Abstract
Methamphetamine (METH) causes partial depletion of central monoamine systems and cognitive dysfunction in rats and humans. We have previously shown and now further show that the positive correlation between expression of the immediate-early gene Arc (activity-regulated, cytoskeleton-associated) in the dorsomedial (DM) striatum and learning on a response reversal task is lost in rats with METH-induced striatal dopamine loss, despite normal behavioral performance and unaltered N-methyl--aspartate (NMDA) receptor-mediated excitatory post-synaptic currents, suggesting intact excitatory transmission. This discrepancy suggests that METH-pretreated rats may no longer be using the dorsal striatum to solve the reversal task. To test this hypothesis, male Sprague–Dawley rats were pretreated with a neurotoxic regimen of METH or saline. Guide cannulae were surgically implanted bilaterally into the DM striatum. Three weeks after METH treatment, rats were trained on a motor response version of a T-maze task, and then underwent reversal training. Before reversal training, the NMDA receptor antagonist -2-amino-5-phosphonopentanoic acid (AP5) or an Arc antisense oligonucleotide was infused into the DM striatum. Acute disruption of DM striatal function by infusion of AP5 impaired reversal learning in saline-, but not METH-, pretreated rats. Likewise, acute disruption of Arc, which is implicated in consolidation of long-term memory, disrupted retention of reversal learning 24 h later in saline-, but not METH-, pretreated rats. These results highlight the critical importance of Arc in the striatum in consolidation of basal ganglia-mediated learning and suggest that long-term toxicity induced by METH alters the cognitive strategies/neural circuits used to solve tasks normally mediated by dorsal striatal function.
Keywords: methamphetamine, Arc, striatum, reversal learning, AP5, monoamines
INTRODUCTION
Methamphetamine (METH) abuse is a significant problem worldwide. METH causes partial loss of dopamine (DA) and serotonin systems in the brain (Morgan and Gibb, 1980; Ricaurte et al, 1980; Seiden et al, 1976; Wagner et al, 1980). In humans, METH-induced neurotoxicity is evident as decreases in DA transporter (DAT) binding in the caudate-putamen that can last for up to 11 months (McCann et al, 1998; Volkow et al, 2001a, 2001b; Wilson et al, 1996). METH-induced toxicity is also evident as decreases in serotonin transporter (SERT) binding across multiple brain regions, including the caudate-putamen and the frontal cortex (Kish et al, 2009; Sekine et al, 2006), as well as loss of glutamatergic neurons in the somatosensory cortex (Eisch et al, 1998; Pu et al, 1996). Cognitive impairments have also been seen in association with METH-induced neurotoxicity, and include deficits in motor sequence learning (Chapman et al, 2001), object recognition (Belcher et al, 2005; Herring et al, 2008; Schröder et al, 2003), visual discrimination and attentional set-shifting (Izquierdo et al, 2010), and novel odor recognition (O'Dell et al, 2011).
In some tasks, however, behavioral impairments associated with METH-induced neurotoxicity are not apparent. Such tasks include those examining conditioned placed aversion (Achat-Mendes et al, 2005), spatial learning on the Morris water maze (Herring et al, 2008; Schröder et al, 2003), and motor response reversal learning on a T-maze (Daberkow et al, 2008). With regard to the response reversal learning task on the T-maze, expression of Arc (activity-regulated cytoskeleton-associated gene), an immediate-early gene important in consolidation of learning, in the dorsomedial (DM) striatum is correlated with number of trials to criterion on the reversal learning task in saline-pretreated rats, but not, interestingly, in METH-pretreated rats (Daberkow et al, 2007, 2008). Thus, although METH-pretreated rats behaviorally appear to be normal on this task, the relation between Arc expression in the striatum and behavior is lost. Guzowski et al (2000; 2001) previously suggested that the correlation between Arc expression and learning reflects the involvement of a brain region in a task. Whether Arc in the DM striatum is necessary for consolidation of response reversal learning has not heretofore been examined; furthermore, whether loss of correlation in METH-pretreated rats indicates a change in the brain regions engaged during the task is unknown.
Therefore, the goal of the present studies was to test whether Arc in the DM striatum is critical for consolidation of response reversal learning and whether loss of the correlation in METH-pretreated rats reflects a loss of dependence of the reversal learning on DM striatal function. We locally infused an Arc antisense oligonucleotide (Guzowski et al, 2000; Hearing et al, 2010) or the N-methyl--aspartate (NMDA) receptor antagonist AP5 into the DM striatum prior to rats engaging in motor response reversal learning on the T-maze task. The results indicate that Arc signaling in the DM striatum is necessary for consolidation of the reversal learning and that METH-induced neurotoxicity is associated with a change in the neural substrates mediating such reversal learning.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles River Laboratories, Raleigh, NC; 275–300 g) were singly housed in tub cages on a 14 : 10-h light cycle. Animal care and experimental procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Utah.
METH Pretreatment
Rats were treated with a neurotoxic regimen of (±)-METH-HCl (4 × 10 mg/kg free base, s.c.; NIDA, Research Triangle Park, NC) over one day as described previously (Daberkow et al, 2008). One hour after the final injection, rats were returned to their home cages and given free access to food and water until behavioral training began (METH-pretreated, n=25; saline-pretreated, n=29).
Surgery
Two weeks after pretreatment, rats were anesthetized with ketamine/xylazine (90/10 mg/kg, i.p.) and placed in a stereotaxic apparatus. A dual, 21-gauge guide cannula (Plastics One, Roanoke, VA) was lowered to end just dorsal to the DM striatum (mm from bregma: AP+0.2; ML±1.9; DV-3.2). The guide was secured with skull screws and dental acrylic, and dummy cannulae were inserted. Subsequent infusions were made through 33-gauge infusion cannulae extending 1.8 mm beyond the guides. The infusion cannulae remained in place for 1 min after infusion before being withdrawn.
Reversal Learning Task
Response reversal learning on the T-maze was conducted as described previously (Daberkow et al, 2007). Beginning 1 week after surgery, rats were food-restricted and habituated to the food reward and maze. The turn bias of each rat was determined, followed by acquisition training for 3 days and then reversal learning. During the reversal learning task, rats had to turn in the opposite direction from acquisition to receive the reward. The criterion for learning on both acquisition and reversal tasks was 9/10 correct turns in a row.
Acute Pharmacological Manipulations
On the day of reversal training, rats were infused through their cannulae with either AP5 (0.5 μl/2 min, 25 nmol in 0.1 M PBS, pH 7.4; Tocris Bioscience, Ellisville, MO) (Palencia and Ragozzino, 2004) or an Arc antisense oligonucleotide. The Arc antisense oligonucleotide was a chimeric phosphorothioate/phosphodiester oligonucleotide against bases 209–228 of the Arc gene (Guzowski et al, 2000). The nonsense/control oligonucleotide was composed of the same bases, but in a scrambled sequence. A 1-μl volume of oligonucleotide (1 nmol/μl, 0.1 M PBS, pH 7.4) (Guzowski et al, 2000) or PBS vehicle was infused (0.39 μl/min) into each DM striatum. After infusions into DM striata, rats were returned to their home cages for 5 min (AP5) or 2 h (Arc antisense) prior to reversal training. Five minutes after reaching criterion, rats infused with AP5 and the corresponding PBS-infused controls were killed, and brains were removed and frozen in isopentane chilled on dry ice. Rats infused with the Arc antisense oligonucleotide and the corresponding controls (PBS or Arc nonsense oligonucleotide) were returned to their home cages upon reaching criterion. The following day, these rats were tested on reversal retention to determine the number of trials needed to again reach criterion on the reversal direction learned the previous day. Five minutes after reaching criterion, rats were killed and brains were removed and frozen.
DAT and SERT Autoradiography
Fresh-frozen brains were sectioned (12 μm), thaw-mounted onto Superfrost Plus (VWR, Aurora, CO) slides, and then stored at −20 °C. Infusion sites were verified during sectioning (Figure 1). DAT levels in the striatum were determined by [125I]RTI-55 (PerkinElmer, Waltham, MA) binding, as reported previously (Boja et al, 1992; O'Dell et al, 2011). SERT binding in the prefrontal cortex was similarly performed except that fluoxetine was omitted. Prefrontal cortex slides incubated in buffer containing fluoxetine showed no binding (data not shown). The slides were apposed to film (Biomax MR; Eastman Kodak, Rochester, NY) for 24 h and developed. Images were digitized and densitometric analysis was performed using the NIH ImageJ software, yielding average, background-subtracted gray values in the DM and dorsolateral (DL) striatum and six prefrontal cortical regions. Two rostral and two middle striatal sections, and four prefrontal cortical sections, were analyzed per rat. DAT and SERT binding in METH-pretreated rats were then converted to percent of average levels in saline-pretreated rats.
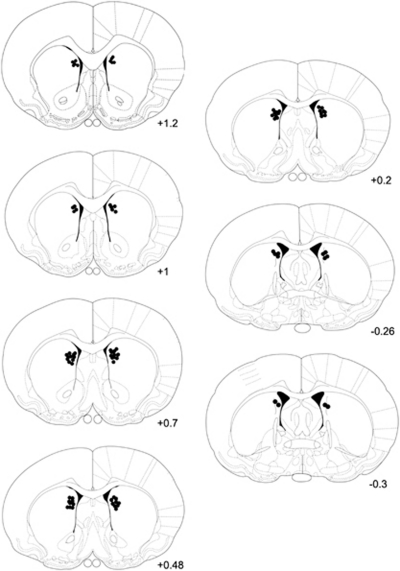
Figure 1.
Infusion sites in the DM striatum. The black dots indicate placement of infusion sites in the DM striatum of rats in AP5 and Arc experiments. The numbers indicate mm from bregma (Paxinos and Watson, 1998).
Error Analysis
The numbers of perseverative and regressive errors made during reversal learning by METH- and saline-pretreated rats were calculated as defined by Palencia and Ragozzino (2004), with modification owing to the task differences. Wrong turns were counted as perseverative errors if they occurred before a rat made more than three turns in the reversal direction. Incorrect turns occurring after the rat had made more than three turns in the new correct direction were counted as regressive errors.
In Situ Hybridization Histochemistry
The left hemisphere from animals used for electrophysiological experiments (see below) was frozen, sectioned (12 μm), and processed for in situ hybridization histochemical determination of Grin2a NMDA receptor subunit expression as described previously (Ganguly and Keefe, 2001) using a full-length ribonucleotide probe synthesized from the cDNA (gift from Dr Peter Seeburg) using 35S-UTP and T7 RNA polymerase (Roche, Indianapolis, IN). Slides were hybridized overnight in humid chambers at 55 °C, washed, treated with Ribonuclease-A (5 μg/ml), washed, dried, and then apposed to X-ray film for 1 week. Images from films were digitized and densitometric analysis was performed using ImageJ, yielding average, background-subtracted gray values in the DM, DL, and ventromedial striatum.
Determination of Striatal DA Content
DA content was determined in striatal tissue punches collected during sectioning of frozen brain hemispheres for in situ hybridization (Chapman et al, 2001). A blunt-tip, 18-gauge needle was used to collect 1-mm3 punches from both the medial and lateral striatum (+0.3 mm anterior to bregma). Punches were sonicated in tissue buffer (0.05 M sodium phosphate/0.03 M citric acid buffer, 25% methanol (v/v), pH 2.5) and centrifuged. A 20-μl volume of the supernatant was injected onto a high-pressure liquid chromatography system coupled to an electrochemical detector (EOx=+0.6 V) for separation and quantification of DA levels. Values were expressed per mg of protein. Protein content was determined by Lowry protein assay.
Striatal Slice Preparation
Acute brain slices were obtained, as described previously (Chapman et al, 2003), from adult rats (375–460 g) killed 3–5 weeks after pretreatment with saline or METH. Rats were anesthetized (pentobarbital, 50 mg/kg) and decapitated. Brains were removed and placed in ice-cold, oxygenated (95% O2–5% CO2) sucrose Ringer solution (pH 7.4) containing (in mM): sucrose (200), KCl (3), NaH2PO4 (1.4), MgSO4 (2), NaHCO3 (26), glucose (10), and CaCl2 (2). The brain was divided along the midline and the right hemisphere was glued caudal-side down to a Vibraslicer chuck (Campden Instruments). Coronal sections (300–350 μm) containing striatum were placed in a holding chamber at room temperature containing oxygenated Ringer solution with 126 mM NaCl in place of sucrose (pH 7.37–7.41). The sections remained in the Ringer solution (osmolality 295–305 mOsm) for ⩾1 h before recording.
Patch-Clamp Recordings
Slices were transferred into the recording chamber perfused with fresh, oxygenated, Mg2+-free Ringer solution at room temperature (∼22 °C) by means of a gravity-feed system (4 ml/min). Whole-cell patch clamp was used to record from single striatal neurons, using previously described inclusion criteria and data acquisition (Chapman et al, 2003). Borosilicate glass microelectrodes (3–6 MΩ resistance) were pulled using a P-87 micropipette puller (Sutter Instruments). The internal recording solution contained (in mM): K gluconate (130), KCl (10), HEPES (10), EGTA (1), CaCl2 (0.1), ATP (2), GTP (1), and glutathione (1). The external solution was the same as that in the holding chamber.
Excitatory post-synaptic currents (EPSCs) were elicited using local, minimal stimulation to mitigate voltage- and space-clamp errors (Stevens and Wang, 1994; Wilcox et al, 1996). A bipolar stimulating electrode was placed near the recording electrode (<300 μm). The stimulating electrode was used to deliver current pulses (100-μs duration) of sufficient amplitude to produce the smallest EPSC (25–40 pA) that could be reliably evoked at low frequency (0.1 Hz). To isolate and maximally activate NMDA receptor-mediated EPSCs, the Ringer solution contained 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione, 50 μM picrotoxin, and 10 μM glycine. Data were acquired with an Axopatch 1D amplifier and the CLAMPEX8 software package interfaced to a Digidata 1200 acquisition board (Axon Instruments). Signals were filtered at 5 kHz and sampled at 10 kHz.
Only recordings not showing substantial changes in holding current or resistance at the electrode tip were used for analysis. All cells required <100 pA to be clamped to −70 mV. Cells with resting membrane potentials above −55 mV were omitted from analysis. The following parameters were determined for averaged NMDA receptor-mediated EPSCs: rise times, peak amplitudes, decay time constants, and weighted τ (τw). The decay time constants were fit with a double exponential equation: I(t)=Ifexp(−t/τf)+Isexp(−t/τs), where If is the amplitude of the fast component, Is is the amplitude of the slow component, and τf and τs are the fast and slow time constants, respectively. Weighted time constants were calculated by using the following equation: τw=[If/(If+Is)]τf+[Is/(If+Is)]τs (Stocca and Vicini, 1998). All data are presented as mean±SEM.
Statistical Analysis
Dependent measures from animals used in the behavioral studies were compared across pretreatment and treatment groups using two-way ANOVAs and post hoc t-tests (JMP v.9.0). Dependent measures from animals used for electrophysiological studies were analyzed using unpaired t-tests for the striatal region of interest.
RESULTS
DAT and SERT Autoradiography
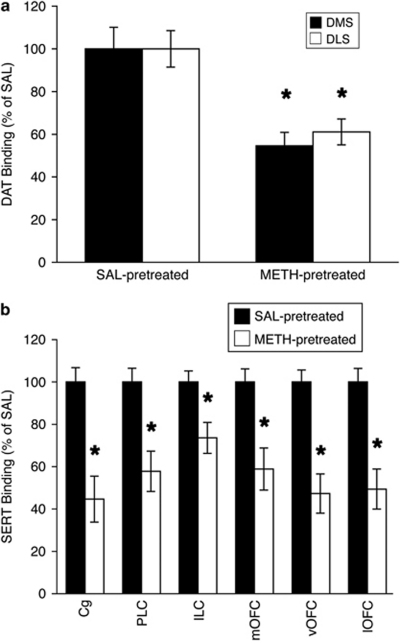
Pretreatment of rats used for the behavioral studies with a ‘binge' regimen of METH resulted in significant decreases in striatal DAT binding. METH-pretreated rats in the AP5 experiment had significantly less DAT binding than saline-pretreated rats in the striatum (Figure 2a; DM striatum: mean±SEM, 54.6±6.4% of saline, F(1, 22)=12.8, p<0.01; DL striatum: 61.1±6.1%, F(1, 22)=12.2, p<0.01). A similar decrease in DAT binding was seen in METH-pretreated rats in the Arc antisense experiment (graph not shown; DM striatum: 58.4±6.9% of saline, F(1, 29)=18.4, p<0.001; DL striatum: 66.4±6.6%, F(1, 29)=14.2, p<0.001). METH-pretreated rats also had significantly decreased SERT binding relative to saline-pretreated controls in all prefrontal regions examined (Figure 2b): prelimbic, 57.8±9.5% of saline, F(1, 17)=14.3, p<0.01; infralimbic, 73.6±7.3%, F(1, 17)=14.5, p<0.01; medial orbitofrontal, 58.9±9.9%, F(1, 17)=13.8, p<0.01; ventral orbitofrontal, 47.3±9.2%, F(1, 17)=25.6, p<0.0001; lateral orbitofrontal, 49.4±9.4%, F(1, 17)=20.0, p<0.001; and cingulate, 44.7±10.9%, F(1, 17)=19.7, p<0.001 cortices.
Figure 2.
DAT and SERT binding. (a) DAT decreases (mean±SEM), expressed as percent of average values in saline-pretreated controls, in rats pretreated with (±)-METH (4 × 10 mg/kg, 2-h intervals; n=25) or saline (SAL; n=29) approximately 7 weeks prior to being killed. (b) SERT decreases (mean±SEM), expressed as percent of average values in saline-pretreated controls, in rats pretreated with METH (n=12) or saline (n=11) approximately 7 weeks after METH pretreatment. *Significantly different from SAL-pretreated values for the same brain region (p<0.01). Cg, cingulate cortex; PLC, prelimbic cortex; ILC, infralimbic cortex; mOFC, medial orbitofrontal cortex; vOFC, ventral OFC; lOFC, lateral OFC.
Effects of Acute NMDA Receptor Blockade in the DM Striatum
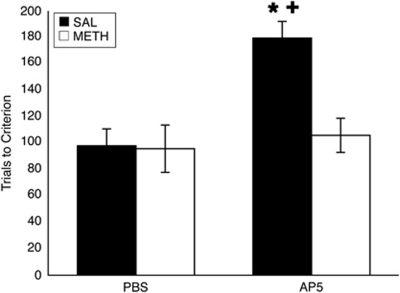
As reported previously by our lab (Daberkow et al, 2008), METH-pretreated rats appear to be behaviorally normal in terms of motor response reversal learning on the T-maze relative to saline-pretreated rats (Figure 3). However, acute disruption of striatal function through bilateral infusion of AP5 into the DM striatum revealed differences in DM striatal involvement in this learning. Analysis revealed a significant overall interaction (pretreatment × infusion; F(1, 1)=4.6, p<0.05), as well as significant main effects of pretreatment (F(1, 1)=5.2, p<0.05) and infusion (F(1, 1)=7.6, p<0.05). Saline-pretreated rats that were infused with AP5 (n=5) required significantly more trials to reach criterion than saline-pretreated, PBS-infused rats (Figure 3; n=8; t(23)=−3.4, p<0.01). METH-pretreated rats (n=5), on the other hand, were unaffected by infusion of AP5, and thus were significantly different from saline-pretreated, AP5-infused rats (t(23)=2.8, p<0.05), but not METH-pretreated, PBS-infused (n=9; t(23)=−0.4, p=0.7) or saline-pretreated, PBS-infused (t(23)=−0.3, p=0.7) rats.
Figure 3.
Effects of acute NMDA receptor blockade in the DM striatum. Mean trials to criterion (9/10 correct consecutive trials; ±SEM) on a motor response reversal task. Rats were given bilateral infusions of AP5 or PBS 5 min prior to the beginning of reversal learning. *Significantly different from SAL-pretreated, PBS-infused rats (p<0.01). +Significantly different from METH-pretreated, AP5-infused rats (p<0.05).
Effects of Acute Arc Disruption in the DM Striatum
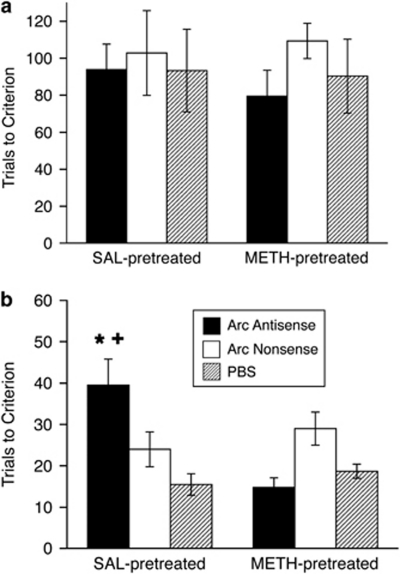
Consistent with prior reports making use of Arc antisense in different brain regions and in different learning and memory paradigms (Guzowski et al, 2000; Hearing et al, 2010), we observed no effects of Arc antisense infusion into the DM striatum on initial reversal learning in either saline- or METH-pretreated rats (Figure 4a). Two-way ANOVA on trials to criterion on the reversal learning task revealed no main effects of pretreatment (F(1, 30)=0.01, p=0.9) or infusion (F(1, 30)=0.69, p=0.5), and no interaction (F(1, 2)=0.09, p=0.9). However, again consistent with prior reports (Guzowski et al, 2000; Hearing et al, 2010), analysis of retention of the reversal learning 24 h after the initial reversal learning task revealed a significant overall interaction (F(1, 2)=4.07, p<0.05) but no main effects of pretreatment (F(1, 30)=0.9, p=0.3) or infusion (F(1, 30)=1.22, p=0.3). Saline-pretreated rats infused with Arc antisense (n=13) took significantly more trials to reach criterion on the retention test (Figure 4b) than controls (saline-pretreated, Arc nonsense-infused: n=4, t(30)=−1.8, p<0.05; saline-pretreated, PBS-infused: n=4, t(30)=−2.8, p<0.01) and METH-pretreated, Arc antisense-infused rats (n=8, t(30)=3.7, p<0.001). METH-pretreated, Arc antisense-infused rats, however, were not significantly different from the control groups (METH-pretreated, Arc nonsense-infused: n=4, t(30)=1.6, p=0.1; METH-pretreated, PBS-infused: n=3, t(30)=0.4, p=0.7). These results indicate that Arc in the DM striatum is necessary for consolidation of response reversal learning under normal conditions, but not in rats with METH-induced neurotoxicity.
Figure 4.
Effects of acute Arc disruption in the DM striatum. Mean trials to criterion (±SEM) on the motor response reversal task (a) and the reversal retention task (b). (a) Rats were given bilateral infusions of an Arc antisense oligonucleotide, an Arc nonsense oligonucleotide, or PBS 2 h prior to beginning reversal training. No significant interactions or main effects of pretreatment or infusion were found. (b) Rats were tested on retention of the previous day's reversal learning. No further infusions were made. *Significantly different from SAL-pretreated, Arc nonsense oligonucleotide and PBS controls (p<0.05). +Significantly different from METH-pretreated, Arc antisense-infused rats (p<0.05).
Error Analysis
As demonstrated previously (Daberkow et al, 2008) and again in this study (Figure 3), METH-pretreated rats perform as well as normal rats on response reversal learning on the T-maze. We also analyzed the types of errors (Palencia and Ragozzino, 2004) made by METH- or saline-pretreated, PBS-infused rats to determine whether the METH-induced monoamine depletions altered behavioral flexibility. Consistent with the lack of effect on trials to criterion, we found no differences between METH- and saline-pretreated rats in numbers of perseverative (saline-pretreated, 25.5±7.4; METH-pretreated, 28.7±6.5; t(15)=−0.3, p=0.8) or regressive (saline-pretreated, 35.9±8.8; METH-pretreated, 45.7±11.0; t(15)=−0.7, p=0.5) errors.
In Situ Hybridization Histochemistry for Striatal Grin2a Subunit
The pharmacological properties of NMDA receptors are determined to a large extent by the subunit composition of the receptors, with the NR2 subunits being of critical importance in this regard. In particular, prior work has shown that NR2a subunit incorporation yields NMDA receptors with higher affinity for competitive antagonists such as AP5 (Buller et al, 1994). Therefore, to assess the possibility that the lack of effect of AP5 infusion into the DM striatum reflects a change in the pharmacological properties of NMDA receptors in the DM striatum in METH-pretreated rats, we examined the expression of the NMDA receptor NR2a subunit in the striatum of saline- and METH-pretreated rats. In this experiment, the METH-pretreated rats had significant depletions of striatal DA (Table 1). These depletions, as determined by HPLC-ECD analysis of tissue DA content in the striatum, are slightly larger than those observed in the cohorts of rats used for the behavioral experiments described above. Other work in our laboratory (unpublished observations) indicates that the magnitude of the DA depletions estimated by DAT binding is typically less than the magnitude measured through determination of DA tissue content, although the two measures are very highly and significantly correlated (r2 values of 0.8–0.9). Thus, although the magnitude of the depletions in this cohort of animals used for determination of NMDA receptor expression and function after METH treatment appears to be greater, we think that they are roughly equivalent degrees of depletion and that any difference simply reflects subtle differences in the actual magnitude of depletion induced in different cohorts of animals treated with METH at different times and by different investigators.
Table 1. Striatal DA Tissue Content 3 Weeks after a Neurotoxic Regimen of METH.
| Treatment | Striatal DA tissue content |
|---|---|
| Dorsolateral | |
| Saline (n=8) | 314±26 |
| METH (n=8) | 98±15a |
| Ventromedial | |
| Saline (n=8) | 282±26 |
| METH (n=8) | 94±26a |
Values are average (±SEM) DA content (ng DA/mg protein) in striatal tissue determined by HPLC-ECD analysis of 1-mm3 tissue punches from the dorsolateral or ventromedial striatum. Values are ng DA/mg protein.
Significantly different from saline (p<0.05).
Analysis of film autoradiograms for Grin2a mRNA expression in striatal sections (+0.7 mm from bregma) from these saline- and METH-pretreated rats revealed a main effect of region (F(2, 39)=6.74, p<0.01), but no main effect of pretreatment (F(1, 39)=0.2, p=0.7) and no significant interaction (F(1, 2)=0.05, p=0.95) (Figure 5g). Post-hoc analysis confirmed previous reports (Buller et al, 1994; Ganguly and Keefe, 2001; Standaert et al, 1999) of greater Grin2a mRNA expression in both the DM (t(39)=−2.1, p<0.05) and DL (t(39)=−3.7, p<0.001) striatum relative to the VM striatum.
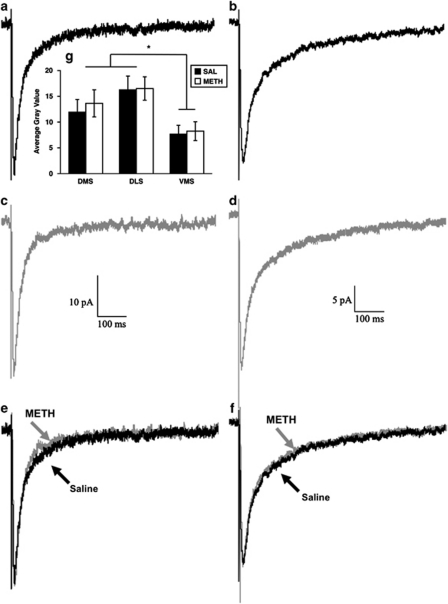
Figure 5.
Grin2a mRNA expression and decay kinetics of NMDA receptor-mediated EPSCs. (a–f) Local, minimal stimulation of the striatum in proximity (<300 μm) to the recorded cell elicits a long-lasting, NMDA receptor-mediated EPSC in the striatum. The average of 35 EPSCs evoked at 0.1 Hz is shown. Representative traces showing the decay-time kinetics of NMDA receptor-mediated EPSCs in the dorsolateral (a, c, e) and ventromedial (b, d, f) striatum of saline- (a, b) and METH- (c, d) pretreated rats are shown, as are normalized, superimposed traces from the DL (e) and VM (f) striatum. (g) Grin2a mRNA expression in the DL, DM, and VM striatum from the hemisphere opposite to that used for electrophysiological recordings. Data are average gray values (±SEM) from densitometric analysis of film autoradiograms. *Both the DM and DL striatum are significantly different from the VM striatum (p<0.01).
Electrophysiological Properties of NMDA Receptor-Mediated EPSCs
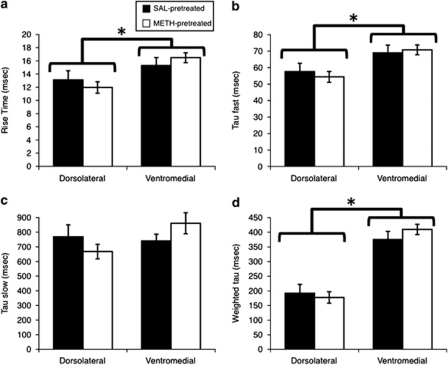
To further assess whether there might be changes in the properties of NMDA receptors in striatal efferent neurons induced by METH exposure and whether this might underlie the differential sensitivity of the METH-pretreated rats to AP5 and Arc antisense oligonucleotide infusion, we compared NMDA receptor-mediated EPSCs from both the DL and VM aspects of the striatum of both saline- and METH-pretreated rats, as there are regional differences in NMDA receptor function in the adult striatum (Chapman et al, 2003). As we have reported previously (Chapman et al, 2003), the kinetics of the NMDA receptor-mediated EPSCs were faster in the DL than the VM striatum; however, prior exposure to a neurotoxic regimen of METH did not change the kinetics (Figures 5 and 6). That is, the rise times (Figure 6a; main effect of region, F(1, 72)=9.64, p<0.01), τf (Figures 5 and 6b; main effect of region, F(1, 78)=11.27, p=0.001), and τw (Figures 5 and 6d; main effect of region, F(1, 78)=72.66, p<0.0001) were significantly faster in the DL striatum, consistent with the greater expression of the NMDA receptor Grin2a subunit in that region of the striatum. There was also a trend for τs to be faster in the DL striatum, although the main effect of region was not statistically significant (F(1, 78)=1.13, p=0.3). However, for none of these kinetic parameters was there a significant main effect of pretreatment (rise times, F(1, 72)=0.0001, p=0.99; τf, F(1, 78)=0.03, p=0.9; τs, F(1, 78)=0.02, p=0.9; τw, F(1, 78)=0.2, p=0.7) or a significant interaction (rise times, F(1, 72)=1.2, p=0.3; τf, F(1, 78)=0.4, p=0.5; τs, F(1, 78)=2.1, p=0.2; τw, F(1, 78)=1.0, p=0.3), indicating that METH-induced neurotoxicity was not associated with changes in the fundamental subunit composition or electrophysiological characteristics of NMDA receptors in the dorsal striatum.
Figure 6.
Kinetic properties of striatal NMDA receptor-mediated EPSCs in saline- and METH-pretreated rats. The values are average kinetic parameters (±SEM) calculated from whole-cell, patch-clamp recordings of NMDA receptor-mediated EPSCs in the dorsolateral and ventromedial striata of rats pretreated with saline (SAL-pretreated; n=10 for DL, n=12 for VM) or a neurotoxic regimen of METH (METH-pretreated; n=25 for DL, n=19 for VM). (a) 10–90% rise time. The decay of the EPSCs was fit with a double exponential equation, I(t)=Ifexp(−t/τf)+Isexp(−t/τs), yielding fast (b; τ fast) and slow (c; τ slow) time constants. Weighted time constants (d; weighted τ) were calculated by using the following equation: τw=[If/(If+Is)]τf+[Is/(If+Is)]τs (Stocca and Vicini, 1998). *Significant main effect of region (p<0.05).
DISCUSSION
This study confirms previous observations that the DM striatum is involved in motor response reversal learning (Palencia and Ragozzino, 2004) and that METH-pretreated rats appear behaviorally normal on this task (Daberkow et al, 2008). However, the present results extend these prior observations in three important ways. First, the present results establish a critical role for Arc in the DM striatum in consolidation of reversal learning in normal rats. Second, they provide additional support, in a novel brain area, for the hypothesis put forth by Guzowski et al (2001) that the correlation between Arc mRNA in a brain region and behavioral performance reflects task-relevant encoding processes occurring in that brain area. Finally, the present results provide the first direct evidence that METH-induced neurotoxicity is associated with a change in the neural substrates engaged to solve a behavioral task normally dependent on the DM striatum. These results therefore highlight the critical importance of striatal Arc for consolidation of basal ganglia-mediated learning and suggest that long-term toxicity induced by METH alters neural circuits and/or cognitive strategies used to solve tasks normally mediated by the dorsal striatum.
The present data provide the first direct evidence that Arc is a critical mediator of consolidation of reversal learning mediated by the DM striatum. This brain region has previously been implicated in cognitive flexibility, including that required for motor response reversal learning. In particular, Ragozzino et al (2002) have established previously that acute blockade of cholinergic muscarinic or glutamatergic NMDA receptors in the DM striatum impairs response reversal learning (Palencia and Ragozzino, 2004; Ragozzino et al, 2002). Additionally, depletion of DA, but not serotonin, in the DM striatum impairs reversal learning (Clarke et al, 2011; O'Neill and Brown, 2007). Furthermore, we have demonstrated previously that, in normal animals, there is a correlation between Arc mRNA in the DM, but not DL, striatum and trials to criterion on a response reversal learning task (Daberkow et al, 2007). Guzowski et al (2001) initially reported such a correlation between Arc expression in the hippocampus and spatial learning on the Morris water maze, leading them to speculate that such correlations reflect the involvement of the encoding processes in that particular brain region in the consolidation of spatial learning. Therefore, we proposed (Daberkow et al, 2007) that the correlation between Arc mRNA in the DM striatum and trials to criterion on the reversal learning task reflected the fact that this reversal is normally dependent on DM striatal function, and that Arc must be a critical mediator of plasticity in the DM striatum underlying consolidation of reversal learning. The present results support this hypothesis, as infusion of an Arc antisense oligonucleotide, but not a scrambled oligonucleotide or a vehicle, into the DM striatum impaired performance in normal rats on a reversal retention test administered 24 h later. Taken together with prior results showing that Arc antisense oligonucleotide infusions into the DL striatum disrupt consolidation of extinction learning occurring during context-induced reinstatement of cocaine-seeking behavior (Hearing et al, 2010), the data strongly implicate Arc as a general, critical mediator of encoding processes underlying striatally based learning and memory functions.
Our previous studies of rats with METH-induced neurotoxicity have shown that, although these rats appear to be behaviorally normal with respect to response reversal learning, Arc induction in the DM striatum is attenuated and no longer correlates with trials to criterion, leading us to hypothesize that METH-induced neurotoxicity promotes a shift in the neural substrates mediating this behavior (Daberkow et al, 2008). The present findings support this hypothesis: in rats with METH-induced neurotoxicity, acute disruption of DM striatal function by infusion of the NMDA receptor antagonist AP5 or an Arc antisense oligonucleotide fails to alter response reversal learning or its retention. Thus, although rats with METH-induced neurotoxicity appear to be normal on the surface, the neural substrates mediating the behavior have changed. These findings are similar to those reported, for example, in Parkinson's disease patients, in which behavior appears unimpaired relative to controls, but functional imaging reveals a change in the brain regions engaged during the task (Moody et al, 2004). These findings highlight the need for studies assessing the impact of neurotoxicity on learning and memory to examine not simply behavioral measures of the learning, but also the processes and brain regions mediating the behavior, before concluding that there is a lack of effect of such toxicity on a particular behavior.
It is conceivable that the lack of effect of acute disruption of NMDA receptor and Arc function in the DM striatum on reversal learning and its consolidation reflects a decrease in sensitivity of the DM striatum to these manipulations, rather than a reorganization of the neural circuitry mediating the behavior. However, we think that this former possibility is unlikely, as in situ hybridization histochemical analysis of Grin2a mRNA expression and electrophysiological determination of the biophysical properties of striatal NMDA receptors failed to reveal any METH-induced changes in these NMDA receptor subunits or properties. The pharmacology of NMDA receptors is heavily influenced by Grin2 subunit incorporation into the receptor (Buller et al, 1994; Traynelis et al, 2010), as are the rise time and decay kinetics of the NMDA receptor-mediated current, with Grin2a-containing receptors showing the fastest kinetics (Dingledine et al, 1999). Striatal efferent neurons, which are the striatal neurons in which Arc is expressed (Vazdarjanova et al, 2006), express the Grin2a and Grin2B subunits (Standaert et al, 1999). The present results confirm our prior observations and those of others that there is greater expression of Grin2a subunits in the DL than VM striatum (Buller et al, 1994; Ganguly and Keefe, 2001; Standaert et al, 1999), and that the rise times and decay kinetics of these currents are correspondingly faster in the DL than in VM striatum (Chapman et al, 2003). These results illustrate our ability to detect differences in the subunit composition of the NMDA receptor using this electrophysiological approach. Importantly, METH-induced neurotoxicity was not associated with changes in Grin2a subunit mRNA expression or in the biophysical properties of the NMDA receptors in the dorsal striatum, strongly suggesting that METH-induced neurotoxicity is not associated with changes in the subunit composition, and thus the pharmacology, of striatal NMDA receptors. It therefore seems unlikely that a change in the sensitivity of NMDA receptors in METH-pretreated rats to AP5 or endogenous glutamate underlies the lack of efficacy of acute AP5 infusion or Arc antisense infusion in those animals in the present studies. Rather, the data suggest that the lack of effect of these agents more likely reflects a change in the neural circuitry engaged in the reversal learning task.
The consequences of METH exposure that lead to this apparent shift in behavioral control are currently unknown; however, the METH-induced partial loss of DA in the DM striatum may be the basis. As is typical (Chapman et al, 2001; Hanson et al, 2009), the binge regimen of METH resulted in an approximately 40% loss of DA tissue content, as measured by DAT levels, in the DM striatum at the end of the behavioral training. Although METH also induces a loss of serotonin in the DM striatum (Haughey et al, 1999), as noted above, DA, not serotonin, neurotransmission in the DM striatum appears to mediate reversal learning (Clarke et al, 2011; Darvas and Palmiter, 2011; O'Neill and Brown, 2007). Thus, one strong possibility is that it is the partial loss of DA in the DM striatum that results in the change in sensitivity of response reversal learning to acute manipulations of DM striatal function in METH-pretreated rats.
An alternative possibility is that METH-induced damage to extra-striatal serotonin systems disrupts the function of afferents to the DM striatum or other neural substrates necessary for reversal learning, thereby altering the circuitry engaged during the reversal learning. The neurotoxic regimen of METH used in the present study also induces a loss of serotonin in the prefrontal cortex (Hotchkiss and Gibb, 1980; Ricaurte et al, 1980), including an approximately 50% loss of SERT binding in the orbitofrontal cortex (OFC) reported here. Serotonin function in the OFC is known to be critical for reversal learning (Clarke et al, 2005, 2007; Robbins and Arnsten, 2009). Thus, changes in the function of the OFC as a consequence of METH-induced neurotoxicity to that region may contribute to the changes in reversal learning observed in the present study. However, the OFC tends to provide afferent innervation to the central and lateral aspects of the dorsal striatum, as well as the nucleus accumbens, and largely does not provide afferents to the DM striatum (Schilman et al, 2008). On the other hand, the prelimbic cortex does project strongly into the DM striatum (Lévesque and Parent, 1998; Vertes, 2006). As presented here, a neurotoxic regimen of METH results in about a 40% loss of SERT in the prelimbic cortex. Furthermore, the prelimbic cortex has a role in reversal learning, although the role is more in controlling complex, higher-order set-shifting tasks, rather than simple one-dimensional reversal learning such as the T-maze task used in this study (Birrell and Brown, 2000; Ragozzino, 2003; Ragozzino et al, 1999). Finally, the centromedian and paracentral nuclei of the thalamus provide excitatory innervation to the DM striatum (Van der Werf et al, 2002). These thalamic nuclei receive relatively dense serotonergic innervation (Vertes et al, 2010), and data obtained from abstinent human METH abusers suggest decreased SERT binding in the thalamus (Sekine et al, 2006). Thus, METH-induced alterations in the function of excitatory afferents from intralaminar cell groups to the DM striatum might also have a role in the disruption of DM striatal control over reversal learning observed in the present studies. However, the extent to which neurotoxic regimens of METH damage the intralaminar nuclei of the thalamus in rodents has not heretofore been reported. Clearly, further studies examining the effects of selective DA depletions induced by substituted amphetamines vs the effects of combined DA/serotonin depletions will be necessary to conclusively rule out a contribution of serotonin loss to the changes in behavioral control observed in the METH-pretreated rats.
An interesting aspect of the present findings is that METH-pretreated rats appear to be behaviorally normal, both in terms of trials to criterion and in the types of errors made during reversal learning. The neural substrates capable of supporting apparently normal reversal learning despite altered DM striatal function remain to be determined. One possibility for an alternate neural substrate is the nucleus accumbens core, which has been implicated in behavioral flexibility (Darvas and Palmiter, 2011; Goto and Grace, 2005; Haluk and Floresco, 2009). The ‘binge' regimen of METH exposure often does not induce as much monoamine loss in the nucleus accumbens as in the dorsal striatum (Eisch et al, 1992; Haughey et al, 1999), and DA signaling in the accumbens has a role in simple reversal learning (Darvas and Palmiter, 2011; Haluk and Floresco, 2009). Future studies thus will be necessary to determine the role of the nucleus accumbens in reversal learning in METH-pretreated rats, the circumstances under which DM striatal vs nucleus accumbens DA signaling normally supports behavioral flexibility, and the cognitive cost associated with loss of DM striatal control over behavioral flexibility.
In summary, the present study provides the first evidence that Arc in the DM striatum is a critical mediator underlying consolidation of motor response reversal learning, thereby further validating its importance as a molecular substrate of learning and memory function. Furthermore, the present results are the first to show that METH-induced neurotoxicity is associated with a change in the neural substrates underlying basal ganglia-mediated learning and memory, despite the fact that behavioral indices of that learning appear to be normal. These findings suggest that METH-induced neurotoxicity may have important ramifications for the ability of individuals with a history of METH abuse to engage in cognitive behavioral therapies for management of drug addiction, as well as the extent to which they can function optimally in tasks related to their employment and personal lives. Further studies are therefore needed to fully understand the molecular, cellular, and systems level substrates mediating learning and memory processes in corticostriatal circuits that are compromised by METH-induced monoamine loss, and to design approaches to mitigate such effects.
Acknowledgments
This work was supported by DA024036 and NS35579 (KAK), DC008553 (EDP), and DA14859 (DEC).
The authors declare no conflict of interest.
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, et al. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12:27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Chapman DE, Keefe KA, Wilcox KS. Evidence for functionally distinct synaptic NMDA receptors in ventromedial versus dorsolateral striatum. J Neurophysiol. 2003;89:69–80. doi: 10.1152/jn.00342.2002. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Arc mRNA induction in striatal efferent neurons associated with response learning. Eur J Neurosci. 2007;26:228–241. doi: 10.1111/j.1460-9568.2007.05630.x. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced arc mRNA expression in identified striatal efferent neurons. Neurotox Res. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol Psychiatry. 2011;69:704–707. doi: 10.1016/j.biopsych.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O'Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Keefe KA. Unilateral dopamine depletion increases expression of the 2A subunit of the N-methyl--aspartate receptor in enkephalin-positive and enkephalin-negative neurons. Neuroscience. 2001;103:405–412. doi: 10.1016/s0306-4522(01)00005-7. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, et al. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Birdsall E, Seferian KS, Crosby MA, Keefe KA, Gibb JW, et al. Methamphetamine-induced dopaminergic deficits and refractoriness to subsequent treatment. Eur J Pharmacol. 2009;607:68–73. doi: 10.1016/j.ejphar.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methampetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2010;14:1–12. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Willams MT. Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology. 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang L-C, Furukawa Y, et al. Brain serotonin transporter in human methamphetamine users. Psychopharmacology. 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Parent A. Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cereb Cortex. 1998;8:602–613. doi: 10.1093/cercor/8.7.602. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Morgan ME, Gibb JW. Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extra-striatal dopaminergic nuclei. Neuropharmacology. 1980;19:989–995. doi: 10.1016/0028-3908(80)90010-6. [DOI] [PubMed] [Google Scholar]
- O'Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- O'Neill M, Brown VJ. The effect of striatal dopamine depletion and the adenosine A2A antagonist KW-6002 on reversal learning in rats. Neurobiol Learn Membr. 2007;88:75–81. doi: 10.1016/j.nlm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Membr. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: Orlando, FL; 1998. [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. Acetylcholine actions in the dorsomedial striatum support the flexible shifting of response patterns. Neurobiol Learn Membr. 2003;80:257–267. doi: 10.1016/s1074-7427(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HBM, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate–putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schröder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Friberg IK, Landwehrmeyer GB, Young AB, Penney JB., Jr Expression of NMDA glutamate receptor subunit mRNAs in neurochemically identified projection and interneurons in the striatum of the rat. Mol Brain Res. 1999;64:11–23. doi: 10.1016/s0169-328x(98)00293-9. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contributions of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Pattern of distribution of serotonergic fibers to the thalamus of the rat. Brain Struct Funct. 2010;215:1–28. doi: 10.1007/s00429-010-0249-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]