Abstract
Background: the rate of performance decline may influence the risk of disability or death.
Methods: for 4,182 Cardiovascular Health Study participants, we used multinomial Poisson log-linear models to assess the contribution of physical performance in 1998–99, and the rate of performance change between 1992–93 and 1998–99, to the risk of death or disability in 2005–06 in three domains: mobility, upper-extremity function (UEF) and activities of daily living (ADL). We evaluated performance in finger-tapping, grip strength, stride length, gait speed and chair stands separately and together for each outcome, adjusting for age, gender, race and years of disability in that outcome between 1992–93 and 1998–99.
Results: participants’ age averaged 79.4 in 1998–99; 1,901 died over 7 years. Compared with the lowest change quintile in stride length, the highest quintile had a 1.32 relative risk (RR) of ADL disability (95% CI: 1.16 –1.96) and a 1.27 RR of death (95% CI: 1.07 –1.51). The highest change quintile for grip strength increased the risk of ADL disability by 35% (95% CI: 1.13 –1.61) and death by 31% (95% CI: 1.16 –1.49), compared with the lowest quintile. The annual change in stride length and grip strength also predicted disability in mobility and UEF.
Conclusion: performance trajectories independently predict death and disability.
Keywords: ageing, motor skills, activities of daily living, disabled persons, mortality, elderly
Introduction
Cross-sectional measures of physical performance, such as gait speed, grip strength, stride length and the short physical performance battery (SPPB), have predicted disability and/or mortality in diverse older populations [1–7]. In the clinical setting, rate of change in performance may be more useful than a cross-sectional measurement, which could misclassify individuals into a high-risk category because of the short-term effects of illness or injury. Previous studies of the effects of performance change on disability and mortality have yielded mixed results. Among 586 subjects with a mean age of 79 at baseline, 3 separate measures of physical performance, but not their respective changes during the preceding year, predicted activities of daily living (ADL) disability 2 years later [8]. Controlling for baseline performance, objectively measured performance change has predicted mortality within 1 year [9] and 5 years [10], whereas self-reported mobility change in a large cohort of non-demented seniors failed to predict mortality [11]. We sought to clarify the relationship between the rate of decline in individual performance measures and long-term disability or death in a large, well-defined, population-based cohort of older men and women.
Methods
We used data from the Cardiovascular Health Study (CHS), an observational, population-based cohort study of persons aged 65 and over recruited from four communities in the USA: Sacramento County, CA; Allegheny County, PA; Forsyth County, NC and Washington County, MD. CHS enrolled 5,201 men and women between 1989 and 1990, and added an additional 687 African-Americans during the fifth study year (1992–93). Details of the study design are described elsewhere [12, 13].
Data collection
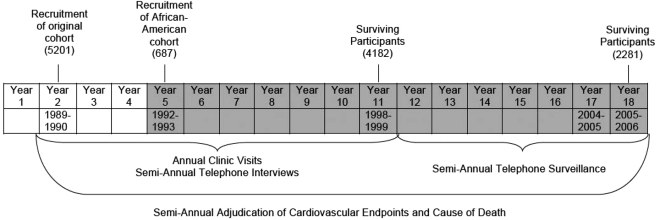
From enrolment through the 11th study year (1998–99), participants returned to the research clinic annually and underwent telephone interviews 6 months after each visit. During these encounters, technicians used standardised questionnaires to obtain a variety of personal and health characteristics. At the annual clinic visit participants underwent a battery of standardised physical performance tests, which included a timed walk, timed chair stands, grip strength and finger tapping. Limited semi-annual telephone surveillance for cardiovascular and functional outcomes has continued since 2000. Deaths were confirmed by reviewing medical records, death certificates and the Health Care Financing Administration database for hospitalisations. There has been 100% ascertainment of participant mortality. The schema for recruitment and data collection is shown in Figure 1.
Figure 1.
Schema for subject recruitment and data collection for the Cardiovascular Health Study. The shaded area denotes the years used for data analysis.
Performance measures
Grip strength
Forearm muscle strength was measured in kilograms by a hand-held Jamar A dynamometer. For this analysis, we used the best of three attempts in the dominant hand.
Timed walk
The time (to 0.1 s) required for a participant to walk a 4.6-m course at his or her usual pace after starting from a standstill was recorded by stopwatch. We converted the results to meters per second.
Number of steps to walk 4.6 m
A technician recorded the number of steps required to walk the 4.6-m course. Hereafter, we refer to stride length, which is derived by dividing the distance walked by the number of steps.
Timed chair stands
This test recorded how quickly the subject could perform five consecutive chair stands (timed to 0.1 s) from a 45-cm-tall chair with arms folded across the chest.
Finger-tapping test
Using their dominant hand, participants tapped on a computer mouse button with their index finger as fast as they could for 15 s.
Outcome measures
Our outcomes of interest were self-reported disability in study year 18 (2005–06) in basic ADL plus self-reported severe impairment in the performance of upper-body and mobility-related tasks. We defined ADL disability as any reported difficulty with one or more of the following: walking around the home, getting out of bed, eating, dressing, bathing or using the toilet. We defined upper-extremity disability as reported inability or great difficulty in lifting 4.5 kg, reaching or gripping. Mobility disability was defined as inability or great difficulty in walking half a mile (0.8 km). To be considered disabled, a participant was required to have a lot of difficulty or disability at both the Year 17 and Year 18 assessments (2004–05 and 2005–06).
Statistical methods
For our analyses, we included the 4,182 CHS participants who were alive at year 11 (1998–99) and had at least three values for each performance measure between years 5 and 11 (1992–93 and 1998–99). Because of varying patterns of missing data, we used linear mixed-effects models with random intercept and slope to estimate the annual rate of change between study years 5 and 11 for each performance measure of each participant [14]. Prior to selecting this approach, we plotted numerous individual trajectories for the five performance variables, using robust splines to smooth the curves. The consistent linearity of the trajectory patterns justified the use of linear models. Gait speed, stride length and chair stands were adjusted to a 50-cm knee-heel length and this adjustment was included in the models when it reached 10% significance. Year-11 values and change slopes were divided into quintiles to permit risk estimates to be linked to a range of performance or performance change. We used multinomial Poisson log-linear models to estimate the relative risk (RR) of disability relative to independence and death relative to independence at Year 18 (2005–06) for ADL. By providing a three-way outcome of death, disability or independence, multinomial models allowed us to use data from all 4,182 participants who were alive at Year 11, including the 1,901 participants who died between years 11 and 18, thereby maximising our sample size. They also allowed us to compare the RRs of death and disability for a given outcome measure. We used similar statistical models to estimate the RR of having a lot of difficulty or inability (relative to no or mild difficulty) and the RR of death (relative to no or mild difficulty) at Year 18 for walking 0.8 km and upper-extremity function (UEF). For a given performance measure, the fitted Year 11 value and the fitted Year 5–11 slope of change were treated as separate predictor variables. For each outcome, a separate regression model was run for each predictor performance variable, adjusting for age, race (black versus non-black), gender, and the number of years of much difficulty with, or disability in, the outcome measure between Years 5 and 11. The sample size for each outcome was determined by the number of survivors to year 18 who had outcome data for both years 17 and 18, plus the number of participants who died between years 11 and 18. Next, we simultaneously entered all predictor performance variables into a second set of models, adjusting for the same covariates. The component variables from each model were entered, in turn, into a stepwise backwards regression for the respective outcomes, with a P-value to enter the model set at <0.10. This procedure yielded a set of simpler, more parsimonious final models.
Results
During 1998–99, the participants’ mean age was 79.4 years, and 63.5% reported good to excellent health despite their burden of co-morbidity (Table 1). The evaluable sample for performance measures ranged from 2,647 for chair stands to 3,609 for grip strength. Participants excluded from analyses because they had fewer than three values for a performance measure tended to be older, female, have greater ADL impairment, and report fair to poor (versus good to excellent) health. Between years 11 and 18, 1,901 participants died.
Table 1.
Characteristics of the study cohort at Study Year 11 (1998–99)
| Women | Men | |
|---|---|---|
| Totals | 2,616 | 1,566 |
| Age ± SD | 79.3 ± 4.8 | 79.5 ± 4.7 |
| Number of blacks (%) | 473 (18.1) | 235 (15.0) |
| Self-reported healtha | ||
| Very good to excellent (%) | 555 (23.1) | 403 (27.8) |
| Good (%) | 1092 (45.4) | 611 (42.1) |
| Fair poor (%) | 759 (31.5) | 438 (30.1) |
| Hypertension (%) | 1451 (69.5) | 776 (60.8) |
| Diabetes mellitus (%) | 253 (13.3) | 188 (15.7) |
| Coronary heart disease (%) | 638 (24.4) | 569 (36.3) |
| Congestive heart failure (%) | 311 (11.9) | 244 (15.6) |
| Mini-Mental State Exam Score (100-point scale) | 88.6 ± 15.2 | 88.8 ± 14.0 |
| Number impaired in | ||
| ADL (%) | 656 (29.5) | 296 (21.9) |
| IADL (%) | 989 (44.6) | 430 (31.8) |
| Fitted performance measureb | ||
| Finger tapping (no. in 15 s) | 56.87 ± 8.23 | 61.6 ± 8.66 |
| Grip strength (kg) | 20.42 ± 5.24 | 34.18 ± 7.73 |
| No. steps to walk 4.6 mc | 10.61 ± 3.37 | 9.74 ± 2.66 |
| Gait speed (m/s)c | 0.83 ± 0.21 | 0.87 ± 0.20 |
| Time to complete five chair stands (s)c | 16.9 ± 6.23 | 16.07 ± 5.62 |
aEvaluable sample size = 2,606 for women, 1,452 for men.
bEstimated by linear mixed-effects models.
cStandardised to a 50 cm knee-heel length.
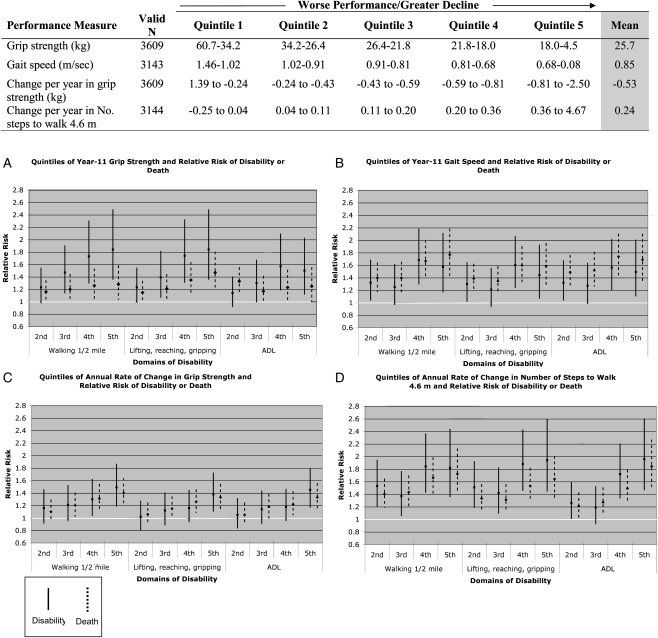
In the prediction models for individual performance measures (not shown), the point estimates generally demonstrated a reduced risk of impairment and mortality for higher Year-11 performance or a lower rate of performance change from years 5 to 11. In the final adjusted models, in which all the performance measures and their slopes of change were entered in a step-wise fashion, the time to complete five chair stands, finger-tapping speed and stride length at Year 11, as well as the slopes of change for chair stands and finger-tapping, failed to enter at a significance of P < 0.10. Compared with the first (strongest) quintile, higher (weaker) quintiles of Year-11 grip strength independently predicted disability at year 18 in all three domains, with similar point estimates for walking 0.8 km and UEF. Weaker grip strength also predicted mortality (Figure 2A). Higher (slower) quintiles of gait speed similarly were associated with a greater RR of disability or death for each outcome, compared with the first (fastest) quintile (Figure 2B). The change slope for gait speed was not associated with any outcome. The highest quintile for the rate of change in grip strength, corresponding to a decline of 0.81–2.50 kg per year, predicted impairment and mortality across the three domains (Figure 2C). Year-11 stride length failed to enter the model. However, the fourth and fifth quintiles for the rate of change in stride length, corresponding to an annual rate of increase of 0.2–4.7 steps to walk a 4.6-m course, predicted disability and mortality for each outcome (Figure 2D).
Figure 2.
The independent contribution of quintiles of physical performance to the relative risk of sustained disability or death at Year 18, compared with the lowest (first) quintile (not shown), in four functional domains. Adjusted for age at Year 5, race (black versus non-black), gender and the number of years of much difficulty with, or dependence in, the outcome measure between Years 5 and 11. All performance measures assessed together. In the figure, the 95% confidence intervals for disability and death are represented by solid and dashed lines, respectively. The range of values that corresponds to each quintile of the performance measures is shown in the table above.
To evaluate whether chronic illness may have mediated the impact of performance change on the outcomes, we re-ran the final models, adding Year-11 values for the presence or absence of hypertension, diabetes, coronary heart disease, congestive heart failure and a Mini-Mental State Exam score in the lowest (worst) quintile. The addition of these co-morbid conditions did not significantly alter the results and are not shown.
Discussion
Few studies have systematically examined the rate of change in physical performance as a predictor of disability or mortality. In 837 men and women with a mean age of 81, followed for an average of 2.2 years, a lower baseline motor performance and a higher annual decline in motor performance independently predicted mortality [9]. However, the interval between the last clinical evaluation and death averaged about 6 months, suggesting that performance decline reflected a pre-terminal condition for many subjects. In contrast, performance decline predicted death or ADL disability 7 years later in our study and death within 5 years in a cohort of 439 older community-dwelling men and women [10]. In the latter study, the 5-year mortality risk doubled in persons experiencing a 1-year decrease in gait speed of ≥0.1 m/s, compared with those with no decline. In a large cohort of non-demented older persons, Schupf et al. [11] found no significant association between change in self-reported mobility and subsequent mortality in their fully adjusted model, which included change in ADL and IADL as predictors. However, if ADL and IADL impairment lie in the causal pathway between performance decline and death, then a deterioration in ADL or IADL would be expected to attenuate the association between change in mobility and mortality.
Gill et al. evaluated risk of ADL dependence as a function of gait speed, timed chair stands and speed of turning 360° [8]. All three performance measures assessed at year 1 predicted ADL dependence 2 years later, but changes in performance over the preceding year were not associated with the development of ADL impairment. The authors relied on just two measurements 1 year apart to calculate performance change, making their change variables vulnerable to measurement error, which could have contributed to the observed non-significant relationship between performance change and ADL dependence. We required at least three valid measurements for each performance variable, and over 85% of participants had 5 or more.
When we evaluated several upper- and lower-body performance measures together in combined models, the rates of change in stride length and grip strength independently predicted mortality or disability. Change in stride length was a more sensitive indicator of risk for disability than change in grip strength, for which only the highest quintile of the change slope was significantly associated with greater risk. Buchman et al. found that a composite measure of muscle strength and change in this strength measure lost its significant associations with mortality when the level of, and the annual rate of change in, motor performance were added to the model [9]. Our detection of a significant relationship between grip strength (and change in grip strength) and disability or death may have been due to the large size of our cohort.
We also found that cross-sectional gait speed and grip strength independently predicted motor disability in both the upper and lower extremities, as well as ADL disability. The paradoxical association of upper-extremity performance with lower-extremity disability, and vice-versa, suggests that lower grip strength, slower gait speed, and higher rates of change in grip strength and stride length may reflect underlying nervous system pathology that increases the risk of generalised disability and death. In normal ageing, the slowing of gait speed is directly related to a shortening of stride length [15, 16]. Slow gait speed and short stride length have been independently associated with a higher probability of having brain infarcts [17], as well as with the extent of white matter hyperintensities (WMH) [17–19]. Impaired mobility performance, as assessed by the SPPB, correlates with the cross-sectional extent of, and rate of increase in, WMH [20, 21]. Over a 5-year period, persons with slow gait are more likely to experience a greater decline in attention and psychomotor speed, as measured by the Digit Symbol Substitution Test (DSST), than individuals who walk faster [22]. Slower gait speed and lower DSST, separately and in combination, have been associated with incident disability and mortality [7]. The interconnectedness of physical performance and brain pathology suggests that nervous-system changes could partially mediate the relationship between declining physical performance and subsequent disability or death.
Age-related alterations in gait speed and stride length also can be explained, in part, by decreased leg strength and range of motion [23], and lower-extremity strength training can improve stride length [24]. Further research is needed to ascertain whether physical therapy or other interventions can delay the time to disability or death in individuals in whom significant downward performance trajectories have been detected.
The failure of chair stands and finger-tapping speed to enter the final models could have been a function of the unreliability of these measures in the CHS. As a performance measure, finger-tapping speed has proved test–retest reliability [25, 26], but, unlike gait speed, is not associated with ventricular volume or white matter changes on MRI [18]. Finger-tapping speed also does not correlate with gait speed [27].
Our study has limitations. Although we used a population-based cohort, the exclusion from the analyses of participants with fewer than three values for a given performance measure may have introduced bias and reduced the generalisability of the results. Although we only observed linear patterns in the many performance trajectories that we plotted, some individual trajectories could have been non-linear, causing inaccurate estimates of annual performance change. Our statistical models contained a limited number of covariates. Although the addition of co-morbid conditions to the models did not significantly alter the results, we may have omitted important confounders.
Conclusions
The rates of change in stride length and grip strength, easily determined in longitudinal practice settings, provide important prognostic information for late-life disability and death that are independent of the predictive value of a performance measurement obtained at a single point in time, which could be inaccurate because of recent injury or illness. By predicting decline in mobility, UEF, and more generalised daily activities, longitudinal changes in stride length and grip strength capture broader deteriorations in function within an individual, suggesting a shared causal pathway.
Key points.
The rate of performance change may be more useful than a single, cross-sectional measurement for estimating disability risk.
Performance trajectories compliment, but do not replace, cross-sectional measurements as predictors of late-life disability.
The rate of change in stride length is a robust predictor of disability or death.
Trajectories for stride length and grip strength may contribute to disability through a shared causal pathway.
Conflicts of interest
None declared.
Funding
The research reported in this article was supported in part by the National Institute on Aging AG-023629 and in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. CHS was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging, R01 HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Ostir GV, Kuo YF, Berges IM, Markides KS, Ottenbacher KJ. Measures of lower body function and risk of mortality over 7 years of follow-up. Am J Epidemiol. 2007;166:599–605. doi: 10.1093/aje/kwm121. doi:10.1093/aje/kwm121. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. doi:10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 4.Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–8. doi: 10.1093/ageing/28.3.283. doi:10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 5.Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004;16:481–6. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 6.Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. J Am Geriatr Soc. 1999;47:1257–60. doi: 10.1111/j.1532-5415.1999.tb05209.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–25. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill TM, Williams CS, Mendes de Leon CF, Tinetti ME. The role of change in physical performance in determining risk for dependence in activities of daily living among nondisabled community-living elderly persons. J Clin Epidemiol. 1997;50:765–72. doi: 10.1016/s0895-4356(97)00065-6. doi:10.1016/S0895-4356(97)00065-6. [DOI] [PubMed] [Google Scholar]
- 9.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–9. doi: 10.1111/j.1532-5415.2006.01032.x. doi:10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 10.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. doi:10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 11.Schupf N, Tang MX, Albert SM, et al. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology. 2005;65:1218–26. doi: 10.1212/01.wnl.0000180970.07386.cb. doi:10.1212/01.wnl.0000180970.07386.cb. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. doi:10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 13.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. doi:10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. doi:10.2307/2529876. [PubMed] [Google Scholar]
- 15.Ferrandez AM, Pailhous J, Durup M. Slowness in elderly gait. Exp Aging Res. 1990;16:79–89. doi: 10.1080/07340669008251531. doi:10.1080/07340669008251531. [DOI] [PubMed] [Google Scholar]
- 16.Elble RJ, Thomas SS, Higgins C, Colliver J. Stride-dependent changes in gait of older people. J Neurol. 1991;238:1–5. doi: 10.1007/BF00319700. doi:10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- 17.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. doi:10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 18.Camicioli R, Moore MM, Sexton G, Howieson DB, Kaye JA. Age-related brain changes associated with motor function in healthy older people. J Am Geriatr Soc. 1999;47:330–4. doi: 10.1111/j.1532-5415.1999.tb02997.x. [DOI] [PubMed] [Google Scholar]
- 19.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–42. doi: 10.1212/01.wnl.0000305959.46197.e6. doi:10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 20.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54:1277–83. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–7. doi: 10.1016/j.jns.2004.12.017. doi:10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–62. doi: 10.1159/000111577. doi:10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait Posture. 2008;27:572–7. doi: 10.1016/j.gaitpost.2007.07.009. doi:10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Lamoureux E, Sparrow WA, Murphy A, Newton RU. The effects of improved strength on obstacle negotiation in community-living older adults. Gait Posture. 2003;17:273–83. doi: 10.1016/s0966-6362(02)00101-7. doi:10.1016/S0966-6362(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 25.Morrison MW, Gregory RJ, Paul JJ. Reliability of the finger tapping test and a note on sex differences. Percept Mot Skills. 1979;48:139–42. doi: 10.2466/pms.1979.48.1.139. doi:10.2466/pms.1979.48.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Gill DM, Reddon JR, Stefanyk WO, Hans HS. Finger tapping: effects of trials and sessions. Percept Mot Skills. 1986;62:675–8. doi: 10.2466/pms.1986.62.2.675. doi:10.2466/pms.1986.62.2.675. [DOI] [PubMed] [Google Scholar]
- 27.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–8. doi: 10.1007/s00221-005-2280-3. doi:10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]