Abstract
The prevalence of type 2 diabetes is increasing worldwide. The majority of currently available glucose-lowering agents work via insulin-dependent mechanisms and have significant limitations. Hence, there is a need for newer treatments utilizing novel therapeutic targets. Drugs which inhibit the sodium glucose cotransporter in the renal tubules (SGLT-2 inhibitors), represent a novel class of drugs under development. By inhibiting SGLT-2, they promote increased renal glucose excretion and thereby calorie loss with improved glycemic control and weight loss. Dapagliflozin is most advanced in development of this new drug class and currently undergoing phase 3 trials. In addition to its glucose lowering effect, dapagliflozin appears to have favorable impacts on weight and blood pressure, with low risk of hypoglycemia. However, as with all new treatments, long-term safety is an issue. Clinical trials showed increased risk of genital and possibly urinary infections with dapgliflozin. Furthermore, concerns have arisen regarding a possible increased incidence of breast and bladder cancer in patients on dapagliflozin. However, it needs further investigation to confirm or refute whether these concerns are concrete.
Keywords: glucose reabsorption, kidney, renal glucose, SGLT, sodium glucose cotransporter, type 2 diabetes
Abbreviations: ADA - American Diabetes Association; DPP-4 - dipeptidyl peptidase 4; FDA - Food and Drug Administration; FPG - fasting plasma glucose; GLP-1 - glucagon-like peptide-1; HbA1c - glycated hemoglobin; HDL - high-density lipoprotein; MAD - multiple ascending dose; SAD - single ascending dose; SEER - Surveillance Epidemiology and End Results; SGLT - sodium glucose cotransporters; SU - sulfonylureas; Tmax - maximum time for drug absorption; T2D - type 2 diabetes; TZD - thiazolidenediones; UTI - urinary tract infections
Introduction
The prevalence of diabetes in the United Kingdom (UK) is 4.26% (Diabetes UK, Oct 2010), with a diagnosed population of 2.8 million people and accounting for 10% of the total National Health Service spend [1]. Add to this figure an estimated half a million people undiagnosed and that by 2025 it is estimated that over 4 million people will have the condition [1] At any one time, between 1 in 5 and 1 in 10 people in hospital has diabetes. This is associated with poorer outcomes and prolonged admissions. Worldwide more than 300 million people have diabetes, the great majority (around 90%) with type 2 diabetes (T2D) [2], and it is anticipated that the figure will reach 438 million by 2030 [3].
T2D is progressive disorder in which the pancreatic beta-cells secrete increasing amounts of insulin to compensate for insulin resistance. Progressive beta-cell failure results in insufficient insulin production which is unable to overcome insulin resistance, leading to glucose intolerance and eventually T2D [4, 5]. Factors affecting insulin resistance include genetic predisposition, age, lack of physical activity, diet, and obesity.
T2D is associated with serious micro- and macrovascular complications which contribute significantly to mortality and morbidity [6]. About 50% of patients show signs of complications at the time of diagnosis. In addition to the management of cardiovascular risk factors (such as hypertension and dyslipidemia), intensive glycemic control has been shown to prevent the development and slow the progression of microvascular complications. Cardiovascular outcomes and mortality may also be improved if such management is begun early in the course of T2D [7]. Hence, good glycemic control (particularly in the early stages of T2D) is a highly important aspect in the management of these patients to reduce the morbidity associated with this condition.
There are several drug classes which improve glycemic control, including biguanides, thiazolidenediones (TZD), sulfonylureas (SU), and alpha-glucosidase inhibitors. All are initially effective, but they fail to maintain normoglycemia as monotherapy in the long term (because of the progressive nature of beta-cell dysfunction), resulting in the requirement for combination therapy and insulin. These agents may be associated with significant side effects including hypoglycemia (SU, insulin), weight gain (SU, TZD, and insulin), edema (TZD), and possibly adverse cardiovascular outcomes (TZD). There is also a safety requirement for glucose self-monitoring (SU, insulin) for some drugs, and the need to use injections (insulin). Finally, none of these agents has been shown to alter the natural history of the disease.
Hence, there is a need for treatments that bring about sustained improvement in glycemic control, slow/reverse the decline in beta-cell function, are weight neutral or cause weight loss, do not cause hypoglycemia, and have no deleterious effects on cardiovascular outcomes. New classes of drugs were introduced around 6 years ago based on their effects on the incretin hormone axis. Incretin-based therapies are either weight neutral (DPP-4 inhibitors) or cause weight loss (GLP-1 analogues), and involve no increased risk of hypoglycemia compared with placebo (unless combined with SU). These newer agents have also shown increased beta-cell survival in animal studies. The drugs work primarily by effects on insulin secretion or insulin sensitivity in the liver, muscle, adipose tissue or by decreasing glucose absorption from the gut.
Another important organ involved in glucose handling is the kidney. Another class of glucose-lowering drugs, which works by effects on the kidney, is currently under development, the sodium glucose cotransporter 2 (SGLT-2) inhibitors. In this article, we will review the available data on these agents.
Renal glucose handling
During the post-absorptive state, the kidney both consumes and releases glucose. Renal glucose release and uptake are under hormonal control. The kidney can compensate, at least partially, for impaired hepatic glucose production, and contributes to the excessive glucose release seen in both patients with types 1 and 2 diabetes.
In normal individuals, approximately 180 g of glucose is filtered by the kidneys [8, 9]. Virtually, all the glucose filtered through the glomeruli is reabsorbed by the proximal renal tubule mediated by SGLT-1 and -2, which are located in the tubule. Because of the presence of the SGLT receptors, no glucose appears in the urine under optimal conditions. During periods of hyperglycemia, the glucose reabsorptive capacity of the kidney increases in proportion to the plasma glucose concentration. It is only when the plasma glucose load is high enough to saturate these transporters (despite their overexpression) that glucose appears in the urine [9].
SGLT-2 is a low-affinity high-capacity transporter, and is present in the S1 segment of the proximal tubule. It accounts for 90 percent of the glucose reabsorbed from the kidneys. SGLT-1 is a high-affinity low-capacity transporter located on the S3 (distal) segment of the proximal tubule. It accounts for 10 percent of the glucose reabsorbed from the kidneys. SGLT-1 is also present abundantly in the intestine and plays an important role in glucose absorption from that site.
Concept of SGLT-2 inhibition
There are rare inherited conditions where there is dysfunction of the SGLT-1 and SGLT-2 transporters [10]. Individuals with the SGLT-1 mutation develop glucose-galactose malabsorbtion. This causes profuse diarrhea which is reversible on controlling glucose and lactose in the diet. These people have only mild glycosuria. Severe diarrhea has limited the utilization of SGLT-1 as a therapeutic target for glucose-lowering treatment. Similarly, the SCL5A2 gene mutation causes dysfunction of the SGLT-2 transporter. Glucose reabsorption is severely impaired, and there is heavy glycosuria. Despite this, the person remains well, and there appear to be no other complications.
Several decades ago, there was interest in a substance called phlorizin isolated from the bark of apple trees. It is a non-specific inhibitor of both SGLT-1 and SGLT-2 transporters, and the results of animal studies were encouraging. Unfortunately, it was poorly absorbed from the intestine and caused additional inhibition of SGLT-1 transporters in the intestine and serious side effects. Hence, the drug was never pursued in humans.
However, given the experience from gene mutations and the potential for SGLT-2 inhibition, a new class of drugs has been developed which inhibit SGLT-2; several of which are now in phase 3 trials. There are various SGLT-2 inhibitors in development (e.g. canagliflozin, sergiflozin, remogiflozin). The most advanced drug in clinical trials is dapagliflozin, a medication being co-developed by Astra Zeneca and Bristol-Myers Squibb. In this article, we will mainly consider the effects of dapagliflozin.
Preclinical studies
In vivo, dapagliflozin caused excretion of glucose in diabetic and normal rats [11]. It improved glucose tolerance in normal rats and reduced hyperglycemia in Zucker Diabetic rats. After single administration of a single dose, high blood glucose was reduced in the rat model of diabetes. Once-daily dapagliflozin treatment over 2 weeks maintained the reductions in lowered fasting and postprandial glucose levels [11].
Pharmacokinetics and pharmacodynamics
Single and multiple ascending dose (SAD and MAD) studies with dapagliflozin in healthy people and those with T2D confirmed a pharmacokinetic profile consistent with once-daily dosing [12, 13]. The half-life is around 17 hours with dose-dependent concentrations. There is rapid absorption of the drug with Tmax of around 1-2 hours. There is minimal renal excretion of the drug as it is protein-bound.
There was a dose-dependent increase in glycosuria in T2D patients and healthy persons reaching a plateau at 20 mg/day. Dapagliflozin can be co-administered with pioglitazone, metformin, glimepiride, or sitagliptin without dose adjustment of either drug [14]. It can also be administered without regard to meal times [15]. The in vivo success led to clinical evaluation in people with diabetes and healthy subjects.
Clinical studies
In a prospective, 12-week, randomized, parallel-group, double-blind, placebo-controlled study on drug-naive T2D patients with HbA1c 7-10%, patients were assigned dapagliflozin (2.5-50 mg), metformin, or placebo. Changes in FPG were dose-related, but there was no dose-related response to postprandial glucose or HbA1c. Even the lowest dose of dapagliflozin produced a near-maximal effect on postprandial glucose [16].
A double-blind triple-arm 12-week randomized controlled trial was performed in patients receiving oral antidiabetes drugs alongside insulin [17]. Basal insulin dose was ≥50 units and stable for ≥6 weeks. Therapies included metformin ≥1,000 mg and/or pioglitazone ≥30 mg, or rosiglitazone 4 mg for ≥6 weeks and insulin therapy for ≥12 weeks. Patients were randomly assigned to placebo, 10 mg dapagiflozin, or 20 mg dapagiflozin in addition to their oral antidiabetes agents, with a 50 % reduction to their daily insulin dose. One event of renal failure occurred during treatment with 10 mg dapagliflozin. The patient was on multiple antihypertensive agents (enalapril, carvedilol and furosemide). Furosemide and enalapril therapy were withheld, and the renal failure resolved with oral rehydration.
The largest phase 3, multi-center, double-blind, parallel-group, placebo-controlled trial involved 546 adults with T2D [18]. They were receiving daily metformin (≥1500 mg per day), and were randomly assigned to add on placebo or dapagliflozin (2.5 mg, 5 mg, or 10 mg). The primary endpoint of the trial was a change in HbA1c after week 24. The secondary endpoints were changes in fasting glucose and change in body weight. There was a dose-dependent reduction in HbA1c with a reduction of 0.84% in patients with 10 mg compared with 0.3% in the placebo group. Fasting glucose was also reduced in the dapagliflozin group (-2.66%, -3.66%, and -3.43% for 2.5, 5, and 10 mg, respectively) compared with -1.02% for placebo. All patients on dapagliflozin had a greater weight loss compared with placebo. Patients on 10 mg had a change in baseline of -2.9 kg compared with placebo (-0.9kg). Also, around 25% of all patients in the dapagliflozin group lost at least 5% of their initial baseline body weight. There was no significant increase in urine infection compared with placebo.
Another trial involved patients who were poorly controlled on insulin alone [19]. Patients were randomized to receive 2.5, 5, 10 mg dapagliflozin or placebo for 48 weeks. Baseline HbA1c was 8.5% with a weight of 85 kg. The dapagliflozin group had a higher rate of urinary tract infections (7.9-10.8%) compared with placebo (5.1%) [19, 20].
In another randomized controlled trial of inadequately controlled patients with T2D, the baseline treatment was metformin which was titrated to control fasting glucose. 406 patients received up to 10 mg/day of dapagliflozin compared with 408 who received up to 20 mg of glipizide based on glycemic response and tolerability. By one year of follow up, there was a decrease in HbA1c of -0.52% in both groups. Patients in the dapagliflozin group lost 3.2 kg weight compared with the sulfonylurea group which gained 1.4 kg. The dapagliflozin group had better improvements in blood pressure and HDL cholesterol (p < 0.0001). The dapagliflozin group also had less hypoglycemia compared with the glipizide group (3.5 % vs. 40.8%), and there were fewer serious adverse events (8.6% vs. 11.1%). Patients had more urinary tract infections with dapagliflozin (10.8% vs. 6.4%) and more genital infections (12.3% vs. 2.7%) [21]. These side effects were more common in women.
New data were presented as a late breaking poster at the ADA this year [22]. This was an extension of the above-mentioned original study to assess long term efficacy and safety. The 2-year results showed a reduction of baseline HbA1c with dapagliflozin of -0.32% vs. -0.14% with glipizide. Dapagliflozin produced a sustained reduction in body weight of -3.7 kg vs. a +1.36 kg increase for glipizide. There was a lower risk for hypoglycemia in the dapagliflozin group (4.2%) as compared to glipizide (45.8%). The overall rate of adverse events was similar after 2 years. Urinary tract infections (UTIs) were 13.5% for dapagliflozin group as compared to 9.1% for glipizide. Genital infections were 14.8% for dapagliflozin group (8% men, 23.3%women) as compared to 2.9% for glipizide (0.4% men, 5.9% women). The majority of events was mild to moderate and responded to standard care. These occurred predominantly in the first year. There was 1 discontinuation in each arm owing to UTI and 3 discontinuations in the dapagliflozin arm because of genital infections occurring in year 1 with none occurring in year 2.
In a 24-week, randomized, parallel-group, double-blind, placebo-controlled phase 3 trial, patients with HbA1c 7.0-10% were assigned to receive once-daily 2.5, 5, or 10 mg dapagliflozin, or placebo [23]. This was given once daily either in the morning (main cohort) or evening (exploratory cohort) for 24 weeks. Patients with HbA1c 10.1-12% (high exploratory cohort) were randomized to receive a morning dose of 5 or 10 mg/day dapagliflozin.
Another 24-week, randomized, placebo-controlled, double-blind trial was conducted on patients with HbA1c 7-10% on glimepiride 4 mg/day for 24 weeks. Patients were assigned to placebo or dapagliflozin (2.5, 5, or 10 mg/day). The primary endpoint was change of HbA1c from baseline. Secondary endpoints were change in body weight and other glycemic parameters. Patients on dapagliflozin had significantly improved HbA1c and reduced weight as compared to placebo (Table 1) [24].
Table 1. Results of trials with dapagliflozin as add-on therapy in diabetic patients with different baseline characteristics.
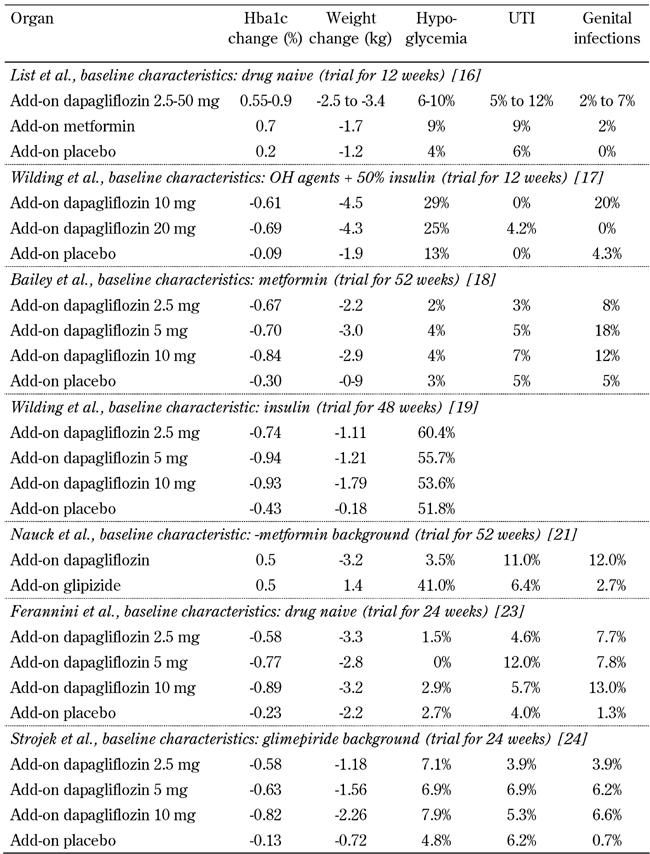
Legend: OH: oral hypoglycemic. UTI: urinary tract infections.
Safety and place in therapy
Most guidelines on management of T2D recommend initial pharmacotherapy with metformin, and then adding in a sulfonylurea, if the agreed target HbA1c is not achieved. This may be followed by addition of pioglitazone or a DPP-4 inhibitor or insulin (although there is also a place for DPP-4 inhibitors in the second line under certain circumstances). Insulin is commonly used as monotherapy or in combination with oral agents, especially metformin if the desired outcome with oral drugs alone is not achieved. GLP-1 agonists are also gaining popularity, particularly where avoidance of weight gain is a significant issue which makes the use of insulin difficult or unacceptable.
Whilst there is still much work to do, the SGLT-2 inhibitors represent a new class of oral antidiabetes agents which appear to have equivalent efficacy to other oral agents, but with the added benefit of weight loss, blood pressure reduction, and low risk of hypoglycemia. The mode of action is insulin-independent which potentially makes them an option at any stage in the disease process and in any combination. In this respect, they could pose a significant threat to the primacy of the sulfonylureas as second-line therapy, and may compete second and third line with other oral agents or even the GLP-1 agonists. The latter may have greater efficacy, but many patients will prefer to try an oral agent before an injectable. Also, SGLT-2 inhibitors are likely to be significantly cheaper with the added benefit of weight loss which appears equivalent to that achieved with GLP-1 agonists.
On the downside, there remain significant questions which will initially limit the use of these agents. These include a lack of durability and safety issues relating to use in renal impairment and the hypothetical possibility of significant side effects in the elderly during acute illness complicated by dehydration. Also, the mechanism of blood pressure-lowering is poorly understood, and, whilst likely to be of long-term benefit, much more work needs to be done in this area, including the potential for interactions with established antihypertensive agents. Issues surrounding increased risk of genital infection (how severe?, how easy to treat?, recurrence rates?) and possibly urinary tract infection also need clarifying.
In the trials, 5 patients on dapagliflozin were reported to have elevation of transaminases by 3 times the upper limit of normal, with accompanying total bilirubin more than twice the upper limit of normal. There was an adequate explanation for these results in all but one case [26]. In the case in question, there was a "probable diagnosis of mild to moderately severe dapagliflozin-induced liver injury".
Overall, there was similarity between the dapagliflozin group (1.4%) vs. control group (1.3%) in the proportion of patients reporting cancers and unspecified tumors [25]. However, there was an imbalance in the number of breast cancer and bladder cancer cases. There were 9 cases of breast cancer in the dapagliflozin group (2223 patients) as compared to 1 case in placebo (1053 patients). All these cases were diagnosed within the first year of the study [25, 26]. However, these figures were higher than the predicted number of 7.1 cases based on the Surveillance Epidemiology and End Results (SEER) program to calculate the expected number of breast cancer cases in a reference US population. It remains uncertain whether the use of dapagliflozin is associated with increased risk of breast cancer. Further studies are needed.
Bladder cancers were reported in 9 out of 5478 patients treated with dapagliflozin compared with 1 case in 3156 patients in the control group [25, 26]. All were men and 5 of the 10 cases were diagnosed within the first year. Six patients had hematuria at the start of the study and before receiving the study drug. Based on the SEER data, only 2 cases were expected in the male dapagliflozin group and one in the male control group. In the female cohort, one would have expected to find 0.5 cases in the dapagliflozin group compared to 0.22 in the control group. The use of an external data source (SEER) as reference population has limitations including the fact that there could be a detection bias because of more frequent urine examinations. In preclinical studies of dapagliflozin in rodents (using 100-fold doses over the maximum recommended dose in humans) over 2 years, there was no evidence of carcinogenicity [25].
Conclusions
SGLT-2-inhibitor drugs appear to be a promising new development for the management of T2D. They could be useful both as monotherapy and in any combination with other antidiabetes agents, including insulin. The novel properties of this drug class are especially welcome at a time when tolerability issues and side effects of several commonly used antidiabetes agents impede their use to maximal potential.
However the incidence of the bladder and breast cancer cases and elevation of hepatic enzymes pose significant safety questions. These were considered by the advisory committee to the FDA recently (July 2011). The voting was 9 to 6 recommending non-approval of the drug because of to these safety concerns.
The FDA hopes to make a final decision by end of October 2011, but if the drug is licensed, it is expected that this will be dependent on an agreement with the manufacturers to do large long-term safety studies. Whether these concerns will affect other SGLT-2 inhibitors in development remains to be seen.
Disclosures: MB is a clinical fellow at Good Hope Hospital. The views expressed in this report are those of the author(s) and not necessarily those of the National Health Service, Good Hope Hospital, or the Department of Health. AAT is a research training fellow supported by the National Institute for Health Research. The views expressed in this report are those of the author(s) and not necessarily those of the National Health Service, National Institute for Health Research, or the Department of Health. AAT has also won research grants from Sanofi-Aventis and Novo Nordisk UK Research Foundation. AHB has received honoraria for lectures and advisory work from BMS/Astra-Zeneca, Takeda, MSD, Novartis, Boehringer Ingelheim, Sanofi-Aventis, Eli Lilly, Novo Nordisk, and Roche.
References
- 1.Diabetes UK website, Reports and Statistics. [July 1, 2011]. http://www.diabetes.org.uk/Professionals/Publications-reports-and-resources/Reports-statistics-and-case-studies/Reports/Diabetes-prevalence-2010.
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.IDF diabetes atlas. International Diabetes Federation; http://www.diabetesatlas.org/book/export/html/36. [Google Scholar]
- 4.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of development. Diabetes Care. 2001;24:89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. Pathogenesis of type 2 diabetes. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 8.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 9.Wright EM. Renal Na (+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280(1):F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 10.Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5(1):133–141. doi: 10.2215/CJN.04010609. [DOI] [PubMed] [Google Scholar]
- 11.Han S, Hagan DL, Taylor JR, Xin L, Meng W, Biller SA, Wetterau JR, Washburn WN, Whaley JM. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723–1729. doi: 10.2337/db07-1472. [DOI] [PubMed] [Google Scholar]
- 12.Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–526. doi: 10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 13.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85(5):513–519. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 14.Kasichayanula S, Liu X, Shyu WC, Zhang W, Pfister M, Griffen SC, Li T, LaCreta FP, Boulton DW. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes Metab. 2011;13:47–54. doi: 10.1111/j.1463-1326.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasichayanula S, Liu X, Zhang W, Pfister M, Reele SB, Aubry AF, LaCreta FP, Boulton DW. Effect of a high-fat meal on the pharmacokinetics of dapagliflozin, a selective SGLT2 inhibitor, in healthy subjects. Diabetes Obes Metab. 2011;13(8):770–773. doi: 10.1111/j.1463-1326.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 16.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose co transport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32(9):1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 19.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes poorly controlled on insulin therapy - efficacy of a novel insulin-independent treatment. Diabetes. 2010;59(Suppl 1):A21–A22. [Google Scholar]
- 20.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Parikh S. Sustained effectiveness of dapagliflozin over 48 weeks in patients with type 2 diabetes poorly controlled with insulin. Diabetes. 2010;59(Suppl 1) Abstract 0021-LB. [Google Scholar]
- 21.Nauck M, Del Prato S, Rohwedder K, Elze M, Parikh S. Dapagliflozin vs glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin: 52-week results of a double-blind, randomized, controlled trial. Diabetologia. 2010;53(Suppl 1) [Google Scholar]
- 22.Nauck MA. San Diego, California. June 24-28, 2011; American Diabetes Association's 71st Scientific Sessions; 0040-LB. [Google Scholar]
- 23.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Care. 2010;33(10):2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomised, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011 doi: 10.1111/j.1463-1326.2011.01434.x. In press. [DOI] [PubMed] [Google Scholar]
- 25.Astrazanacea-investigational compound dapagliflozin sustained glycaemic control and weight reduction. Eur Pharmaceut Rev; [Aug 1, 2011]. 2011. http://www.europeanpharmaceuticalreview.com/7918/news/industry-news/bristol-myers-squibb-and-astrazeneca-announce-investigational-compound-dapagliflozin-sustained-glycemic-control-and-weight-reduction-in-study-of-type2-diabetes-patients-inadequately-controlled-with-me/ [Google Scholar]
- 26.FDA briefing document NDA 202293. Advisory committee meeting July 19, 2011. [Aug 5, 2011]. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM262994.pdf.


