 | 8.5 | 25 | 27 | Yes | Yes |
| Tourian et al (2009) | DLX | 60 | 93.8 | 8 | 458 | 21 | 62 | HDRS≥50% | 298 | 47.1 | 160 | 38.1 | 1.24 | HDRS17 | 47.9 | 45 | 50 | 36 | 7.8 | 26 | 27 | Yes | Yes |
| Hewett et al (2010) | BUP | 180 | 90 | 8 | 390 | 65 | 66 | MADRS | 203 | 57.1 | 187 | 49.2 | 1.16 | MADRS | 51.7 | 48 | 51.7 | 43 | 4.9 | 22 | 22 | No | No |
| Hewett et al (2010) | VNX | 85 | 76.5 | 8 | 385 | 65 | 66 | MADRS | 198 | 66.2 | 187 | 49.2 | 1.34 | MADRS | 50 | 56 | 51.7 | 43 | 13.4 | 23 | 22 | No | — |
| Means/sums | 142 Trials | — | 156±67 | 7.2±1.8 | 27 127a | 16±15 | 62±9.9 | — | 17 059 | 53.8±10.9 | 9925a | 36.6±10.9 | 1.57±0.49 | — | 45.3±6.4 | 48.6±8.4 | 45.1±36.0 | 36±9.5 | 12.6±8.2 | 29.8±12.3 | 33.3±15.7 | 78.90% | 81.7% |
Abbreviations: AMI, amitriptyline; BUP, bupropion; CMI, clomipramine; CTP, citalopram; S-CTP, escitalopram; Dep, depression rating; DLX, duloxetine; DMI, desipramine; FLX, fluoxetine; IMI, impramine; ITT, findings based on intent-to-treat: at least one dose and one assessment; MTZ, mirtazapine; N, responders or cases treated; NRT, (nortriptyline:); Pbo, placebo; PNZ, phenelzine; PRX, paroxetine; RD, response difference, %-Improvement, drug—placebo; RR, response rate ratio, drug–placebo; Rx, drug; SLG, selegiline; SRT, sertraline; TCP, tranylcypromine; TZD, trazodone; VNX, venlafaxine; dVNX, desvenlafaxine.
Corrected for 3677 repeated placebo cases in 35 trials.
There are a total of 142 drug–placebo comparisons from 107 studies, involving a total of 26 948 patients.
Initial depression scores (as percentage of scale maxima were similar in drug- (45.6±6.5%) and placebo-arms (48.6±8.4%)). There were 120±90 subjects (range: 11–521) per antidepressant arm and 96±57 per placebo arm (range: 18–273) or 216±136 participants per trial, and 16.1±15.3 collaborating sites per trial. Treatment lasted approximately 7.2±1.8 weeks, uncorrected for early dropouts at unspecified times, at rates of approximately 29.8±12.3% or 4.54±2.58% per week with drugs, and 33.3±15.7% or 5.06±3.02% per week with placebos (paired-t=2.71, p=0.007). Supplemental use of moderate doses of sedative-anxiolytics was permitted in 59.1% of all trials. Most trials (81.7%) included at least brief periods to allow previously administered drugs to ‘wash-out,' and most (78.9%) employed intention-to-treat methods; 97.4% of trials were sponsored by pharmaceutical manufacturers. The overall estimated IMI-eq standardized dose was 158±68 mg/day, and did not differ by drug-type or between older (TCAs, MAO-inhibitors: 155±49 mg/day) and modern antidepressants (SRIs, SNRIs, and atypical agents: 159±77 mg/day).
Meta-Analysis
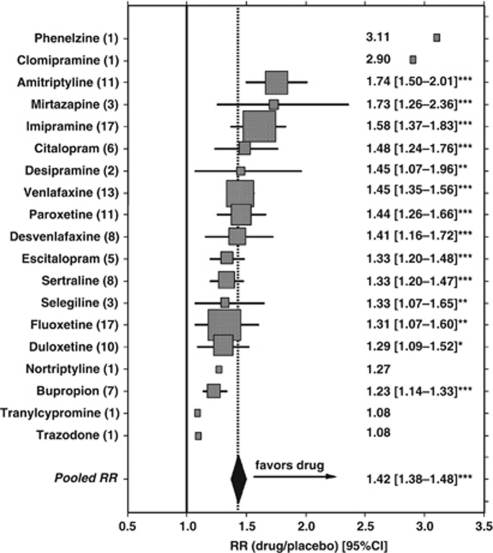
Meta-analyses with the 122 trials reporting on responder rates yielded pooled drug–placebo RRs (RR with CIs) for each agent, and an overall pooled RR value of 1.42. (95% CI: 1.38–1.48; z=16.3, p<0.0001). Among agents with more than one trial, amitriptyline ranked highest in apparent efficacy, and bupropion lowest; however, CIs for most agents overlapped, indicating the need for caution in attempting to rank drugs by efficacy (Figure 1). Single-trial data available for phenelzine, clomipramine, nortriptyline, trazodone, and tranylcypromine are likely to be unstable and unreliable (Figure 1). Construction of a ‘funnel plot' (1/standard-error-of-RR vs 1/RR) for all reports with data on responder rates yielded a V-shaped distribution of values that was symmetrically distributed around the pooled value of 1/RR (not shown); this finding may provide evidence against selective reporting of positive trials results.
Figure 1.
Summary of meta-analytically computed relative rates (RR) of response after randomization to drug vs placebo) with 95% confidence intervals (CI, horizontal bars when n⩾2 trials per drug) for controlled trials of each of 19 antidepressants (with numbers of trials on the left axis, and numerical values on the right). Drugs are listed by descending apparent efficacy, with symbol-size approximately proportional to weighting by trials per drug. The vertical solid line=null (1.0); vertical dotted line and solid diamond (width=CI)=pooled RR for all agents tested (*p<0.05; **p⩽0.01; ***p⩽0.001). Overall pooled RR=1.42 (CI: 1.38–1.48), indicating an average of 42% superiority of antidepressants over placebos. Note that phenelzine, clomipramine, tranylcypromine and trazodone (n=1 trial each) appear to be outliers.
We also compared antidepressants by types with pooled data, and compared apparent efficacy by three outcome measures. These included meta-analytically computed response RRs and responder rate-differences (RD), as well as relative differences (RD) in changes in depression ratings with drug–placebo pairs. Although these outcome measures yielded slightly different rankings, TCAs consistently ranked as the most effective antidepressants considered, and atypical agents, seemingly least effective (Table 2). Trials carried out before the median reporting year (1998) yielded higher values of all efficacy measures (Table 2). Median years of trial-reporting ranked: TCAs (1991) < MAO-inhibitors (1997) < atypical agents (1998) = SRIs (1998) < SNRIs (2003). Efficacy based on responder-rate RR values was much greater for TCAs than other types of antidepressants (1.83±0.62 vs 1.48±0.41; F=11.8, p=0.0008). Moreover, when the numbers of placebo-responders and nonresponders in the TCA trials were substituted for corresponding placebo data for trials of modern antidepressants, the meta-analytically pooled RR value was identical to that found in the TCA trials, supporting the impression that apparent differences response rates with the two classes of antidepressants was accounted for by secular changes in placebo responses.
Table 2. Comparisons Among Antidepressant Types and Reporting Years.
| Measures | All drugs | TCAs | MAO inhibitors | SRIs | SNRIs | Atypicals | Early (1983–1997) | Late (1998–2010) |
|---|---|---|---|---|---|---|---|---|
| Trials (n): | 124 | 31 | 5 | 47 | 30 | 11 | 57 | 67 |
| Responder RR | ||||||||
| Pooled RR | 1.42 | 1.62 | 1.39 | 1.37 | 1.40 | 1.25 | 1.63 | 1.32 |
| 95% CI | 1.38–1.48 | 1.47–1.78 | 1.11–1.48 | 1.27–1.48 | 1.30–1.51 | 1.15–1.35 | 1.49–1.78 | 1.26–1.38 |
| z-Score | 15.7 | 9.86 | 2.88 | 8.28 | 8.60 | 5.50 | 10.9 | 12.6 |
| p-Value | <0.0001 | 0.0001 | 0.004 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Responder RD | ||||||||
| Pooled RD | 16.3% | 21.4% | 12.1% | 14.6% | 16.4% | 11.9% | 20.7% | 13.4% |
| 95% CI | 14.4–18.2 | 17.7–25.1 | 3.58–20.5 | 11.5–17.7 | 12.3–20.5 | 8.15–15.7 | 17.5–23.8 | 11.1–15.6 |
| z-Score | 16.6 | 11.3 | 2.79 | 9.21 | 7.81 | 6.19 | 12.9 | 11.7 |
| p-Value | <0.0001 | 0.0001 | 0.005 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Improvement RD | ||||||||
| Pooled RD | 12.5% | 16.2% | 16.0% | 11.5% | 9.80% | 12.8% | 16.8% | 9.80% |
| 95% CI | 11.0–14.1 | 13.3–19.1 | 0.98–33.0 | 8.70–14.2 | 7.14–12.5 | 8.19–17.4 | 14.5–19.2 | 7.17–10.2 |
| Paired-t | 16.1 | 11.4 | 2.62 | 8.40 | 7.54 | 6.18 | 14.5 | 11.3 |
| p-Value | <0.0001 | <0.0001 | 0.05 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| NNT | 8.0 | 6.2 | 6.2 | 8.7 | 10.2 | 7.8 | 6.0 | 10.2 |
| 95%CI | 7.1–9.1 | 5.2–7.5 | 3.0–102 | 7.0–11.5 | 8.0–14.0 | 5.7–12.2 | 5.2–6.9 | 9.8–13.9 |
Abbreviations: MAO, monoamine oxidase; NNT, number-needed-to-treat (reciprocal of RD); SNRI, serotonin-norepinephrine reuptake inhibitors; SRI, serotonin-reuptake inhibitor; TCA, tricyclic antidepressants.
Based on meta-analytic computation of ratios of responder rates with antidepressants/placebos (RR) or their differences (RD), and on differences in percentage-improvement in initial depression ratings with drug—placebo for 19 antidepressants tested for efficacy in 124 trials summarized in Table 1. Note that most CIs overlap between agents, and that ranking by apparent potency varies among the three outcome measures, but that TCAs appear to be consistently more effective than other types of antidepressants, including SNRIs, SRIs, MAO inhibitors, or atypical agents (bupropion, mirtazapine, and trazodone). Also, early trials (reported in 1983–1997 vs 1998–2010) yield consistently greater drug–placebo differences.
Factors Associated with Trials Results
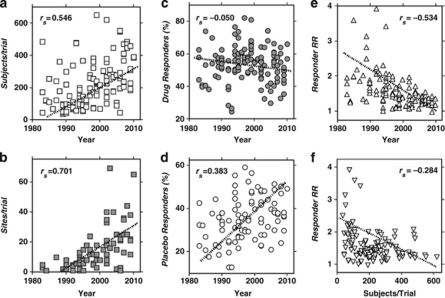
Given the preceding findings suggesting that older agents, specifically TCAs, might appear to be somewhat more effective than modern antidepressants in general, and that older trials yielded consistently greater drug–placebo differences, we carried out several correlational analyses to further examine effects of reporting-year on numbers of sites and subjects per trial, on responses to drugs and placebos and their ratio (Figure 2). Both sites and subjects per trial increased between 1983 and 2010 (Figures 2a and b). Responses in placebo-arms of trials increased across the same era, but responses to antidepressant drugs decreased slightly (Figures 2c and d) to yield highly significant decreases in the drug–placebo responder rate-ratio (RR) across the same years (Figure 2e); drug–placebo differences in rates of response or of percentage improvement also declined (not shown). Responder RR values also declined significantly as the number of subjects per trial (Figure 2f) as well as sites per trial (not shown) increased. Trial-duration also increased significantly across the years sampled (rs=0.603, p<0.0001), and longer-trials led selectively to larger responses with placebos (slope, 1.90 (CI: 0.85–2.95), p=0.0005) than with drugs (0.92 (–0.15 to 1.99)).
Figure 2.
Correlations with Spearman nonparametric correlation coefficients (rs): (a) Subjects per trial vs year of trial reporting (p<0.0001); (b) Collaborating sites per trial vs year (p<0.0001); (c) Meta-analytic responder rate (% of subjects) for antidepressants vs year (p=0.58); (d) Responder rate (%) for placebo vs year (p<0.0001); (e) Responder rate ratio (RR: drug–placebo) vs year (p<0.0001); (f) RR vs subjects per trial (p=0.002). In addition RR decreased significantly with more sites per trial (rs=−0.302, p=0.004). Note that response after randomization to placebo but not antidepressant drugs selectively increased over years, as subject and site counts per trial increased, with corresponding decreases in drug–placebo relative response rate-ratio (RR).
Multivariate linear regression modeling indicated that the following factors were associated significantly and independently with more recent reporting-years, as follows: (a) more drugs other than TCAs, (b) larger numbers of subjects per trial (or sites per trial), (c) lower response to drugs, (d) greater responses to placebo, (e) higher proportions of depressed women, and (f) longer trials. However, there was no evidence of secular changes in ratings of depression-severity at intake, IMI-eq drug-dose, or dropout rates (Table 3).
Table 3. Multivariate Linear Regression Model: Factors Associated with Year of Publication of Trial Reports.
| Factors | Slope function (β) (95% CI) | t-Score | p-Value |
|---|---|---|---|
| More subjects per trial | +0.013 (+0.006 to +0.021) | 3.40 | 0.001 |
| More placebo response | +0.183 (+0.067 to +0.300) | 3.13 | 0.002 |
| Less drug response | –0.179 (–0.288 to –0.071) | 3.27 | 0.001 |
| Less use of TCAs | –5.73 (–7.97 to –3.50) | 3.13 | 0.002 |
| More women subjects | +0.155 (+0.053 to +0.258) | 3.00 | 0.003 |
| Longer trials | +0.807 (+0.268 to +1.345) | 2.97 | 0.004 |
Factors listed are independently and significantly associated with more recent reports among reporting-years (1983–2010) as the continuous outcome measure. Factors not associated with more recent trials included: initial depression severity rating, IMI-eq drug dose, and dropout-rate.
Finally, we carried out a preliminary, hypothesis-generating post hoc analysis of deciles of meta-analytically determined drug–placebo responder RR values as well as depression-improvement RD values vs trial-sizes (not shown). By both outcome measures, the apparently optimal number was 2–10 sites per trial, and 30–75 subjects per trial, with lower efficacy found at both lower and higher counts.
DISCUSSION
The present findings are congruent with reviews discussed above indicating that antidepressant drug-vs-placebo differences in published reports of controlled trials are generally moderate (Baldessarini, 2005; Gartlehner et al, 2008; Kirsch et al, 2008; Tsapakis et al, 2008; Bridge et al, 2009; Wooley et al, 2009; Masi et al, 2010; Pigott et al, 2010; Khin et al, 2011). This conclusion was reached in the previous literature despite typical reliance on initial improvement on scale ratings rather than less readily achieved clinical remission, and despite growing evidence of publication bias toward underreporting of studies without significant drug–placebo differences (Ioannidis, 2008; Turner et al, 2008). Following nearly identical mid-range, initial depression ratings across drug and placebo arms and reporting-years, the crude response rates in the reports reviewed here averaged 54% with FDA-approved antidepressants that are employed clinically to treat major depression in the United States, compared with 37% with placebo. These differences consistently favor active drugs, but by only 17%.
The present findings also support the broad consensus that drug–placebo differences have been declining for a variety of psychotropic drugs in recent decades, making it increasingly difficult to demonstrate efficacy (Khin et al, 2011; Yildiz et al, 2011a, 2011b). This trend probably has encouraged increased reliance on larger trials (more subjects and collaborating sites) in order to maintain statistical power. Moreover, increasing reliance on complex trials carried out in varied geographic locations and cultures may tend to limit the reliability of research findings (Vázquez et al, 2011).
It is evidently widely held that differences in efficacy among specific drugs or types of antidepressants in the treatment of acute episodes of major depressive disorder are generally minor (Healy, 1997; Baldessarini, 2005, 2012; Cipriani et al, 2007; Gartlehner et al, 2008; Ghaemi, 2008; Pigott et al, 2010; Khin et al, 2011). The present findings support the conclusion that pooling of data from placebo-controlled trials does not yield clear rankings of specific drugs or drug-types by apparent efficacy (Figure 1). Unexpectedly, however, there were significant differences in reported apparent efficacy between TCAs and newer antidepressants (Table 2). We propose that this outcome may reflect important changes in characteristics of clinical trials for depression over the past three decades. These include increasing size and complexity, with selective increases in response rates with placebos and somewhat decreasing responses with antidepressants (Figure 2). It is particularly noteworthy that when placebo-response data from the generally older TCA trials were substituted for those in more recent trials of modern drugs, both types of agents yielded identical meta-analytically pooled RR values. In contrast, we did not find evidence of significant changes over the years in initial ratings of depression-severity (adjusted for variance among rating scales), in approximate IMI-eq antidepressant doses, or in several other measured characteristics of trials (Table 3).
It is increasingly clear that drug–placebo differences in trials of antidepressants and other psychotropic agents have been declining (Gartlehner et al, 2008; Ioannidis, 2008; Kirsch et al, 2008; Tsapakis et al, 2008; Turner et al, 2008; Bridge et al, 2009; Masi et al, 2010; Khin et al, 2011; Vázquez et al, 2011; Yildiz et al, 2011a, 2011b). In accord with recent findings in controlled treatment trials for mania (Yildiz et al, 2011a, 2011b), a secular increase in sites and participants per trial was associated, selectively, with rising placebo-associated response rates, resulting in declining drug–placebo contrasts or effect-size (Figure 2; Table 3). We propose that this tendency may, at least in part, reflect declining quality-control and greater heterogeneity of diagnostic and clinical assessments in large, complex, multi-site trials, particularly when dissimilar cultures are involved and local standardization of assessment methods is limited (Yildiz et al, 2011a, 2011b; Vázquez et al, 2011). We propose that selective increases in response rates associated with randomized placebo-treatment might reflect ‘regression-to-mean' effects (Anderson, 1990; Bland and Altman, 1994) or random outcomes. Placebo-associated responses have increased from former levels of 20 to 30% to current levels of 30 to 50%, and to as high as 59.2% in a 1997 trial involving paroxetine (Lecrubier et al, 1997).
Alternative factors that may contribute to the observed secular trends include changes in the types of patients recruited into antidepressant trials, including less severely ill patients willing to accept potential randomization to a placebo, and even partially treated subjects. Levels of training and expertise of personnel providing diagnostic and symptom-rating assessments may also have declined. In addition, trials have become longer over the years sampled (Table 2), requiring more clinical assessments with greater risk of measurement-variance, and providing more clinical contact and more time for spontaneous improvement—all of which may favor responses associated with placebo treatment. Additional technical factors may include less reliance on expert raters, with greater risk of less stable assessments in a very heterogeneous disorder (Healy, 1997).
If the preceding interpretation of the present findings is correct, it suggests several practical considerations for the design and conduct of therapeutic trials for major depression and perhaps other disorders. These include seeking an optimal range of trial-sizes, with redoubled efforts to maximize quality-control, limit placebo-associated responses, and maximize drug–placebo differences. Preliminary analyses of the present data suggest that an optimal range of collaborating sites per trial may be 2–10, and of subjects per trial, about 30–75. Such conservative considerations for the design of future trials may improve outcomes. Additional potential benefits may include reduced time, complexity, and costs, as well as limiting exposure of as many acutely depressed patient-subjects to placebo-treatment as possible.
Limitations of this study include a lack of relevant details in many reports of controlled trials, sometimes including inconsistent reporting of definitions and outcomes for responder rate and percentage improvement, of the number of rating-scale items and of their maximum attainable scores in a few trials. Also, in most trials, exposure times are estimated from nominal protocol requirements since precise, subject-based actual weeks of treatment usually are not stated. Also, numbers of patients with defined outcomes are usually, but not always, based on prevalent intention-to-treat methods, which can limit responses owing to early dropout. Routine reporting of such details would greatly benefit future meta-analyses. Additional limitations to generalization arise from our requirements of peer-review and publication of findings in placebo-controlled trials concerning antidepressants approved and marketed in the United States for acute adult, major depression.
In conclusion, the present meta-analytic review of outcomes of placebo-controlled trials of antidepressants for acute episodes of major depressive disorder found evidence that older antidepressants, particularly TCAs, yielded somewhat superior apparent efficacy to some modern, second-generation agents. However, such nominal differences appear to have been influenced by secular changes in the nature of such trials over the past three decades. These include rising subject- and site-numbers and increasing placebo-associated responses, leading to falling drug–placebo differences or effect-size. We hypothesize that more conservative numbers of subjects and sites, with improved quality-control of trial methods, may paradoxically yield superior results in controlled trials of some psychotropic drugs, and do so more economically. Finally, the lack of major and compelling differences in apparent efficacy among specific antidepressants, and moderate differences among drug-types, suggest that meta-analyses of controlled trials may have limited value in efforts to develop an evidence-basis (Sackett et al, 1996) for identifying superior treatments.
Acknowledgments
This work was supported by a grant from the Bruce J Anderson Foundation and by the McLean Private Donors Psychopharmacology Research Fund (to RJB), and by a Traveling Research Fellowship from the University of Barcelona and the Fundación Española de Psiquiatría y Salud Mental (to JU). Leonardo Tondo, MD provided helpful technical advice.
The authors declare no conflict of interest.
References
- Amsterdam JD. Double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry. 2003;64:208–214. doi: 10.4088/jcp.v64n0216. [DOI] [PubMed] [Google Scholar]
- Anderson B. Methodological Errors in Medical Research: Incomplete Catalog. Blackwell: New York; 1990. [Google Scholar]
- Anderson IM. Meta-analytical studies on new antidepressants. Br Med Bull. 2001;57:161–178. doi: 10.1093/bmb/57.1.161. [DOI] [PubMed] [Google Scholar]
- Andreoli V, Caillard V, Deo RS, Rybakowski JK, Versiani M. Reboxetine, new noradrenaline selective antidepressant, is at least as effective as fluoxetine in the treatment of depression. J Clin Psychopharmacol. 2002;22:393–399. doi: 10.1097/00004714-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ.2005Drug therapy of depression and anxiety disorders. Chapter 17In: Brunton LL, Lazo JS, Parker KL (eds).Goodman and Gilman's The Pharmacological Basis of Therapeutics11th edn.McGraw-Hill: New York; 429–459. [Google Scholar]
- Baldessarini RJ.2012Chemotherapy in Psychiatry3rd edn.New York: Springer-Verlag; in press). [Google Scholar]
- Baldessarini RJ, Tondo L, Ghiani C, Lepri B. Discontinuation rate vs recurrence risk following long-term antidepressant treatment in major depressive disorder patients. Am J Psychiatry. 2010;167:934–941. doi: 10.1176/appi.ajp.2010.09060880. [DOI] [PubMed] [Google Scholar]
- Bakish D, Bradwejn J, Nair N, McClure J, Remick R, Bulger L. Comparison of moclobemide, amitriptyline and placebo in depression: Canadian multicenter study. Psychopharmacology. 1992;106 (Suppl:S98–S101. doi: 10.1007/BF02246248. [DOI] [PubMed] [Google Scholar]
- Ban TA, Aguglia E, Batista R, Castillo A, Lipcsey A, Macher JP, et al. Clinical efficacy of reboxetine: comparative study with desipramine, with methodological considerations. Hum Psychopharmacol. 1998;13 (Suppl:S29–S39. [Google Scholar]
- Bjerkenstedt L, Edman GV, Alken RG, Mannel M. Hypericum extract LI-160 and fluoxetine in mild to moderate depression: randomized, placebo-controlled multi-center study in outpatients. Eur Arch Psychiatry Clin Neurosci. 2005;255:40–47. doi: 10.1007/s00406-004-0532-z. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499–1500. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin A, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry. 2002;159:1869–1875. doi: 10.1176/appi.ajp.159.11.1869. [DOI] [PubMed] [Google Scholar]
- Boyer P, Montgomery S, Lepola U, Germain JM, Brisard C, Ganguly R, et al. Efficacy, safety, and tolerability of fixed-dose desvenlafaxine 50 and 100 mg/day for major depressive disorder in a placebo-controlled trial. Int Clin Psychopharmacol. 2008;23:243–253. doi: 10.1097/YIC.0b013e32830cebed. [DOI] [PubMed] [Google Scholar]
- Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Double-blind comparison of Org-3770, amitriptyline, and placebo in major depression. J Clin Psychiatry. 1995;56:519–525. [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166:42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- Carman JS, Ahdieh H, Wyatt-Knowles E, Warga E, Panagides J. Controlled study of mianserin in moderately to severely depressed outpatients. Psychopharmacol Bull. 1991;27:135–139. [PubMed] [Google Scholar]
- Cassano GB, Heinze G, Lôo H, Mendlewicz J, Sousa MP. Double-blind comparison of tianeptine, imipramine and placebo in the treatment of major depressive episodes. Eur Psychiatry. 1996;11:254–259. doi: 10.1016/0924-9338(96)82332-7. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Geddes JR, Furukawa TA, Barbui C. Metareview on short-term effectiveness and safety of antidepressants for depression: evidence-based approach to inform clinical practice. Can J Psychiatry. 2007;52:553–562. doi: 10.1177/070674370705200903. [DOI] [PubMed] [Google Scholar]
- Claghorn J, Gershon S, Goldstein BJ, Behrnetz S, Bush DF, Huitfeldt B. Double-blind evaluation of zimelidine in comparison to placebo and amitriptyline in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:367–382. doi: 10.1016/0278-5846(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Claghorn JL, Earl CQ, Walczak DD, Stoner KA, Wong LF, Kanter D, et al. Fluvoxamine maleate in the treatment of depression: single-center, double-blind, placebo-controlled comparison with imipramine in outpatients. J Clin Psychopharmacol. 1996;16:113–120. doi: 10.1097/00004714-199604000-00003. [DOI] [PubMed] [Google Scholar]
- Claghorn JL, Kiev A, Rickels K, Smith WT, Dunbar GC. Paroxetine vs placebo: double-blind comparison in depressed patients. J Clin Psychiatry. 1992;53:434–438. [PubMed] [Google Scholar]
- Claghorn JL, Lesem MD. Double-blind placebo-controlled study of Org-3770 in depressed outpatients. J Affect Disord. 1995;34:165–171. doi: 10.1016/0165-0327(95)00014-e. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Croft HA, Horrigan JP, Wightman DS, Krishen A, Richard NE, et al. Bupropion extended release compared with escitalopram: effects on sexual functioning and antidepressant efficacy in two randomized, double-blind, placebo-controlled studies. J Clin Psychiatry. 2006;67:736–746. doi: 10.4088/jcp.v67n0507. [DOI] [PubMed] [Google Scholar]
- Cohn CK, Robinson DS, Roberts DL, Schwiderski UE, O'Brien K, Ieni JR. Responders to antidepressant drug treatment: study comparing nefazodone, imipramine, and placebo in patients with major depression. J Clin Psychiatry. 1996;57 (Suppl 2:15–18. [PubMed] [Google Scholar]
- Cohn JB, Wilcox C. Comparison of fluoxetine, imipramine, and placebo in patients with major depressive disorder. J Clin Psychiatry. 1985;46:26–31. [PubMed] [Google Scholar]
- Cohn JB, Wilcox CS. Paroxetine in major depression: double-blind trial with imipramine and placebo. J Clin Psychiatry. 1992;53 (Suppl:52–56. [PubMed] [Google Scholar]
- Coleman CC, Cunningham LA, Foster VJ, Batey SR, Donahue RM, Houser TL, et al. Sexual dysfunction associated with the treatment of depression: placebo-controlled comparison of bupropion sustained release and sertraline treatment. Ann Clin Psychiatry. 1999;11:205–215. doi: 10.1023/a:1022309428886. [DOI] [PubMed] [Google Scholar]
- Coleman CC, King BR, Bolden-Watson C, Book MJ, Segraves RT, Richard N, et al. Placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clin Ther. 2001;23:1040–1058. doi: 10.1016/s0149-2918(01)80090-4. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL. Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety. 2000;11:58–65. doi: 10.1002/(sici)1520-6394(2000)11:2<58::aid-da2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Croft H, Settle E, Jr, Houser T, Batey SR, Donahue RM, Ascher JA. Placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther. 1999;21:643–658. doi: 10.1016/S0149-2918(00)88317-4. [DOI] [PubMed] [Google Scholar]
- Cunningham LA. Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Ann Clin Psychiatry. 1997;9:157–164. doi: 10.1023/a:1026277907818. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Borison RL, Carman JS, Chouinard G, Crowder JE, Diamond BI, et al. Comparison of venlafaxine, trazodone, and placebo in major depression. J Clin Psychopharmacol. 1994;14:99–106. [PubMed] [Google Scholar]
- Cutler AJ, Montgomery SA, Feifel D, Lazarus A, Astrom M, Brecher M. Extended release quetiapine fumarate monotherapy in major depressive disorder: placebo- and duloxetine-controlled study. J Clin Psychiatry. 2009;70:526–539. doi: 10.4088/jcp.08m04592. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Effect of Hypericum perforatum (St John's wort) in major depressive disorder: randomized controlled trial. JAMA. 2002;287:1807–1814. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- DeMartinis NA, Yeung PP, Entsuah R, Manley AL. Double-blind, placebo-controlled study of the efficacy and safety of desvenlafaxine succinate in the treatment of major depressive disorder. J Clin Psychiatry. 2007;68:677–688. doi: 10.4088/jcp.v68n0504. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002a;63:308–315. doi: 10.4088/jcp.v63n0407. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res. 2002b;36:383–390. doi: 10.1016/s0022-3956(02)00060-2. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Wiltse CG, Mallinckrodt CH, McNamara RK, Demitrack MA, Bitter I. Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Neuropsychopharmacol. 2004;14:457–470. doi: 10.1016/j.euroneuro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Doogan DP, Langdon CJ. Double-blind, placebo-controlled comparison of sertraline and dothiepin in the treatment of major depression in general practice. Int Clin Psychopharmacol. 1994;9:95–100. doi: 10.1097/00004850-199400920-00005. [DOI] [PubMed] [Google Scholar]
- Fabre LF. Six-week, double-blind trial of paroxetine, imipramine, and placebo in depressed outpatients. J Clin Psychiatry. 1992;53 (Suppl:40–43. [PubMed] [Google Scholar]
- Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, et al. Sertraline safety and efficacy in major depression: a double-blind fixed-dose comparison with placebo. Biol Psychiatry. 1995;38:592–602. doi: 10.1016/0006-3223(95)00178-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, Zajecka J, et al. Double-blind, randomized trial of St John's wort, fluoxetine, and placebo in major depressive disorder. J Clin Psychopharmacol. 2005;25:441–447. doi: 10.1097/01.jcp.0000178416.60426.29. [DOI] [PubMed] [Google Scholar]
- Fava M, Amsterdam JD, Deltito JA, Salzman C, Schwaller M, Dunner DL. Double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Ann Clin Psychiatry. 1998;10:145–150. doi: 10.1023/a:1022337927842. [DOI] [PubMed] [Google Scholar]
- Feiger AD. Double-blind comparison of gepirone extended release, imipramine, and placebo in the treatment of outpatient major depression. Psychopharmacol Bull. 1996;32:659–665. [PubMed] [Google Scholar]
- Feiger AD, Rickels K, Rynn MA, Zimbroff DS, Robinson DS. Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry. 2006;67:1354–1361. doi: 10.4088/jcp.v67n0905. [DOI] [PubMed] [Google Scholar]
- Feiger AD, Tourian KA, Rosas GR, Padmanabhan SK. Placebo-controlled study evaluating the efficacy and safety of flexible-dose desvenlafaxine treatment in outpatients with major depressive disorder. CNS Spectrums. 2009;14:41–50. doi: 10.1017/s1092852900020046. [DOI] [PubMed] [Google Scholar]
- Feighner JP. Double-blind comparison of paroxetine, imipramine and placebo in depressed outpatients. Int Clin Psychopharmacol. 1992;6 (Suppl 4:31–35. doi: 10.1097/00004850-199206004-00007. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Aden GC, Fabre LF, Rickels K, Smith WT. Comparison of alprazolam, imipramine, and placebo in the treatment of depression. JAMA. 1983;249:3057–3064. [PubMed] [Google Scholar]
- Feighner JP, Boyer WF, Merideth CH, Hendrickson GG. Double-blind comparison of fluoxetine, imipramine and placebo in outpatients with major depression. Int Clin Psychopharmacol. 1989a;4:127–134. doi: 10.1097/00004850-198904000-00004. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Boyer WF, Meredith CH, Hendrickson GG. Placebo-controlled inpatient comparison of fluvoxamine maleate and imipramine in major depression. Int Clin Psychopharmacol. 1989b;4:239–244. doi: 10.1097/00004850-198907000-00006. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Cohn JB, Fabre LF, Jr, Fieve RR, Mendels J, Shrivastava RK, et al. Study comparing paroxetine, placebo, and imipramine in depressed patients. J Affect Disord. 1993;28:71–79. doi: 10.1016/0165-0327(93)90035-i. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Overo K. Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. J Clin Psychiatry. 1999;60:824–830. doi: 10.4088/jcp.v60n1204. [DOI] [PubMed] [Google Scholar]
- Fontaine R, Ontiveros A, Elie R, Kensler TT, Roberts DL, Kaplita S, et al. Double-blind comparison of nefazodone, imipramine, and placebo in major depression. J Clin Psychiatry. 1994;55:234–241. [PubMed] [Google Scholar]
- Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVaugh-Geiss A, Krebs EE, et al. Comparative benefits and harms of second-generation antidepressants. Ann Intern Med. 2008;149:734–750. doi: 10.7326/0003-4819-149-10-200811180-00008. [DOI] [PubMed] [Google Scholar]
- Gastpar M, Singer A, Zeller K. Comparative efficacy and safety of a once-daily dosage of hypericum extract STW3-VI and citalopram in patients with moderate depression: double-blind, randomized, multicentre, placebo-controlled study. Pharmacopsychiatry. 2006;39:66–75. doi: 10.1055/s-2006-931544. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Wojcik JD, Falk WE, Baldessarini RJ, Zeisel SH, Schoenfeld D, et al. Tyrosine for depression: double-blind trial. J Affect Disord. 1990;19:125–132. doi: 10.1016/0165-0327(90)90017-3. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN. Why antidepressants are not antidepressants. Bipolar Disord. 2008;10:957–968. doi: 10.1111/j.1399-5618.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM. Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. J Clin Psychiatry. 2002;63:577–584. doi: 10.4088/jcp.v63n0707. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C, Demitrack MA. Duloxetine in the treatment of depression: double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharmacol. 2004;24:389–399. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: double-blind clinical trial. J Clin Psychiatry. 2002;63:225–231. doi: 10.4088/jcp.v63n0309. [DOI] [PubMed] [Google Scholar]
- Guelfi JD, White C, Hackett D, Guichoux JY, Magni G. Effectiveness of venlafaxine in patients hospitalized for major depression and melancholia. J Clin Psychiatry. 1995;56:450–458. [PubMed] [Google Scholar]
- Guy W.1976ECDEU Assessment Manual for Psychopharmacology US Department HEW publication (ADM) 76-338: Rockville, MD; National Institute of Mental Health, pp 218–222. [Google Scholar]
- Hamilton M. Rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D. The Antidepressant Era. Harvard University: Cambridge, MA; 1997. [Google Scholar]
- Hewett K, Gee MD, Krishen A, Wunderlich HP, Le Clus A, Evoniuk G, et al. Double-blind, placebo-controlled comparison of antidepressant efficacy and tolerability of bupropion-XR and venlafaxine-XR. J Psychopharmacol. 2010;24:1209–1216. doi: 10.1177/0269881109106953. [DOI] [PubMed] [Google Scholar]
- Hollyman JA, Freeling P, Paykel ES, Bhat A, Sedgwick P. Double-blind placebo-controlled trial of amitriptyline among depressed patients in general practice. J R Coll Gen Pract. 1988;38:393–397. [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials. Philos Ethics Humanit Med. 2008;3:14–22. doi: 10.1186/1747-5341-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itil TM, Shrivastava RK, Mukherjee S, Coleman BS, Michael ST. Double-blind placebo-controlled study of fluvoxamine and imipramine in out-patients with primary depression. Br J Clin Pharmacol. 1983;15 (Suppl 3:S433S–S4338. doi: 10.1111/j.1365-2125.1983.tb02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson JW, Rush AJ, Nelson JC, VanMeter SA, Krishen A, Hampton KD, et al. Extended-release bupropion for patients with major depressive disorder presenting with symptoms of reduced energy, pleasure, and interest: findings from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67:865–873. doi: 10.4088/jcp.v67n0602. [DOI] [PubMed] [Google Scholar]
- Khan A, Fabre LF, Rudolph R. Venlafaxine in depressed outpatients. Psychopharmacol Bull. 1991;27:141–144. [PubMed] [Google Scholar]
- Khan A, Upton GV, Rudolph RL, Entsuah R, Leventer SM. Use of venlafaxine in the treatment of major depression and major depression associated with anxiety: dose-response study. J Clin Psychopharmacol. 1998;18:19–25. doi: 10.1097/00004714-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Khan M. Randomized, double-blind, placebo-controlled, 5-weeks study of Org-3770 (mirtazapine) in major depression. Hum Psychopharmacol. 1995;10 (Suppl:S119–S124. [Google Scholar]
- Khin NA, Chen YF, Yang Y, Yang P, Laughren TP. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applicatons. J Clin Psychiatry. 2011;72:464–472. doi: 10.4088/JCP.10m06191. [DOI] [PubMed] [Google Scholar]
- Kiev A. Double-blind, placebo-controlled study of paroxetine in depressed outpatients. J Clin Psychiatry. 1992;53 (Suppl:S27–S29. [PubMed] [Google Scholar]
- Kirsch I, Deacon B, Huedo-Medina TB, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45–e53. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakman G, Faltermaier-Temizel M, Bossert-Zaudig S, Baghai T, Lorkowski G. Treatment of depressive outpatients with lorazepam, alprazolam, amitriptyline and placebo. Psychopharmacology. 1995;120:109–115. doi: 10.1007/BF02246151. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Holm P, Høyer E, Mejlhede A, Mikkelsen PL, Olesen A, et al. Moclobemide and clomipramine in reactive depression: placebo-controlled randomized clinical trial. Acta Psychiatr Scand. 1989;79:530–536. doi: 10.1111/j.1600-0447.1989.tb10299.x. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Bourin M, Moon CA, Schifano F, Blanchard C, Danjou P, et al. Efficacy of venlafaxine in depressive illness in general practice. Acta Psychiatr Scand. 1997;95:485–493. doi: 10.1111/j.1600-0447.1997.tb10136.x. [DOI] [PubMed] [Google Scholar]
- Lepola UM, Loft H, Reines EH. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Lieberman DZ, Montgomery SA, Tourian KA, Brisard C, Rosas G, Padmanabhan K, et al. A pooled analysis of two placebo-controlled trials of desvenlafaxine in major depressive disorder. Int Clin Psychopharmacol. 2008;23:188–197. doi: 10.1097/YIC.0b013e32830263de. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Manley AL, Padmanabhan SK, Ganguly R, Tummala R, Tourian KA. Efficacy, safety, and tolerability of desvenlafaxine 50 mg/day and 100 mg/day in outpatients with major depressive disorder. Curr Med Res Opin. 2008;24:1877–1890. doi: 10.1185/03007990802161923. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Yeung PP, Entsuah R. Randomized, double-blind, placebo-controlled trial of desvenlafaxine succinate in adult outpatients with major depressive disorder. J Clin Psychiatry. 2007;68:1663–1672. doi: 10.4088/jcp.v68n1105. [DOI] [PubMed] [Google Scholar]
- Lineberry CG, Johnston JA, Raymond RN, Samara B, Feighner JP, Harto NE, et al. Fixed-dose (300 mg) efficacy study of bupropion and placebo in depressed outpatients. J Clin Psychiatry. 1990;51:194–199. [PubMed] [Google Scholar]
- Lydiard RB, Stahl SM, Hertzman M, Harrison WM. Double-blind, placebo-controlled study comparing the effects of sertraline vs amitriptyline in the treatment of major depression. J Clin Psychiatry. 1997;58:484–491. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- Masi G, Liboni F, Brovedani P. Pharmacotherapy of major depressive disorder in adolescents. Expert Opin Pharmacother. 2010;11:375–386. doi: 10.1517/14656560903527226. [DOI] [PubMed] [Google Scholar]
- Massana J. Reboxetine vs fluoxetine: overview of efficacy and tolerability. J Clin Psychiatry. 1998;59 (Suppl 14:8–10. [PubMed] [Google Scholar]
- Mendels J, Kiev A, Fabre LF. Double-blind comparison of citalopram and placebo in depressed outpatients with melancholia. Depress Anxiety. 1999;9:54–60. [PubMed] [Google Scholar]
- Mendels J, Schless AP. Comparative efficacy of alprazolam, imipramine, and placebo administered once a day in treating depressed patients. J Clin Psychiatry. 1986;47:357–361. [PubMed] [Google Scholar]
- Miller SM, Naylor GJ, Murtagh M, Winslow G. Double-blind comparison of paroxetine and placebo in the treatment of depressed patients in a psychiatric outpatient clinic. Acta Psychiatr Scand Suppl. 1989;350:143–144. doi: 10.1111/j.1600-0447.1989.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. New depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moreno RA, Teng CT, Almeida KM, Tavares JH. Hypericum perforatum vs fluoxetine in the treatment of mild to moderate depression: randomized double-blind trial in a Brazilian sample. Rev Bras Psiquiatr. 2006;28:29–32. doi: 10.1590/s1516-44462006000100007. [DOI] [PubMed] [Google Scholar]
- Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D. Randomized controlled trial comparing problem-solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995;310:441–445. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Thase ME. Double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. J Psychiatr Res. 2007;41:351–359. doi: 10.1016/j.jpsychires.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson GD, et al. Duloxetine vs escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, non-inferiority study. Curr Med Res Opin. 2007;23:401–416. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? Meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Perahia DG, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ. Duloxetine in the treatment of major depressive disorder: placebo- and paroxetine-controlled trial. Eur Psychiatry. 2006;21:367–378. doi: 10.1016/j.eurpsy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Philipp M, Kohnen R, Hiller KO. Hypericum extract vs imipramine or placebo in patients with moderate depression: randomized multicenter study of treatment for eight weeks. BMJ. 1999;319:1534–1538. doi: 10.1136/bmj.319.7224.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: current status of research. Psychother Psychosom. 2010;79:267–279. doi: 10.1159/000318293. [DOI] [PubMed] [Google Scholar]
- Pitts WM, Fann WE, Halaris AE, Dressler DM, Sajadi C, Snyder S, et al. Bupropion in depression: tri-center placebo-controlled study. J Clin Psychiatry. 1983;44 (Part 2:95–100. [PubMed] [Google Scholar]
- Quitkin FM, McGrath PJ, Stewart JW, Harrison W, Wager SG, Nunes E, et al. Phenelzine and imipramine in mood reactive depressives: further delineation of the syndrome of atypical depression. Arch Gen Psychiatry. 1989;46:787–793. doi: 10.1001/archpsyc.1989.01810090029005. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, et al. Antidepressant efficacy of sertraline: double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry. 1990;51 (Suppl B:S18–S27. [PubMed] [Google Scholar]
- Reimherr FW, Cunningham LA, Batey SR, Johnston JA, Ascher JA. Multicenter evaluation of the efficacy and safety of 150 and 300 mg/day sustained-release bupropion tablets vs placebo in depressed outpatients. Clin Ther. 1998;20:505–516. doi: 10.1016/s0149-2918(98)80060-x. [DOI] [PubMed] [Google Scholar]
- Rickels K, Amsterdam J, Clary C, Fox I, Schweizer E, Weise C. Efficacy and safety of paroxetine compared with placebo in outpatients with major depression. J Clin Psychiatry. 1992;53 (Suppl:S30–S32. [PubMed] [Google Scholar]
- Rickels K, Chung HR, Csanalosi IB, Hurowitz AM, London J, Wiseman K, et al. Alprazolam, diazepam, imipramine, and placebo in outpatients with major depression. Arch Gen Psychiatry. 1987;44:862–866. doi: 10.1001/archpsyc.1987.01800220024005. [DOI] [PubMed] [Google Scholar]
- Rickels K, Feighner JP, Smith WT. Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Arch Gen Psychiatry. 1985;42:134–141. doi: 10.1001/archpsyc.1985.01790250028004. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Clary C, Fox I, Weise C. Nefazodone and imipramine in major depression: a placebo-controlled trial. Br J Psychiatry. 1994;164:802–805. doi: 10.1192/bjp.164.6.802. [DOI] [PubMed] [Google Scholar]
- Roth D, Mattes J, Sheehan KH, Sheehan DV. Double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:929–939. doi: 10.1016/0278-5846(90)90078-u. [DOI] [PubMed] [Google Scholar]
- Rudolph RL, Fabre LF, Feighner JP, Rickels K, Entsuah R, Derivan AT. Randomized, placebo-controlled, dose-response trial of venlafaxine hydrochloride in the treatment of major depression. J Clin Psychiatry. 1998;59:116–122. doi: 10.4088/jcp.v59n0305. [DOI] [PubMed] [Google Scholar]
- Rudolph RL, Feiger AD. Double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. J Affect Disord. 1999;56:171–181. doi: 10.1016/s0165-0327(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;12:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer E, Feighner J, Mandos LA, Rickels K. Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. J Clin Psychiatry. 1994;55:104–108. [PubMed] [Google Scholar]
- Septien-Velez L, Pitrosky B, Padmanabhan SK, Germain JM, Tourian KA. Randomized, double-blind, placebo-controlled trial of desvenlafaxine succinate in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2007;22:338–347. doi: 10.1097/YIC.0b013e3281e2c84b. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Nemeroff CB, Thase ME, Entsuah R. Placebo-controlled inpatient comparison of venlafaxine and fluoxetine for the treatment of major depression with melancholic features. Int Clin Psychopharmacol. 2009;24:61–86. doi: 10.1097/YIC.0b013e32831980f2. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Multicenter comparative trial of moclobemide, imipramine and placebo in major depressive disorder. Int Clin Psychopharmacol. 1994;9:109–113. doi: 10.1097/00004850-199400920-00007. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Ravindran A. Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry. 1999;60:22–28. doi: 10.4088/jcp.v60n0105. [DOI] [PubMed] [Google Scholar]
- Smith WT, Glaudin V, Panagides J, Gilvary E. Mirtazapine vs amitriptyline vs placebo in the treatment of major depressive disorder. Psychopharmacol Bull. 1990;26:191–196. [PubMed] [Google Scholar]
- Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biol Psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- Thase ME. Efficacy and tolerability of once-daily venlafaxine extended release (XR) in outpatients with major depression. J Clin Psychiatry. 1997;58:393–398. doi: 10.4088/jcp.v58n0904. [DOI] [PubMed] [Google Scholar]
- Tourian KA, Padmanabhan SK, Groark J, Brisard C, Farrington D. Desvenlafaxine 50 and 100 mg/day in the treatment of major depressive disorder: an 8-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial and a post hoc pooled analysis of three studies. Clin Ther. 2009;31:1405–1423. doi: 10.1016/j.clinthera.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Pigotti TA, Perera P, Dillingham KE, Carfagno ML, Pitts CD. Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1356–1364. doi: 10.4088/jcp.v65n1010. [DOI] [PubMed] [Google Scholar]
- Tsapakis EM, Soldani F, Tondo L, Baldessarini RJ. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008;193:10–17. doi: 10.1192/bjp.bp.106.031088. [DOI] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Vartiainen H, Leinonen E. Double-blind study of mirtazapine and placebo in hospitalized patients with major depression. Eur Neuropsychopharmacol. 1994;4:145–150. doi: 10.1016/0924-977x(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Vázquez G, Baldessarini RJ, Yildiz A, Tamayo J, Tondo L, Salvatore P. Multi-site international collaborative clinical trials in mania: commentary. Int J Neuropsychopharmacol. 2011;14:1013–1016. doi: 10.1017/S1461145711000447. [DOI] [PubMed] [Google Scholar]
- Wade A, Michael-Lemming MO, Bang-Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Dunlop SR, Dornseif BE, Bosomworth JC, Humbert M. Low-dose fluoxetine therapy for depression. Psychopharmacol Bull. 1988;24:183–188. [PubMed] [Google Scholar]
- Wernicke JF, Dunlop SR, Dornseif BE, Zerbe RL. Fixed-dose fluoxetine therapy for depression. Psychopharmacol Bull. 1987;23:164–168. [PubMed] [Google Scholar]
- White K, Razani J, Cadow B, Gelfand R, Palmer R, Simpson G, et al. Tranylcypromine vs nortriptyline vs placebo in depressed outpatients: a controlled trial. Psychopharmacology. 1984;82:258–262. doi: 10.1007/BF00427786. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Cohn JB, Katz BB, Mijares CP, Guarino JJ, Panagides J, et al. Double-blind, placebo-controlled study comparing mianserin and amitriptyline in moderately depressed outpatients. Int Clin Psychopharmacol. 1994;9:271–279. doi: 10.1097/00004850-199400940-00006. [DOI] [PubMed] [Google Scholar]
- Wooley SB, Cardoni AA, Goethe JW. Last-observation carried forward imputation method in clnical efficacy trials: review of 352 antidepressant studies. Pharmacotherapy. 2009;29:1408–1416. doi: 10.1592/phco.29.12.1408. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Vieta E, Leucht S, Baldessarini RJ. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011a;36:375–389. doi: 10.1038/npp.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz A, Vieta E, Tohen M, Baldessarini RJ. Placebo effects in treatment trials for acute mania. Int J Neuropsychopharmacol. 2011b;14:863–875. doi: 10.1017/S1461145710001641. [DOI] [PubMed] [Google Scholar]