Abstract
Renal and cardiovascular complications make type 2 diabetes one of the most morbid conditions in medicine. The kidney frequently gets involved in this "multi-organ disease". Of the large proportion of patients who progress with further loss of renal function, most prematurely die or end up in dialysis. Many interventions have targeted a decelerated progression of renal function loss, including metabolic control, blood pressure, and lipid management. Recently, modulation of the renin-angiotensin-aldosterone-system (RAAS) have been combined with the existing therapeutic armamentarium. RAAS inhibitors lower blood pressure and decrease albuminuria which leads to additionally protective renal and cardiovascular effects. Although this has been the success story of the last two decades, it has still made a relatively small contribution to patient welfare, since the residual risk in patients that received this optimal care remains extremely high. New treatment strategies are required that further slow the progression of renal and cardiovascular functions. Recently, several pathways have been investigated, targeting traditional risk factors such as blood pressure- and lipid-lowering strategies with unexpected results. Furthermore, novel targets and drugs have been identified. Preliminary studies on surrogate markers for renal outcome show a great potential for additive renal protection, such that in many cases hard endpoint trials are initiated. Novel interventions, which are reviewed here, include vitamin D receptor activators, RAASi with direct renin inhibitors or aldosterone antagonists, endothelin-antagonist, inflammation suppression with pentoxyfillin, MCP-1 synthesis inhibitors, or with Nrf2 agonists. Despite the current depressing situation of type 2 diabetic patients with nephropathy, new treatment options are under development to reduce the high morbidity and mortality associated with this universal ever-increasing disease threat.
Keywords: ACE-inhibitor, albuminuria, angiotensin receptor blockade, endothelin, inflammation, nephropathy, novel drug, type 2 diabetes, vitamin D
Abbreviations: ACCOMPLISH - Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (trial); ACCORD - Action to Control Cardiovascular Risk in Diabetes (trial); ACE - angiotensin-converting enzyme; ACEi - angiotensin-converting enzyme inhibitor; ADVANCE - Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; ALTITUDE - Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (trial); ARB - angiotensin II receptor blocker; ASCEND - A Study of Cardiovascular Events iN Diabetes; ASCOT - Anglo-Scandinavian Cardiac Outcomes Trial; AVOID - Aliskiren in the eValuation of prOteinuria in Diabetes (trial); BENEDICT - BErgamo NEphrologic DIabetes Complications Trial; CANVAS - Canagliflozin Cardiovascular Assessment Study; DCCT - Diabetes Control and Complications Trial; DPP-4 - dipeptidyl peptidase 4; DRI - direct renin inhibition; ECH - erythroid cell-derived; eGFR - estimated glomerular filtration rate; ESRD - end-stage renal disease; ETA/B - endothelin type A/B; FIELD - Fenofibrate Intervention and Event Lowering in Diabetes (study); GFR - glomerular filtration rate; GLP-1 - glucagon-like peptide-1; HbA1c - glycated hemoglobin A1c; IDNT - Irbesartan in Diabetic Nephropathy Trial; IRMA-2 - Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (study); Keap1 - Kelch like-ECH-associated protein 1; LDL - low-density lipoprotein; LIRICO - Long-term Impact of RAS Inhibition on Cardiorenal Outcomes (study); MCP-1 - monocyte chemoattractant protein-1; MRB - mineralocorticoid receptor blockade; NCT - national clinical trial; Nrf2 - nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2; ONTARGET - Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial; PLANET - Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial; PREDIAN - Pentoxifylline for Renoprotection in Diabetic Nephropathy (trial); RAAS - renin-angiotensin-aldosterone-system; RAASi - renin-angiotensin-aldosterone-system inhibition; RADAR - Reducing residual Albuminuria in subjects with type 2 Diabetes and nephropathy with Atrasentan (trial); RAS - renin-angiotensin system; RENAAL - Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (study); ROADMAP - Randomised Olmesartan And Diabetes Microalbuminuria Prevention (trial); SEER - Surveillance Epidemiology and End Results; SGLT-2 - sodium glucose cotransporter 2; SHARP - Study of Heart and Renal Protection; UKPDS - UK Prospective Diabetes Study; VADT - Veteran Affairs Diabetes Trial; VA-NEPHRON-D - NEPHROpathy iN Diabetes (study); VITAL - Selective Vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (study)
Introduction
The incidence of diabetes is growing rapidly. Estimates indicate that 285 million people worldwide had diabetes in 2010. This number represents 6% of the adult population aged between 20 and 79 years. It is expected that the prevalence will increase to nearly 8% in 2030, amounting to 438 million diagnosed patients [1].
The kidney frequently gets involved in type 2 diabetes, starting with signs of hyperfiltration followed by microalbuminuria (>30 mg of albumin in the urine per 24 hr). The presence of microalbuminuria occurs in 30% to 50% of patients with type 1 diabetes, and it is estimated to occur in approximately 20% to 30% of all patients with type 2 diabetes at the time of diagnosis. The progression to further involvement of the kidney is shown when urinary albumin excretion rises to macroalbuminuria (> 300 mg/24 hr), which occurs in ~3 % of the microalbuminuric type 2 diabetic patients per year. Patients with macroalbuminuria are at risk of progressive renal function decline and loss of hormonal functions that are regulated by the kidney. These functions include volume homeostasis, blood pressure, vitamin D metabolism, and erythropoietin production. Finally, ~10% of patients who survived death (mostly) from cardiovascular disease end up in dialysis or with transplantation.
The high risk for end-stage renal disease (ESRD) or premature mortality in patients with diabetes and nephropathy is illustrated in Figure 1. The incidence of ESRD is simulated by multiplying transition probabilities from normo- to micro- to macroalbuminuria and eventually to ESRD. For this purpose, data are used from different landmark trials in type 2 diabetic patients with normoalbuminuria (BENEDICT and ROADMAP), microalbuminuria (IRMA-2), and macroalbuminuria (RENAAL and IDNT). Figure 1 illustrates that the risk for ESRD or death is particularly high in subjects with macroalbuminuria and nephropathy. In many parts of the world, renal replacement programs are not routinely available for all patients. Therefore, these patients die when they enter ESRD. Comparing these figures with the average annual rate for all kinds of cancer, it becomes clear that the mortality risk of patients with diabetes and nephropathy is higher than the average mortality rate of all types of cancer.
Figure 1.

Upper part: comparison of death rates of patients with diabetes at early and advanced stages of diabetic nephropathy. In the absence of dialysis or renal transplantation, patients with end-stage-renal-disease (ESRD) would die. Cancer data are from the US Cancer Institute Surveillance Epidemiology and End Results (SEER) database (1996-2003). Bottom part: comparison of the effect of drug intervention in the RAAS system on ESRD at different stages of type 2 diabetes and kidney disease. Early intervention appears to be most beneficial in reducing the risk of ESRD.
The increasing prevalence of diabetes in combination with the relentlessly progressive course of diabetic complications, despite optimal treatment, emphasizes the need for novel treatment options. This is a challenging task as evidenced by a lack of new treatment strategies since the introduction of angiotensin II receptor blockers (ARBs), more than a decade ago. Fortunately, various novel therapeutic options are currently in development. This article reviews current treatment options and provides an overview of novel therapeutic agents that hold great potential for the treatment of diabetic kidney disease.
Current drugs to prevent the progression of renal disease
Glycemic control
Achieving optimal glucose control is essential to delay the progression of renal complications. A meta-analysis of small clinical studies showed that intensified HbA1c targeting reduced the relative risk of diabetic nephropathy by 66% [2]. These results were subsequently confirmed in a larger type 1 diabetic population by the DCCT trial [3], and in the type 2 diabetic population by the UKPDS trial [4]. The recent ADVANCE trial examined a more contemporary type 2 diabetic population and found similar results [5].
The consistent association between HbA1c and renal and cardiovascular outcomes, in combination with beneficial results of randomized controlled trials targeting HbA1c, has prompted the initiation of new trials investigating whether targeting HbA1c levels of below 6% would further delay renal and cardiovascular disease progression. The ACCORD trial investigated this question in 10,251 patients but the trial was terminated early after a median follow-up of 3.4 years because of excess mortality in the intensive therapy group [6]. At the time of discontinuation, the rate of cardiovascular events was not significantly different between the intensively and conventionally HbA1c-targeted treatment group (6.9% intensive versus 7.9% conventional therapy group). Similarly, in the Veteran Affairs Diabetes Trial (VADT), no significant reduction in the risk of developing micro- or macrovascular complications was observed with very strict HbA1c control in 1791 type 2 diabetic patients [7].
Extensive post-hoc analyses have been conducted to identify an explanation for the unexpected higher mortality rates in ACCORD. It has been suggested that drug-induced increases in body weight and fluid retention caused the excess mortality. Furthermore, frequent hypoglycemic episodes during very strict glycemic control may have occurred. Indeed, post-hoc analyses from the ADVANCE trial showed that severe hypoglycemia significantly increased the adjusted risks of macrovascular events [8]. These recent experiences have taught us that the prevention of progressive renal function loss is only achievable through the development of novel therapeutic agents that omit episodes of hypoglycemia and other side-effects that potentially counteract any beneficial effects.
Blood pressure
Conventional blood pressure control. A range of studies in patients with diabetes have shown that optimal blood pressure control decelerates the progression of nephropathy [9, 10]. Parving et al. showed that strict blood pressure control results in a two-fold reduction in albuminuria in combination with a more than two-fold decrease in the rate of renal function decline (reduction in the decline of glomerular filtration rate (GFR) from 0.9 ml/min/month to 0.3 ml/min/month).
Interventions in the renin-angiotensin-aldosteron-system (RAAS). Although optimal blood pressure control is important, identifying drugs that lower blood preassur is critical when targeting renoprotection. Some classes of drugs exert specific renoprotective effects not observed with other drug classes. It appears that agents that directly intervene in the RAAS have outstanding renoprotective effects. The BENEDICT, IRMA-2, and recent ROADMAP trials have shown that blockade of the RAAS in early stages of renal disease is a beneficial intervention [11-13].
Large-scale prospective trials in patients with type 2 diabetes and overt nephropathy (RENAAL and IDNT) have shown that intervention with an ARB is effective in preventing the transition from overt nephropathy to ESRD [14, 15]. The beneficial effects of angiotensin-converting enzyme inhibitors (ACEi) and ARB are largely attributable to their anti-albuminuric effect. Post-hoc analyses from the RENAAL and IDNT trials showed that the ARB-induced reduction in albuminuria explained most of the long-term renal and cardioprotective effects of ARBs in patients with late-stage diabetes and nephropathy [16, 17]. A new analysis from the IRMA-2 trial confirmed these results in diabetic patients with earlier stages of nephropathy [18]. Despite these promising results from intervention in RAAS, the absolute residual risk for advancing into end-stage renal disease is still extremely high, as is shown in Figure 1. Assuming that ESRD is equivalent to death in the absence of dialysis or transplantation, then the risk for dying is double that of death from treated cancer (Figure 1). Thus, additional protective treatments are clearly needed.
Combination of drugs. Based on the idea that a combination of ACEi and ARB would result in more effective inhibition of the RAAS, large-scale trials have been initiated and conducted to test whether this strategy reduces the progression of renal disease. The ONTARGET trial was conducted in a population at high cardiovascular risk treated by a combination of an ACEi and an ARB. Unexpectedly, it showed that the treatment increased the risk of renal and cardiovascular disease and adverse events, despite an additional albuminuria-lowering effect [19, 20]. An explanation for this unexpected finding is not available, but it has been postulated that the increase in hyperkalemic events and hypotensive periods may have counteracted the protective blood pressure- and albuminuria-lowering effects [21]. Results from future trials such as the LIRICO [22] and VA-NEPHRON-D [23] will clarify the value of dual-agent RAAS blockade in other populations including those with diabetic nephropathy.
The combination of an ACEi or an ARB with diuretic therapy has been investigated to reduce the risk of diabetic nephropathy and ESRD. The rationale of this strategy is based on the idea that diuretic therapy potentiates the beneficial effects of ACEi and ARB therapy. Indeed, it has been shown that combining a diuretic agent with half-dose ACEi and ARB lowers blood pressure and albuminuria more effectively than a combination of full-dose ACEi and ARB [24]. The ADVANCE trial showed that the combination of an ACEi (perindopril) with a diuretic agent (indapamide) significantly reduces blood pressure and the risk of renal and cardiovascular complications compared with placebo therapy in 11,140 type 2 diabetic patients [25]. Unfortunately, no comparison was made between ACEi alone versus the ACEi/diuretic combination in this trial.
Bakris et al. compared the blood pressure- and albuminuria-lowering effects of hydrochlorathiazide versus amlodipine in combination with ACEi therapy. In a 6 month trial, it was shown that combining hydrochlorothiazide with ACEi is more effective in reducing albuminuria than adding amlodipine [26]. However, the long-term randomized trial (ACCOMPLISH) that followed this relatively small study reported contradictory results. Despite the larger reduction in albuminuria seen in combination with hydrochlorothiazide, the rate of renal events was significantly higher compared with the combination with amlodipine [27].
Based on these results, one could question the validity of albuminuria as a surrogate for renal outcomes. However, flaws have been described regarding analysis and interpretation of the ACCOMPLISH data. Therefore, the question of whether the combination with hydrochlorothiazide confers specific renoprotective effects is still not adequately addressed [28].
Lipid metabolism
Cholesterol-lowering has undoubtedly contributed to reducing the risk of cardiovascular events in various patient populations, including the diabetic population. Whether cholesterol-lowering therapies reduce the risk of renal events has been a subject of debate for many years. Meta-analyses of initial studies have reported significant reductions in proteinuria in chronic kidney disease patients or patients with micro- or microalbuminuria. However, the effect appeared to be very heterogeneous for different statins [29]. Recently, the SHARP trial showed that the risk of major vascular events in individuals with chronic kidney disease (randomly assigned to simvastatin/ezetimibe or placebo) was markedly reduced by 16% [30]. However, in the subgroup of 6247 individuals who did not require dialysis at study entry, simvastatin/ezetimibe did not lower the risk of ESRD. The lack of a reduction in the risk of ESRD is concordant with the notion that the combination of simvastatin/ezetimibe did not reduce albuminuria in the SHARP trial [31].
Can the SHARP-trial results be generalized to each statin or do different statins exert different renoprotective effects? The PLANET trials gave more insight into this question. The PLANET I (diabetics) and II (non-diabetics) trials compared the effects of atorvastatin and rosuvastatin on renal parameters at equal lipid-lowering doses. The trial showed that atorvastatin reduced proteinuria in diabetic and non-diabetic subjects during a one-year treatment, but did not considerably change the estimated glomerular filtration rate (eGFR) over time [32]. In contrast, rosuvastatin did not change proteinuria, but was associated with a significant reduction in eGFR. This illustrates that different statins may have different renal effects, and suggests that the SHARP results cannot be extrapolated directly to each statin. In line with the PLANET results is a post-hoc analysis from the ASCOT lipid-lowering arm. In ASCOT, atorvastatin significantly improved eGFR over time compared with placebo treatment in 10,305 subjects at cardiovascular risk [33]. Unfortunately, no adequate analysis was performed in this study to investigate whether the slower rate in eGFR decline with atorvastatin could be attributed to its anti-albuminuric effect. In conclusion, these trials illustrate that different statins may have different effects on renal function.
Not only statins, but also fenofibrates, which also intervene in lipid metabolism, appear to have renoprotective effects in patients with diabetes. A post-hoc analysis from the FIELD trial showed that fenofibrate reduced albuminuria after 2 years of follow-up (first post-randomization measurement). This was accompanied by a slower decline in eGFR during the 5 years of follow-up versus placebo [34]. Detailed analyses should be conducted to assess whether the reduction in albuminuria or triglyceride/LDL-cholesterol was the primary driver for renoprotection. Given the accumulating body of evidence indicating that any drug that reduces albuminuria slows renal function decline, it is tempting to speculate that the reduction in albuminuria was the primary determinant of renoprotection.
New and future drugs to prevent the progression of renal disease
Although optimizing glucose, blood pressure, and lipid control has been the favored treatment of the last few decades, it has made a relatively small contribution to the prevention of renal disease. The residual risk of type 2 diabetic patients still remains extremely high even with optimal care. Several novel approaches aimed at halting the progression of renal disease are currently being tested in clinical trials, as outlined in Table 1 and reviewed in more detail below.
Table 1. Potential approaches and trials on additive renal and cardiovascular protection therapy.
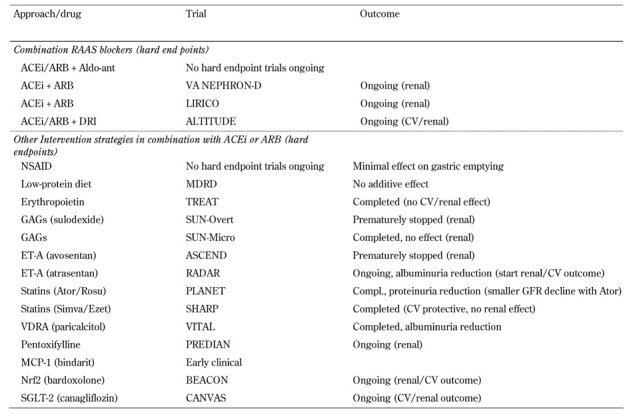
Legend: ACEi : angiotensin-converting enzyme inhibitor. Aldo-ant: aldosterone antagonist. ALTITUDE: Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints. ARB: angiotensin II receptor blocker. ASCEND: A Study of Cardiovascular Events iN Diabetes. Ator: atorvastatin. BEACON: Bardoxolone methyl EvAluation in patients with Chronic kidney disease and type 2 diabetes: the Occurrence of renal eveNts. CANVAS: Canagliflozin Cardiovascular Assessment Study. CV: cardiovascular. DRI: direct renin inhibition. ET-A: endothelin type Ezet: ezetimibe. A. GAG: glycosaminoglycan. GFR: glomerular filtration rate. LIRICO: Long-term Impact of RAS Inhibition on Cardiorenal Outcomes. MCP-1: monocyte chemoattractant protein-1. MDRD: Modification of Diet in Renal Disease. Nrf2: nuclear factor (erythroid-derived 2)-like 2, also known as NFE2L2. NSAID: nonsteroidal anti-inflammatory drug. PLANET: Prospective Evaluation of Proteinuria and Renal Function in Diabetic Patients With Progressive Renal Disease Trial. PREDIAN: Pentoxifylline for Renoprotection in Diabetic Nephropathy. RADAR: Reducing residual Albuminuria in subjects with type 2 Diabetes and nephropathy with Atrasentan. Rosu: rosuvastatin. SGLT-2: sodium glucose cotransporter 2. SHARP: Study of Heart and Renal Protection. Simva: simvastatin. SUN-Micro: Sulodexide Microalbuminuria. SUN-Overt: Sulodexide in Overt Type 2 Diabetes Patients. TREAT: Trial to Reduce Cardiovascular Events with Aranesp Therapy. VA NEPHRON-D: NEPHROpathy iN Diabetes. VDRA: vitamin D receptor activators. VITAL: Selective Vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes.
Glycemic control
Dipeptyl-peptidase-4 inhibitiors. Binding of glucagon-like peptide 1 (GLP-1) to its receptor enhances insulin secretion in the pancreas and reduces glucagon release. Secretion of GLP-1 is impaired in patients with type 2 diabetes. The recognition of GLP-1 disturbances in patients with type 2 diabetes has initiated the development of GLP-1 analogues. In vivo, GLP-1 is rapidly degraded by dipeptyl peptidase-4 (DPP-4). Thus, direct infusion of GLP-1 is not possible for clinical intervention because of its very short half-life. To overcome this problem, DPP-4-resistant GLP-1 analogues have been manufactured and successfully applied in clinical practice. The GLP-1 receptor can also be activated by the inhibition of DPP-4, which decreases GLP-1 degradation. Various DPP-4 inhibitors have been developed and become available for clinical use such as sitagliptin and saxagliptin. Other DPP-4 inhibitors such as linagliptin and alogliptin are still in development and currently being assessed in phase 3 clinical trials (NCT01243424 and NCT00968708).
The beneficial effects of DPP-4-inhibitors may go beyond improving blood glucose control. A small Japanese study showed that six months treatment with the DPP-4 inhibitor sitagliptin reduced albuminuria by approximately 20% [35]. This off-target effect was accompanied by a reduction in systolic blood pressure of 11 mmHg and a 0.7% reduction in HbA1c. The mechanisms causing the reduction of albuminuria are not well understood, but it may be possible that DPP-4 inhibition suppresses inflammatory markers and improves endothelial function, resulting in reduced urinary albumin excretion. Indeed, in a recent mechanistic study with the DPP-4 inhibitor vildagliptin, improvements in endothelial function were observed [36]. However, further well-designed randomized placebo-controlled trials are needed to confirm the albuminuria-lowering effect of DPP-4 inhibitors. If true, DPP-4 inhibitors could be powerful renoprotective agents with a range of applications beyond their on-target glucose-related effects.
Finally, DPP-4 inhibitors currently in development seem to exert similar effects on HbA1c. However, pharmacokinetic properties vary among different agents which could make a specific compound particularly useful for a certain sub-population. For example, linagliptin is mainly metabolized and eliminated by the liver, making it particularly useful for a diabetic patient with nephropathy [37].
Sodium glucose transport 2 inhibitors. The sodium glucose cotransporter 2 (SGLT-2) located in the proximal tubule of the kidney is an effective transporter system that is responsible for the nearly complete reabsorption of glucose to maintain appropriate glucose levels. Each glucose molecule that is reabsorbed is accompanied by reabsorption of a sodium molecule in a 1:2 ratio. SGLT-2 inhibitors inhibit the SGLT-2 transporter, and this leads to enhanced glucose and sodium excretion and a reduction in plasma glucose and HbA1c. Bailey et al. showed that dapagliflozin in doses up to 10 mg/day combined with metformin (≥ 1500 mg/day) reduced HbA1c by up to 0.84% relative to placebo [38].
Furthermore, trends towards increases in sodium excretion and hematocrit, and declines in body weight and blood pressure have been noted during SGLT-2 inhibition [38]. These additional (off-target) effects could well be the result of combined proximal inhibition of glucose and sodium resulting in enhanced natriuresis and diuresis. Prospective studies, designed to investigate the mechanisms of blood pressure and body weight reductions during SGLT-2 inhibition, are needed to clarify these mechanisms. As described above, volume correction measures, by means of diuretic treatment, potentiate the blood pressure- and albuminuria-lowering effects of ACEis and ARBs. As most patients with diabetes are treated either with ACEi or ARB therapy, SGLT-2 inhibition may, just like diuretics, potentiate the blood pressure- and albuminuria-lowering effects of ACEi and ARB because of their natriuretic/diuretic effects. These beneficial effects may occur in addition to the glucose- and body weight-lowering effects of SGLT-2 inhibitors.
Despite the beneficial effects of SGLT-2 inhibition on cardiovascular risk markers, recent safety concerns have been expressed with the use of the SGLT-2 inhibitor (dapagliflozin); most notably is a non-significant increased risk of bladder and breast cancer. Given the lack of statistical significance, it is questionable whether this increased risk is a true finding or merely a coincidental. It has been suggested that, in cases of undiagnosed bladder cancer, increased glucose in the urine (secondary to SGLT-2 inhibition) elicits urinary tract infections that in turn increase the likelihood that patients visit their doctor and are diagnosed with bladder cancer after a routine urine examination [39]. Clearly, long-term follow-up data are needed to clarify these safety concerns. Phase III trials with the SGLT-2 inhibitors canagliflozin (CANVAS; NCT01032629) and empagliflozin (NCT01131676) are currently ongoing. These trials will provide more insight into the long-term renal and cardiovascular protective effects of SGLT-2 inhibitors.
Blood pressure
Direct renin inhibition. Renin-angiotensin-aldosterone-system inhibition (RAASi) by means of ACEi or ARB causes a compensatory increase in plasma renin activity which may lead to activation of the RAAS and blunt the efficacy of ACEi or ARBs. Direct renin inhibition (DRI) has therefore been considered a logical target to enhance the effects of ACEi or ARBs. Indeed, the Aliskiren in the eValuation of prOteinuria in Diabetes (AVOID) trial in 599 subjects with type 2 diabetes and nephropathy showed that combining the DRI aliskiren 300 mg/day with losartan 100 mg/day further reduced albuminuria by 20% [40]. This effect appears to be independent of changes in blood pressure observed in the placebo and aliskiren treatment arms. The currently ongoing ALTITUDE trial determines whether treatment with aliskiren prevents renal and cardiovascular events in addition to the maximum recommended ACEi or ARB therapy. Furthermore, it will provide additional information on the long-term efficacy and safety of direct renin inhibition [41].
Mineralocorticoid receptor blockade (MRB). RAAS-activation causes sodium and water retention that is mediated by aldosterone. Aldosterone was thought to have a primary role in maintaining the sodium and potassium balance, but during the last decade, it has become increasingly clear that aldosterone exerts deleterious effects on renal and vascular tissue. Aldosterone promotes inflammatory processes, increases fibrosis, and causes podocyte injury leading to albuminuria and renal dysfunction [42].
Inhibition of aldosterone has been shown to exert beneficial effects on albuminuria. For example, Mehdi et al. investigated the comparative anti-albuminuric effects of combining spironolactone or losartan with lisinopril. They showed that, after 48 weeks treatment, the combination with spironolactone 25 mg/day resulted in a 34% reduction in albuminuria; twice as high as the combination of losartan with lisinopril [43]. Despite these encouraging results a note of caution is required. MRB with spironolactone or eplerenone is associated with a high incidence of hyperkalemia, especially in patients with diabetes and/or nephropathy [44]. The development of hyperkalemia is in turn associated with a higher risk of developing ESRD [45]. Thus, close potassium monitoring and appropriate treatment is warranted when combining a MRB with the therapeutic regimen.
Measures to manage hyperkalemia include limiting potassium rich food consumption, discontinuation of drugs that impair potassium excretion (e.g. non-steroidal anti-inflammatory drugs), or prescribing potassium binders. With respect to the latter, a new non-absorbable polymer (RLY5016), designed to bind potassium in the gastrointestinal tract, was recently tested in patients with heart failure [46]. The results indicated that this polymer caused significant reductions in serum potassium, prevented hyperkalemia, and was well tolerated. Long-term studies are needed to characterize the long-term safety of this therapy, but at least these results suggest that RLY5016 is an attractable candidate to improve the management of hyperkalemia in patients with diabetic nephropathy in the future. This would then lead to studies that test the long-term renal effects of combining MRBs with current renal protective treatments.
Other targets
Vitamin-D receptor activation. Approximately 25 years ago Resnick et al. reported that serum levels of 25-vitamin D are inversely associated with plasma renin activity in normal and hypertensive subjects, suggesting an interaction between the RAAS and vitamin D axes [47]. It was only in 1998 that Schwartz and co-workers found that 1,25 Vitamin D reduced albuminuria in sub-totally nephrectomized rats [48]. Since then, there has been mounting evidence that Vitamin D receptor activation has a potent inhibitory effect on RAAS. This is likely induced by suppressing renin biosynthesis via direct inhibition of renin gene expression [49].
A post-hoc analysis of three combined trials assessed the effects of the Vitamin D receptor activator, paricalcitol, in patients with secondary hyperparathyroidism. The analysis demonstrated that a larger proportion of patients treated with paricalcitol had a reduction in proteinuria, measured by dipstick methodology, compared with placebo treatment [50]. The effects appeared to be independent of concomitant RAAS blockade. More recently, the results of the VITAL study were published. This trial was specifically designed to investigate the anti-albuminuric properties of 1µg or 2µg paricalcitol versus placebo in patients with diabetes and nephropathy [51]. The results showed that paricalcitol in addition to ACEi or ARB therapy reduced albuminuria in a dose-dependent fashion and was well tolerated [52]. Whether paricalcitol delays the progression to ESRD was not determined, and still needs to be demonstrated.
Endothelin blockade. The endothelin system is chronically activated in patients with diabetic nephropathy. Binding of endothelin to the endothelin type A receptor (ETA receptor) elicits pronounced vasoconstriction, sodium retention, and promotes podocyte dysfunction leading to glomerular damage, proteinuria, and renal function loss. Endothelin type B receptor activation counteracts the downstream effects of ETA receptor activation, and causes vasodilatation and sodium excretion [53]. Specific blockade of the ETA receptor, but not the ETB receptor, may thus be a promising target to ameliorate diabetes-related renal complications.
Avosentan, an endothelin receptor blocker, significantly reduced proteinuria in patients with type 2 diabetes and nephropathy [54]. However, a large phase 3 trial, which tested the effect of avosentan on hard renal outcomes (ASCEND), was terminated prematurely because of an excess of drug-related adverse events in the avosentan treatment arm, including a high incidence of fluid retention [55]. Despite the selectivity of avosentan for the ETA receptor (ETA:ETB = 50:1), the high dose of avosentan used in this trial may have partially blocked the ETB receptor, resulting in the high incidence of fluid retention.
Recently, the anti-albuminuric effects of atrasentan, another ETA receptor antagonist, were reported in patients with diabetic nephropathy [56]. In this randomized, controlled double-blind trial, 89 subjects who were on stable doses of RAASi were treated with atrasentan for 8 weeks. The results showed significant reductions in albuminuria from baseline for subjects receiving 0.75 mg/day atrasentan (42% reduction, p = 0.023) and 1.75 mg/day (36% reduction, p = 0.073) compared to placebo (11% reduction). Whereas, subjects receiving 0.25 mg/day had a non-significant albuminuria reduction of 21% (p = 0.291) [56]. Pre-clinical studies demonstrate that atrasentan has a higher selectivity for the ETA receptor (ETA:ETB = 1800:1) compared to avosentan. Theoretically, this could mean that adverse events related to edema are less likely to happen with atrasentan. The larger RADAR trial, which is currently ongoing (NCT01356849), will further characterize the efficacy and safety of atrasentan in patients with diabetes and nephropathy.
Anti-inflammatory drugs. Recently, more and more evidence indicates an important role of underlying inflammatory processes in the pathogenesis of diabetic nephropathy. Consequently, research in anti-inflammatory strategies may offer effective approaches to prevent the progression of renal disease. Pentoxyfilline is a methylxanthine derivative that acts in vivo as a phosphodiesterase inhibitor. A number of clinical studies have assessed the anti-albuminuric properties of pentoxifylline. A combined meta-analysis of these trials documented that oral pentoxifylline reduced albuminuria by nearly 300 mg/day versus control therapy [57]. Sub-group analysis showed that the anti-albuminuric effects were mainly present in subjects with macroalbuminuria, and were markedly reduced (and statistically non-significant) in subjects with microalbuminuria. The duration of follow-up in the previous studies was too short to assess the effects of pentoxifylline on progression of renal disease. The ongoing Pentoxifylline for Renoprotection in Diabetic Nephropathy (PREDIAN) trial will address whether 2-year treatment with pentoxifylline reduces the rate of progression to renal function loss in subjects with type 2 diabetes and nephropathy. The results of this trial are expected in 2012.
Emerging data show that monocyte chemoattractant protein-1 (MCP-1), a potent cytokine, plays a very important role in initiating and sustaining chronic inflammation in renal tissues. MCP-1 is secreted in response to high glucose concentrations. Also, MCP-1 attracts blood monocytes and macrophages through binding to the chemokine receptor 2, and it facilitates inflammation [58]. Urinary MCP-1 levels correlate with the degree of albuminuria in patients with diabetes. Interestingly, the reduction of albuminuria induced by ACEi correlates with the degree of urinary MCP-1 reduction [59]. These findings support the idea that inhibition of MCP-1 reduces albuminuria and improves long-term renal function. A prospective randomized controlled study showed that inhibition of MCP-1 synthesis reduced albuminuria in addition to ACEi or ARB therapy relative to placebo in subjects who had macroalbuminuria, but did not change albuminuria in subjects with microalbuminuria [60]. To the best of our knowledge, there are no ongoing studies investigating specific renal outcomes.
Finally, bardoxolone methyl is an anti-inflammatory drug which became available for clinical studies a few years ago. Bardoxolone methyl activates the Nrf2-Keap1 pathway, which results in inhibition of the pro-inflammatory cytokine nuclear factor κB (NF-κB). In a previous non-randomized clinical study, treatment with bardoxolone methyl for 8 weeks resulted in a significant increase in eGFR [61]. A subsequent 52-week follow-up study showed that the initial bardoxolone-methyl-induced rise in eGFR was sustained throughout the 52-week follow-up period [62]. A long-term rigid outcome study is currently ongoing (NCT 01351675), determining whether bardoxolone methyl truly delays the progression to ESRD.
Conclusions
Despite the successful use of lifestyle changes, metabolic control, and blood pressure control including ACEi and ARB therapy, the residual renal risk remains very high, leaving the diabetic population with a clear unmet need for novel treatment options. Novel treatments are imperative to improve the prognosis of these patients. As outlined in this review, various drugs are in development. They may offer additional renoprotection, and have the potential to reduce the high morbidity and mortality. Most of these strategies have only been tested as single addition to ACEi or ARB. It would be interesting to define the most favorable combination of these novel drugs to improve the outlook for this high risk population.
Disclosures: DDZ is consultant at Astra Zeneca, Abbott, Amgen, BMS, Hemocue, J&J, MSD, Novartis, Noxxon, and Reata. HLH is consultant at Abbott, J&J, Reata, and Vitae. All payments are directed to the authors' institution.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–1309. doi: 10.1016/0140-6736(93)90816-y. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr. et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S. et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen EB, Mogensen CE. Effect of antihypertensive treatment on urinary albumin excretion, glomerular filtration rate, and renal plasma flow in patients with essential hypertension. Scand J Clin Lab Invest. 1976;36:231–237. [PubMed] [Google Scholar]
- 10.Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet. 1983;1(8335):1175–1179. doi: 10.1016/s0140-6736(83)92462-5. [DOI] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M. et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 12.Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM. et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 13.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 14.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 15.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 17.Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, DeFerrari G, Drury P, Locatelli F, Wiegmann TB. et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Hellemons M, Persson F, Bakker SJ, Rossing P, Parving HH, De Zeeuw D, Lambers Heerspink HJ. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: a post hoc analysis of the IRMA-2 trial. Diabetes Care. 2011;34(9):2078–2083. doi: 10.2337/dc11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 20.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 21.Lambers Heerspink HJ, de Zeeuw D. Dual RAS therapy not ON TARGET, but fully alive. Nephron Clin Pract. 2011 doi: 10.1159/000315882. In press. [DOI] [PubMed] [Google Scholar]
- 22.Maione A, Nicolucci A, Craig JC, Tognoni G, Moschetta A, Palasciano G, Pugliese G, Procaccini DA, Gesualdo L, Pellegrini F. et al. Protocol of the Long-term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO) randomized trial. J Nephrol. 2007;20:646–655. [PubMed] [Google Scholar]
- 23.Fried LF, Duckworth W, Zhang JH, O'Connor T, Brophy M, Emanuele N, Huang GD, McCullough PA, Palevsky PM, Seliger S. et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D) Clin J Am Soc Nephrol. 2009;4:361–368. doi: 10.2215/CJN.03350708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esnault VL, Ekhlas A, Nguyen JM, Moranne O. Diuretic uptitration with half dose combined ACEI + ARB better decrease proteinuria than combined ACEI + ARB uptitration. Nephrol Dial Transplant. 2010;25(7):2218–2224. doi: 10.1093/ndt/gfp776. [DOI] [PubMed] [Google Scholar]
- 25.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 26.Bakris GL, Toto RD, McCullough PA, Rocha R, Purkayastha D, Davis P. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int. 2008;73:1303–1309. doi: 10.1038/ki.2008.102. [DOI] [PubMed] [Google Scholar]
- 27.Bakris GL, Sarafidis PA, Weir MR, Dahlof B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V. et al. Renal outcomes with different fixed-dose combination therapies in high-risk hypertensive patients. Lancet. 2010;375(9721):1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 28.Lambers Heerspink HJ, de Zeeuw D. Composite renal endpoints: was ACCOMPLISH accomplished? Lancet. 2010;375(9721):1140–1142. doi: 10.1016/S0140-6736(10)60098-0. [DOI] [PubMed] [Google Scholar]
- 29.Strippoli GF, Navaneethan SD, Johnson DW, Perkovic V, Pellegrini F, Nicolucci A, Craig JC. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis D, Baigent C, Landray MJ. Effects of simvastatin/ezetimibe on renal function. ERA-EDTA meeting; Prague. June 2010. [Google Scholar]
- 32.de Zeeuw D, Anzalone D, Cain V, Cressman M, Molitoris B, Monyak J, Parving HH. Different renal protective effects of atorvastatin and rosuvastatin in patients with proteinuric diabetic and non-diabetic renal disease; result from the PLANET Trials. ERA-EDTA meeting; Munich. June 2010. [Google Scholar]
- 33.Gupta K. The relationship between statin therapy and progression of renal damage among 10305 hypertensive patients randomised in the ascot-lipid-lowering arm. World Congress Nephrology meeting; Vancouver. April 2011. [Google Scholar]
- 34.Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O'Connell RL, Whiting MJ, Glasziou PP. et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011;54(2):280–290. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 35.Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J. 2011;58(1):69–73. doi: 10.1507/endocrj.k10e-382. [DOI] [PubMed] [Google Scholar]
- 36.van Poppel PC, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care. 2011;34(9):2072–2077. doi: 10.2337/dc10-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes Obes Metab. 2011;13(10):939–946. doi: 10.1111/j.1463-1326.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 38.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–2233. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 39.Jones D. Diabetes field cautiously upbeat despite possible setback for leading SGLT2 inhibitor. Nat Rev Drug Discov. 2011;10(9):645–646. doi: 10.1038/nrd3546. [DOI] [PubMed] [Google Scholar]
- 40.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 41.Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z. et al. Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant. 2009;24:1663–1671. doi: 10.1093/ndt/gfn721. [DOI] [PubMed] [Google Scholar]
- 42.Bertocchio JP, Warnock DG, Jaisser F. Mineralocorticoid receptor activation and blockade: an emerging paradigm in chronic kidney disease. Kidney Int. 2011;79(10):1051–1060. doi: 10.1038/ki.2011.48. [DOI] [PubMed] [Google Scholar]
- 43.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–2650. doi: 10.1681/ASN.2009070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Meiracker AH, Baggen RG, Pauli S, Lindemans A, Vulto AG, Poldermans D, Boomsma F. Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24:2285–2292. doi: 10.1097/01.hjh.0000249708.44016.5c. [DOI] [PubMed] [Google Scholar]
- 45.Miao Y, Dobre D, Heerspink HJ, Brenner BM, Cooper ME, Parving HH, Shahinfar S, Grobbee D, de Zeeuw D. Increased serum potassium affects renal outcomes: a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Diabetologia. 2011;54(1):44–50. doi: 10.1007/s00125-010-1922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E. Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int. 1998;53:1696–1705. doi: 10.1046/j.1523-1755.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 51.Lambers Heerspink HJ, Agarwal R, Coyne DW, Parving HH, Ritz E, Remuzzi G, Audhya P, Amdahl MJ, Andress DL, de Zeeuw D. The selective vitamin D receptor activator for albuminuria lowering (VITAL) study: study design and baseline characteristics. Am J Nephrol. 2009;30:280–286. doi: 10.1159/000225903. [DOI] [PubMed] [Google Scholar]
- 52.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 53.Kohan DE. Biology of endothelin receptors in the collecting duct. Kidney Int. 2009;76:481–486. doi: 10.1038/ki.2009.203. [DOI] [PubMed] [Google Scholar]
- 54.Wenzel RR, Littke T, Kuranoff S, Jurgens C, Bruck H, Ritz E, Philipp T, Mitchell A. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20:655–664. doi: 10.1681/ASN.2008050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22(4):763–772. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCormick BB, Sydor A, Akbari A, Fergusson D, Doucette S, Knoll G. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am J Kidney Dis. 2008;52:454–463. doi: 10.1053/j.ajkd.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 59.Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26:2421–2425. doi: 10.2337/diacare.26.8.2421. [DOI] [PubMed] [Google Scholar]
- 60.Ruggenenti P. Effects of MCP-1 inhibition by bindarit therapy in type 2 diabetes subjects with micro- or macroalbuminuria. American Society of Nephrology meeting; Denver. November 2010. [Google Scholar]
- 61.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011;33(5):469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 62.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]


