Abstract
Background: advance care planning (ACP) allows a patient to state their preferences for care, so that if in future they cannot make decisions their wishes are known. Our aim was to review systematically the effectiveness of ACP interventions in people with cognitive impairment and dementia.
Methods: systematic searches of key electronic databases, supplemented by hand searches of reference lists and consultation with experts. Two independent reviewers undertook screening, data extraction and quality assessment.
Results: four studies were included; three allocated providers randomly to intervention or control arm. All took place in nursing homes. Three studies reported formal processes of capacity assessment, only up to 36% of participants were judged to have capacity. Three studies reported positive findings in terms of documentation of patient preferences for care. Two studies reported significant reductions in hospitalisation rates; a third found increased use of hospice services in the intervention group. A meta-analysis could not be carried out due to heterogeneity of outcome measures.
Conclusions: there is limited evidence for the effectiveness of ACP in people with cognitive impairment/dementia in terms of ACP documentation and health-care use. In terms of capacity to discuss ACP, nursing home settings may be too late for people with dementia.
Keywords: systematic review, advance care planning, advance directives, dementia, nursing homes, elderly
Older people may wish to plan ahead, so that if in future they cannot make decisions, their wishes about their care will be known; this is especially relevant in dementia, where patients experience an extended period of mental incapacity but may retain physical health. Advance directives (ADs), or living wills are documents in which an adult could record wishes for future care, including refusal to receive certain treatments or interventions. More recently the broader concept of advance care planning (ACP), a multi-stage process whereby a patient and their carers achieve a shared understanding of their goals and preferences for future care has been introduced. Patients can document their wishes as advance statements (patient preferences for care), as ADs, also known as advance decisions to refuse treatment (ADRTs) and/or nominate a power of attorney to make decisions on their behalf should they lose mental capacity.
The implementation of ACP has been influenced by legislation in some countries such as the USA [1] and by policy in others, as in the UK [2]. Evidence shows that one to one discussions with a trained professional over a period of time are the most successful [3–8], but in practice ACP decisions may not be adequately disseminated to influence care [6]. People with dementia consistently receive suboptimal care at the end of life [9, 10]; ACP may thus provide an opportunity for more person-centred care [11], although such discussions should occur while the person still has capacity [12, 13]. This aim of this systematic review was to determine the effectiveness of ACP interventions in people with cognitive impairment and dementia and also to identify factors influencing the implementation of ACP in this area [11]; this paper presents the findings of the effectiveness review.
Methods
Search strategy and study selection criteria
Details of the electronic search strategy, search terms and selection criteria used are given in Appendix 1 (available as Supplementary data in Age and Ageing online); this was enhanced by hand searching reference lists of retrieved articles and grey literature, and consultation with experts [11]. Only studies reported in English were considered for inclusion.
The review followed recommended best practice [14]. Abstracts were scrutinised by two independent reviewers (C.D., N.R.); when high levels of agreement were achieved, screening was completed by one reviewer (C.D.). Disagreements were resolved through discussion or via a third reviewer (L.R.), with a presumption of inclusion in cases of doubt. Full text copies of the papers included were assessed via a similar process.
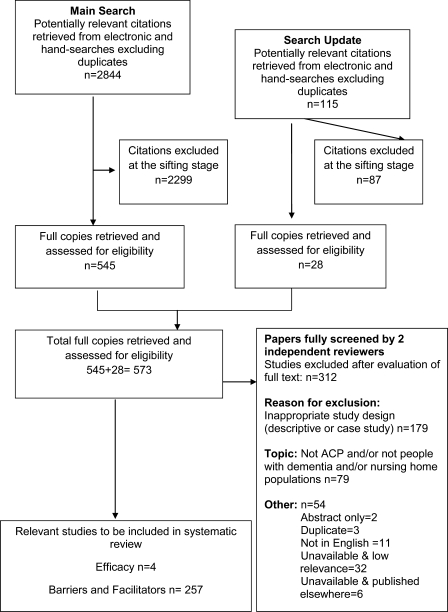
Figure 1 summarises the flow of studies through the review. The results of the second part of the review, factors which influence the implementation of ACP in practice, will be reported separately. An update was carried out in January 2010; no additional studies were found.
Figure 1.
Flow chart demonstrating study selection process.
Data extraction and quality assessment
Data extraction followed recommendations from the Cochrane Effective Practice and Organisation of Care (EPOC) Review Group [15]. Because the studies were expected to be heterogeneous in terms of the interventions, methods and outcomes, a detailed narrative of the key findings and results was also extracted. Two independent reviewers (N.R., C.D.) rated each study included in the review using the risk of bias criteria recommended by the EPOC Review Group [16].
Data synthesis
It was not possible to pool the results of the four included studies in a meta-analysis due to substantial differences in the methods and outcome measures employed, despite two of the studies evaluating similar interventions based on the Let Me Decide programme [17, 18]. A narrative summary of the outcomes of the included studies was constructed [14].
Results
The main characteristics and results of the included studies are summarised in Table 1.
Table 1.
Setting, study characteristics and key findings of included studies
| Study | Study design | Country | Setting | Sample | Intervention and professional involved | Primary outcome measure | ACP outcomes | Health-care outcomes | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Caplan et al. [17] | Controlled before and after study | Australia | Nursing homes (NHs) (n = 32) and hospitals (n = 3) | Nursing home residents (NHRs), their families, staff and general practitioners | Education for residents, families, staff and GPs | Not specified | Number of ACP referrals | Number of Emergency Department presentations and hospital admissions | ACP. In the first year, 63 NHRs referrals were received; 45 NHRs agreed to proceed with ACP. Five were judged to have capacity: discussions and decisions documented in their notes |
| Potential nursing home participants n = 1,344 | Number of ACP discussions | Number of emergency ambulance calls from all the nursing homes (NHs) | Of the 40 without capacity: 3 ‘persons responsible’ completed a plan of treatment, 20 NHRs had discussions and preferences were recorded in notes but no document was signed, 10 NHRs completed discussions but nothing formally documented, 6 persons responsible declined discussion of ACP, 1 NHR had previously completed ACP | ||||||
| Number of discussions that proceeded to a written form by either the resident or a proxy | Number of emergency and elective admissions to hospital and bed-days occupied by residents in hospital | Mortality. No significant change in mortality until the final third year when mortality rose in the control nursing homes (30.4 versus 41.6 deaths per 100 nursing home beds; P = 0.0425) | |||||||
| Number of deaths registered to the address of each NH | Health care use. Annual rate of admission of residents per nursing home bed initially higher in the intervention hospitals, but lower by the final year (0.865 versus 1.254; RR = 0.89; 95% CI: 0.85–0.93; P < 0.0001). Risk of a resident being in an intervention hospital bed for a day compared with control group fell by a quarter from being initially similar (RR = 1.01; 95% CI: 0.98–1.04; P = 0.442) to being lower (RR = 0.74; 95% CI 0.72–0.77; P ≤ 0.0001) | ||||||||
| Hanson et al. [19] | Controlled before and after study | USA | Nursing homes (n = 9) | Nursing home residents (n = 458) | Recruitment and training (in palliative care practices) of palliative care leadership in each facility | Not specified | Number of documented ACP discussions | Percentage of residents receiving hospice or palliative services, pain assessment, pain treatment among residents in pain | ACP. Documented ACP discussions were rare at baseline. Intervention NHRs had a significant increase in documented discussions from 4 to 17% (P < 0.001) |
| Two nursing homes were chosen at random for training | Number of orders on life-sustaining treatments, living wills and health-care powers of attorney | DNR orders increased slightly in intervention nursing homes (58–65%, P = 0.04), as did use of DNR flags. Living wills (31% versus 30%) and health-care powers of attorney (27% versus 33%) were not significantly changed | |||||||
| Patient health. Significant increase in the intervention group in pain assessment from 18 to 60% (P ≤ 0.001); no change in orders for pain medication | |||||||||
| Health-care use. Significant increase in hospice enrolment in the intervention group from 4.0% at baseline to 6.8% post intervention (P ≤ 0.01) | |||||||||
| Molloy et al. [18] | Randomised control trial | Canada | Nursing homes (n = 6) | Nursing home residents (n = 1,133) | Two days educational workshop for nurses in nursing homes who then trained other staff and counselled patients. Used Let Me Decide. Nurses carried out the discussions; Doctors reviewed and signed the forms | Residents’ and families’ satisfaction with health care and health-care utilisation over 18 months | Rates of completion of advance directives | Residents’ and families’ satisfaction with health care and | Satisfaction with healthcare. No significant differences between intervention and control homes |
| Six nursing homes were randomly allocated to the two trial arms | Health care utilisation over 18 months | ACP . Overall, 49% of participants completed ADs. At the end of the study, 70% of NHRs in the intervention group and 57% in the control group completed ADs. In the control homes 71% of ADs were DNR orders and in the intervention homes 89% of directives were Let Me Decide | |||||||
| Mortality. Similar in both intervention and control groups (24% versus 28%; P = 0.20) | |||||||||
| Health-care use. Mean hospitalisation rates per patient lower in the intervention group compared with the control (0.27 versus 0.48; P = 0.001) | |||||||||
| Average total cost per patient (health-care resource use) significantly less in the intervention group compared with the control group (CAD 3,490 versus CAD 5,239; P = 0.01); hospital costs per patient also less in the intervention group (CAD 1,772 versus CAD 3,869; P = 0.003). | |||||||||
| Morrison et al. [20] | Non-randomised controlled trial | USA | Nursing home | Nursing home residents (n = 139) | Education, using interactive methods, in ACP for intervention group social workers. Social workers (usual care) received a talk on state law and medical decision-making | Not specified | Nursing home chart documentation of advance directives and DNR orders | Concordance of treatments received with documented preferences | ACP. Intervention residents were more likely to have their preferences documented in nursing home chart than control group residents. Significant increases in documentation of patent preferences in the intervention group compared with control: cardio pulmonary resuscitation (40% versus 20%; P = 0.005); artificial nutrition and hydration (47 versus 9%; P ≤ 0.01); intravenous antibiotics (44 versus 9%; P = 0.01) and hospitalisation (49 versus 16%; P ≤ 0.01) |
| Four social workers were randomly allocated to two arms | Documentation of patient preferences for care | Health-care use. Control residents were significantly more likely than intervention residents to receive treatments discordant with their prior stated wishes (P = 0.04) |
*Int, intervention; **Con, control; ***CI, confidence interval; RR, relative risk.
Study characteristics
Four studies met the inclusion criteria for the efficacy review [17–20] (see Table 1). Two studies were from the USA [19, 20], one from Canada [18] and one from Australia [17]. All studies were in nursing home settings.
The mean age of participants varied from 78 to 87 years; one study did not report details of participants’ ages [17]. Although none of the four studies specifically targeted participants with dementia, two studies included people with a range of cognitive impairment from mild to severe [19, 20] and one included people with mild and/or moderate dementia, but excluded those with severe dementia [17].
Three studies reported formally assessing the capacity of nursing home residents to make advance care plans [17, 18, 20] (see Appendix 2; available as Supplementary data in Age and Ageing online). Two studies reported a two stage process undertaken by nurses with an initial screen to exclude residents with severe cognitive impairment [17, 18, 21]. In the third study, capacity was assessed by the social worker [20]. The proportion of participants who were judged to have capacity to make decisions varied upto a maximum proportion of 36% [18].
Methodological quality
The quality of the included studies was variable (see Appendix 3; available as Supplementary data in Age and Ageing online). In terms of methodological quality, studies which randomised had small numbers at the unit of randomisation level [18, 19]; although Molloy et al. included over 1,000 participants, this was a cluster randomised controlled trial comprising only six care homes. None of the four studies prospectively carried out a power calculation at the design stage.
Characteristics of the interventions
Two of the included studies evaluated a specific advance directive programme, ‘Let Me Decide’, with trained nurses providing education to nursing home residents and their families, as well as staff in participating nursing homes and hospitals [17, 18]. Caplan also trained the medical practitioners providing care to participating nursing homes [17]. Morrison et al. trained social workers in ACP, via small group workshops and role play sessions [20]. Hanson et al. trained palliative care teams to deliver educational sessions to nursing home staff [19].
Two of the studies focused solely on the implementation of ACP [18, 20]; in the other two studies, ACP was part of a wider implementation of hospital in the home or palliative care [17, 19].
Reported outcome of the interventions
Only one of the RCTs [18] specified a main outcome measure, ‘residents’ and families’ satisfaction with health care and health-care utilisation (see Table 1). Additional outcome measures focused on ACP outcomes, patient health and/or health-care use.
ACP outcomes
Three studies found the intervention led to an increase in the number of ACP-related written outputs [18–20]. Hanson et al. reported a slight, but significant, increase in Do Not Resuscitate (DNR) orders, but no significant change in the rate of living wills or power of attorney documents [19]. In Malloy's study, the completion of ADs was greater in the intervention group compared with the control although the level of significance was not reported [18]. Morrison et al. found significant increases in the intervention group in documentation of participants’ preferences for care (resuscitation, artificial nutrition, intravenous antibiotics and hospitalisation) recorded in their nursing home charts with respect to cardio-pulmonary [20].
Patient health
Only one study evaluated the effect of the ACP intervention on participant health: Hanson et al. found a significant increase in the intervention group in pain assessment, but no significant differences in the use of pain medication [19].
Health-care utilisation
Three of the studies provided empirical data on the effects of ACP intervention on health-care use [17–19]. In terms of significant effects, Molloy et al. (2000) found lower hospitalisation rates and hospital costs per patient in the intervention group than in the control [18]. Caplan et al., also found significant health-care resource reduction in the intervention nursing homes, compared with control, in the annual rate of hospital admissions, the use of hospital day beds and the number of calls to the ambulance service [17]. Hanson et al. found a significant increase in hospice use in the intervention group compared with the control [19].
Discussion
There is some evidence, albeit of variable quality, which shows that ACP has the potential to reduce inappropriate hospital admissions and health-care costs for people with cognitive impairment and dementia. Further high-quality research would strengthen the argument for ACP to become an evidence-based part of routine dementia care; however, it must be acknowledged that this is a difficult and challenging area in which to carry out randomised controlled trials. Equally important would be whether people with dementia and their families would chose to undertake ACP, and at which point in their illness, before it is presumed by professionals to be an essential part of good quality care for all [12].
A key limitation of this review is that only papers written in English were included; consequently important studies may have been excluded. Another difficulty was that papers were not always clear about the stage at which people with dementia were excluded. Siegert et al. stated that a diagnosis of dementia was a explicit exclusion criteria, then later specified ‘significant cognitive impairment’ [22] but did not clarify whether or not people with mild/moderate cognitive impairment were included: additional data could not be found. It should also be noted that in two of the studies [17, 19] significant outcomes may have been related to factors other than the ACP intervention for example, in the Caplan study (2006), ACP occurred alongside implementation of a hospital-in-the-home scheme to reduce hospitalisation [17].
In the UK, guidance recommends that in dementia ACP should be carried out in the earlier stages of the illness before capacity is lost [12], after which seeking proxy views to facilitate best interest decision-making is recommended. All of the studies included in this review were set in nursing home populations. In the three studies which formally assessed participants’ mental capacity, the majority of participants were deemed incapable of making their own health-care decisions; timing of ACP in dementia is thus important, with discussions started earlier, if possible, in the illness trajectory and reviewed on entry to a nursing home.
Key points.
ACP allows a patient to discuss and write down their preferences for care, so that if in future they cannot make decisions their wishes are known; in dementia ACP should be carried out before mental capacity is lost.
This systematic review identified four studies involving people with cognitive impairment/dementia; all were set in nursing homes.
Three studies formally assessed the capacity of participants to take part in ACP; proportions varied, with a maximum of only up to one-third judged to have the capacity to do so.
There is limited evidence of variable quality that ACP has the potential to positively influence patient preferences for future care and reduce hospitalisation rates.
Authors’ contribution
L.R. was principal investigator. Study concept and design: L.R., C.E., J.H., N.R. F.B. designed and conducted the searches. C.D. and N.R. were the principal reviewers carrying out screening, selection and data extraction, with assistance from L.R. Statistical support: D.H. All authors contributed to the interpretation of results, writing of the paper and have seen and approved the final manuscript.
Conflicts of interest
None declared.
Funding
This work was supported by a research grant (PB-PG-0807-11073) from the National Institute of Health Research for Patient Benefit Programme. This paper presents independent research commissioned by the National Institute for Health Research (NIHR); the views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
References
- 1.Patient Self Determination Act: 1990. (Accessed online 1 May 2011). Available at: http://www.americanbar.org/groups/public_education/resources/law_issues_for_consumers/patient_self_determination_act.html . [Google Scholar]
- 2.Department of Health. End of life care strategy: Promoting High Quality Care for All Adults at the End of Life. 2008. (Accessed online 1 May 2011). Available at: http://www.endoflifecareforadults.nhs.uk/publications/eolc-strategy .
- 3.Bravo G, Dubois M-F, Wagneur B. Assessing the effectiveness of interventions to promote advance directives among older adults: a systematic review and multi-level analysis. Soc Sci Med. 2008;67:1122–32. doi: 10.1016/j.socscimed.2008.06.006. doi:10.1016/j.socscimed.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Hanson L, Tulskey J, Danis M. Can clinical interventions change care at the end of life? Ann Int Med. 1997;126:381–8. doi: 10.7326/0003-4819-126-5-199703010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Jezewski M, Meeker M, Sessanna L, Finnell D. The effectiveness of interventions to increase advance directive completion rates. J Aging Health. 2007;19:519–35. doi: 10.1177/0898264307300198. doi:10.1177/0898264307300198. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: a systematic review. Ann Int Med. 2008;148:147–58. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 7.Patel RV, Sinuff T, Cook DJ. Influencing advance directive completion rates in non-terminally ill patients: a systematic review. J Crit Care. 2004;19:1–9. doi: 10.1016/j.jcrc.2004.02.002. doi:10.1016/j.jcrc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Ramsaroop SD, Reid M, Adelman RD. Completing an advance directive in the primary care setting: what do we need for success? Am Geriatric Soc. 2007;55:277–83. doi: 10.1111/j.1532-5415.2007.01065.x. doi:10.1111/j.1532-5415.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 9.Robinson L, Hughes JC, Daley S, et al. End-of-life care and dementia. Rev Clin Gerontol. 2005;15:135–48. doi:10.1017/S0959259806001833. [Google Scholar]
- 10.Sampson EL, Gould V, Lee D, Blanchard M. Differences in care received by patients with and without dementia who died during acute hospital admission: a retrospective case note study. Age Ageing. 2006;35:187–9. doi: 10.1093/ageing/afj025. doi:10.1093/ageing/afj025. [DOI] [PubMed] [Google Scholar]
- 11.Robinson AL, Bamford C, Beyer F, et al. Patient preferences for future care—how can advance care planning become embedded into dementia care: a study protocol. BMC Geriatric. 2010;10:1471–6. doi: 10.1186/1471-2318-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Clinical Excellence (NICE) Dementia: supporting people with Dementia and their carers in health and social care. 2006.
- 13.Mitchell SL, Kiely DK, Hamel MB. Dying with advanced dementia in the nursing home. Arch Int Med. 2004;164:321–6. doi: 10.1001/archinte.164.3.321. doi:10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Centre for Reviews and Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: University of York; 2009. [Google Scholar]
- 15.Cochrane Effective Practice and Organisation of Care(EPOC) Review Group. Data collection checklist. 2002. (Accessed online 1 May 2011). http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf .
- 16.Cochrane Effective Practice and Organisation of Care (EPOC) Review Group. Resources for Review Authors: 6.4. Risk of Bias. 2009. http://epoc.cochrane.org/epoc.cochrane.org/epoc-resources-reviews-authors. (1 April 2011, date last accessed)
- 17.Caplan G, Meller A, Squires B, Chan S, Willett W. Advance care planning and hospital in the nursing home. Age Ageing. 2006;35:581–5. doi: 10.1093/ageing/afl063. doi:10.1093/ageing/afl063. [DOI] [PubMed] [Google Scholar]
- 18.Molloy DW, Guyatt GH, Russo R, et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial [see comment] JAMA. 2000;283:1437–44. doi: 10.1001/jama.283.11.1437. doi:10.1001/jama.283.11.1437. [DOI] [PubMed] [Google Scholar]
- 19.Hanson LC, Reynolds KS, Henderson M, Pickard CG. A quality improvement intervention to increase palliative care in nursing homes. J Palliat Med. 2005;8:576–84. doi: 10.1089/jpm.2005.8.576. doi:10.1089/jpm.2005.8.576. [DOI] [PubMed] [Google Scholar]
- 20.Morrison RS, Chichin E, Carter J, Burack O, Lantz M, Meier DE. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatric Soc. 2005;53:290–4. doi: 10.1111/j.1532-5415.2005.53116.x. doi:10.1111/j.1532-5415.2005.53116.x. [DOI] [PubMed] [Google Scholar]
- 21.Molloy DW, Silberfeld M, Darzins P, et al. Measuring capacity to complete an advance directive. J Am Geriatric Soc. 1996;44:660–4. doi: 10.1111/j.1532-5415.1996.tb01828.x. [DOI] [PubMed] [Google Scholar]
- 22.Siegert EA, Clipp EC, Mulhausen P, Kochersberger G. Impact of advance directive videotape on patient comprehension and treatment preferences [see comment] Arch Fam Med. 1996;5:207–12. doi: 10.1001/archfami.5.4.207. doi:10.1001/archfami.5.4.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.