Abstract
The dynamics of synaptic transmission between neurons plays a major role in neural information processing. In the cochlear nucleus, auditory nerve synapses have a relatively high release probability and show pronounced synaptic depression that, in conjunction with the variability of interspike intervals, shapes the information transmitted to the postsynaptic cells. Cellular mechanisms have been best analyzed at the endbulb synapses, revealing that the recent history of presynaptic activity plays a complex, non-linear, role in regulating release. Emerging evidence suggests that the dynamics of synaptic function differ according to the target neuron within the cochlear nucleus. One consequence of hearing loss is changes in evoked release at surviving auditory nerve synapses, and in some situations spontaneous release is greatly enhanced. In contrast, even with cochlear ablation, postsynaptic excitability is less affected. The existing evidence suggests that different modes of hearing loss can result in different dynamic patterns of synaptic transmission between the auditory nerve and postsynaptic neurons. These changes in dynamics in turn will affect the efficacy with which different kinds of information about the acoustic environment can be processed by the parallel pathways in the cochlear nucleus.
1. Introduction
The dynamics of synaptic transmission between neurons plays a major role in neural information processing (Abbott and Regehr, 2004). While transmission is fundamentally probabilistic, the release of neurotransmitters is also highly dependent on the recent history of activity at a particular synapse, and both the release probability and the temporal dynamics of release can be modulated. Changes in the strength of synapses can be divided into those transient effects that last for seconds to minutes, or short-term plasticity, and those that last for tens of minutes to days (or even years), or long-term plasticity. In this review, we will focus on the role of short-term plasticity, and on the changes in release and short-term plasticity that follow hearing loss, at the first synapses in the central auditory pathway.
Short-term plasticity can appear as either depression or facilitation of synaptic strength, and a single synapse can exhibit either or both of these phenomena at any given time. In general, depressing synapses have a high release probability at rest, while facilitating synapses have a low release probability. The depression and facilitation between sequential presynaptic action potentials can be viewed as a combination of the time-dependent changes in the availability of releasable vesicles, in vesicular release probability and of postsynaptic receptor sensitivity to the released transmitter (i.e., desensitization). In depressing synapses, repeated presynaptic activation of a synapse over a time frame of tens of millseconds to seconds results in reduced transmitter release and/or a reduced postsynaptic response over time. For constant-rate stimulation (i.e., regular trains), depression is generally rate-dependent (model calculations of the strength of the synaptic response are shown for different rates in Figure 1A, B), so that greater depression is seen at high stimulus rates (corresponding to shorter interstimulus intervals). Consequently, depressing synapses tend to decrease the strength of synaptic transmission after the onset of a burst of action potentials at a high rate. From the standpoint of the postsynaptic cell, this means that high-rate information is suppressed, relative to low-frequency events. Depressing synapses can be viewed as low-pass filters of presynaptic spike trains, and thus can contribute to the adaptation of neural responses to sustained sensory input. On the other hand, some synapses show facilitation for certain rates of presynaptic activity. In this case, as the presynaptic rate increases (up to a point), the release probability increases. Such synapses can enhance responses to higher-frequency presynaptic activity, and thus act as high-pass filters (Grande and Spain, 2005). From an information theoretic standpoint, these differences in synaptic dynamics convey (or emphasize) specific aspects of the information contained in the timing of presynaptic action potentials to the postsynaptic neuron (Cook et al., 2003; Fuhrmann et al., 2002).
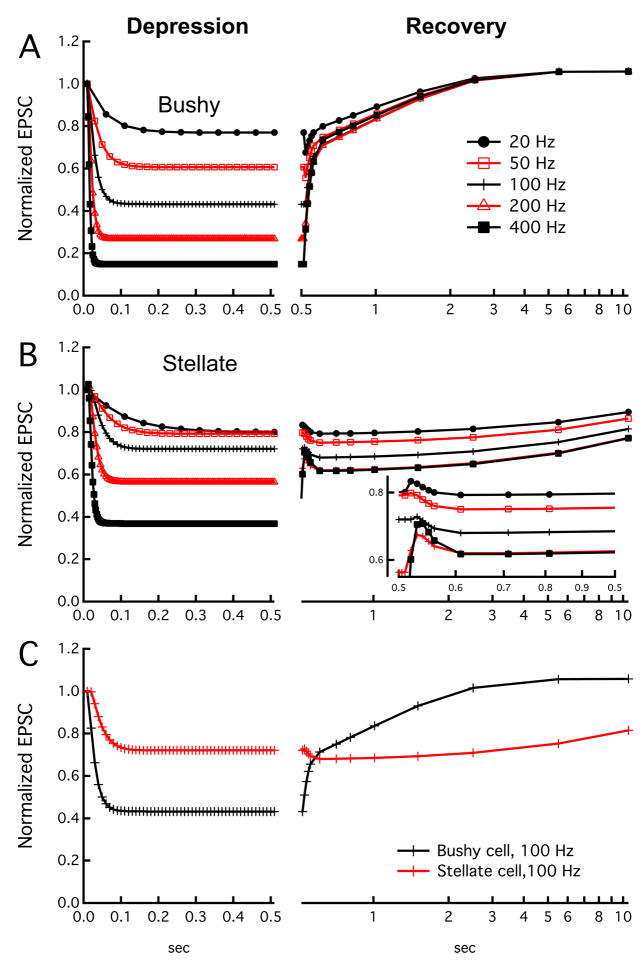
Figure 1.
Schematic of synaptic depression at normal auditory synapses as modeled from measurements in mouse cochlear nucleus. Each plot shows the normalized EPSC amplitude during stimulation with regular trains at different frequencies from 20 to 400 Hz, with a linear time scale on the left, and recovery kinetics measured by single test pulses after the train with a logarithmic time scale on the right. A. Typical parameters for auditory nerve synapses onto VCN bushy cells. Note that there is substantial depression at high frequencies, but rapid recovery after the train, and that recovery occurs in 2 phases (see text). B. Typical parameters for auditory nerve synapses onto VCN T-stellate cells. Note that the magnitude of depression during the stimulus is less than for bushy cells, but that recovery is slower. The inset shows the early portion of the recovery on an expanded time scale. C. Direct comparison of the plots for bushy and stellate cells at 100 Hz. The kinetic models are based on the formalizations of Dittman et al. (2000) and Yang and Xu-Friedman (2008), and parameters were obtained from fits to data measured from auditory nerve root stimulation in identified cells in brain slices from CBA mice (R. Xie and P.B. Manis, unpublished data).
What determines the release probability of a synapse, and in turn, whether it facilitates or depresses during changes in the firing rate of the parent axon? Synapses from a single presynaptic cell that innervate different types of target cells can have very distinct release probabilities (Mancilla and Manis, 2009; Markram et al., 1998), and the release probabilities from synapses of one class of afferent cell that innervate a specific population of target cells often are more similar than those innervating different targets (Dittman et al., 2000). This relationship seems to hold across both excitatory and inhibitory synapses, and across multiple regions of the brain. Recent studies in the avian and mammalian cochlear nucleus also suggest that auditory nerve endings on different sets of target cells may show different release dynamics (Cao and Oertel, 2010; MacLeod et al., 2007). In particular, endings on bushy cells depress more rapidly than endings on non-bushy cells (T-stellate cells or regular-spiking neurons of the avian nucleus angularis). This is summarized in Figure 1C, which shows a comparison between the rates and magnitudes of depression in a model based on measurements made in bushy and T-stellate cells of the mouse ventral cochlear nucleus (VCN).
The presence of ongoing spontaneous or background discharge may also chronically bias release mechanisms (Hermann et al., 2007; Wang et al., 2010). Even relatively low afferent rates, such as 10–20 Hz, which is well within the rate of the spontaneous activity rates of auditory nerve fibers (in mouse Taberner and Liberman, 2005), can lead to a modest depression of release (Wang et al., 2010). It is also likely that the longer-term history of activity in an afferent may engage slowly varying homeostatic mechanisms that regulate how transmitter is released. In the context of the auditory system, sensory deprivation or hearing loss might be expected to influence the transfer of information, as might the introduction of artificial stimulus patterns by cochlear implants. Such changes in presynaptic function are presaged by demonstrations of the plasticity of endbulb morphology in cats and mice with congenital hearing loss (Lee et al., 2003; Ryugo et al., 1997). As discussed below, hearing loss can alter synaptic efficacy and synaptic dynamics at auditory nerve synapses, although the details and overall consequences appear to depend on the etiology of the loss.
In this review, we will focus on short-term plasticity of auditory nerve synapses in the cochlear nucleus, and their potential modulation by hearing loss. Most of the review will focus on the endbulb synapse in the VCN in rodents, although we will also touch upon endbulbs from other model systems and other synapses in the cochlear nucleus whose release parameters might be affected by hearing loss.
2. Short-term plasticity at end-bulb synapses
2.1. Methodological Considerations
We begin this section by raising three caveats. First, almost all studies examining biophysical properties of synaptic transmission at central auditory nerve synapses have been performed in brain slices, because of the tremendous technical advantages afforded by the ability to perform stable, long term recordings from postsynaptic cells, and the ability to manipulate the bathing and intracellular solutions. However, the extracellular solution used in most of these experiments contains a mixture of divalent ions that likely differ from that found in cerebrospinal fluid in vivo (Lorteije et al., 2009), and this in turn influences both the basal release probability as well as the dynamics of the synapses. Such solutions are used because they improve yield in patch clamp recordings, and in some regions of the brain also help stabilize network activity in part by indirectly modulating ion channel function. The most commonly used mixtures contain 2.0–2.5 mM calcium, and 1.5–3 mM magnesium, whereas CSF contains about 1.5 mM calcium (Jones and Keep, 1988) and 1.0 mM magnesium (Sun et al., 2009). Because of the elevated calcium concentration, these slice solutions raise the basal release probability, which in turn decreases facilitation and emphasizes synaptic depression, particularly at high frequencies. In some studies, the extracellular calcium levels have been manipulated to measure release probabilities (Oleskevich et al., 2000; Wang and Manis, 2005), and from these experiments, the differences between experimental conditions and the conditions in vivo can be estimated. While the use of elevated extracellular divalent ions does not invalidate studies of mechanisms that modulate release, it should be considered that the strength of synaptic depression might be less in vivo than would be assumed from a cursory examination of the literature.
The second caveat is recording temperature. While most practitioners record at near-physiological temperatures (e.g., 32–34 °C) as a compromise between slice longevity and physiological synaptic function, some investigators perform experiments at lower (room ~22 °C) or higher (37 °C) temperatures, which can result in both quantitatively and qualitatively different results. When examining evoked release, the temperature greatly influences the shape of the presynaptic action potential as well as the kinetics of calcium channels, and thus the calcium influx with each action potential. Temperature will also influence the kinetics of transport mechanisms that clear calcium within the terminal and that clear released neurotransmitters from the synaptic cleft. Since it is unlikely that any of these processes have exactly the same temperature dependence, it can be difficult to compare experiments that are performed at different temperatures.
The third caveat is the distinction between spherical and globular bushy cells. These two cell types share a number of common mechanisms, including the presence of presynaptic terminals with multiple release sites, postsynaptic expression of low-voltage activated potassium channels, and rapidly desensitizing postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. However, they differ in four prominent respects (and undoubtedly others). The first is the convergence of auditory nerve fibers: spherical bushy cells have relatively low convergence of 2–4 auditory nerve fibers (ANFs) in cat; globular bushy cells receive more convergent ANFs (6–69: (Spirou et al., 2005) 3–50: (Liberman, 1991)), with a mean of 17 (Spirou et al., 1990). The second is their axonal targets (Cant and Benson, 2003): Spherical bushy cells principally innervate the lateral superior olive ipsilaterally and the medial superior olive bilaterally, while globular bushy cells principally innervate the contralateral medial nucleus of the trapezoid body (MNTB), and in rodents, the ipsilateral lateral superior olive. Third, the intrinsic excitability of different bushy cell classes may be quantitatively different, as suggested by the wide variation in the strength of the low-voltage activated potassium conductance across bushy cells (Cao et al., 2007; Rothman and Manis, 2003). Finally, there are important species-specific differences in the relative numbers of spherical and globular bushy cells, in part governed by the extent of low-frequency hearing. While in the discussion that follows we generally treat these two cell types as a single group, it is important to keep in mind that there may be differences between studies that arise from differences (or selection) in sampling, species or even tonotopic location.
2.2. Mechanisms of synaptic depression
As illustrated above, auditory nerve synapses exhibit synaptic depression at all stimulus frequencies, at least when measured in the slightly elevated calcium levels used in slice studies. There are a number of cellular mechanisms that can drive synaptic depression. These include: 1) depletion of the readily-releasable pool of synaptic vesicles, 2) decreases in presynaptic calcium influx due to voltage or calcium-dependent inactivation of presynaptic calcium channels, 3) activity-dependent signaling from the postsynaptic cell, 4) activity-dependent signaling from molecules released by the presynaptic terminal (including autoreceptor activation), 5) receptor saturation by released transmitter (Chanda and Xu-Friedman, 2010b), and 6) postsynaptic receptor desensitization. It is important to note that glutamate receptor desensitization can occur with micromolar concentrations of glutamate and in the absence of receptor channel opening (Trussell and Fischbach, 1989), which can confound the interpretation of release as inferred from postsynaptic receptor currents.
The principal mechanism that regulates synaptic depression at auditory nerve synapses appears to be presynaptic calcium dynamics. Stimulation of endbulb synapses at 100 Hz or higher causes a rate-dependent depression of postsynaptic responses (Oleskevich and Walmsley, 2002; Wang and Manis, 2008; Yang and Xu-Friedman, 2009). Both the magnitude of the depression and the initial release probability depend on the external Ca2+ concentration. Another factor that probably contributes to depression is presynaptic calcium channel inactivation by accumulated intraterminal calcium (Wang et al., 2010). While facilitation can be seen at the beginning of short high-frequency stimulus trains under specific experimental conditions (Chanda and Xu-Friedman, 2010b; Wang and Manis, 2008), postsynaptic receptor desensitization often occludes early facilitation. Synaptic depression is usually adequately described by a single exponential function, but a second slower decay can be detected with trains more than a few hundred milliseconds long (Wang and Manis, 2008).
The importance of glutamate receptor desensitization at central auditory synapses depends on age. At young ages, receptors show more pronounced desensitization, and this desensitization contributes to the overall depression of the synaptic response. At older ages, receptor desensitization, though still present, is less important (Wang et al., 2010), and appears to recover more quickly after transmitter release. Consequently, desensitization in older animals may only be apparent or functionally important when the terminal is repeatedly activated at short intervals (Chanda and Xu-Friedman, 2010b). Desensitization can be difficult to detect, and the manipulations usually used to study this process in intact synapses often have confounding influences on release as well.
Recovery from depression
While many studies have emphasized the depression that occurs during bouts of high frequency activity, the mechanisms and kinetics of recovery following synaptic depression are far more than a biological curiosity. Recovery is a process that occurs continuously between presynaptic spikes, and thus it’s dynamics are integral to understanding the dynamic behavior of the release process. A full description of the responses of synapses to naturalistic, time-varying afferent spikes, depends critically on the mechanisms that are engaged after release. During ongoing activity, release represents a dynamic equilibrium between multiple processes. On the one hand presynaptic activity can affect rate-dependent release probabilities, potassium and sodium channel availability (inactivation for example can affect the shape of presynaptic action potentials and therefore the opening and closing of voltage-gated calcium channels), calcium channel inactivation, and postsynaptic receptor desensitization, all of which contribute to regulate synaptic strength. On the other hand, in between presynaptic action potentials and during lulls in activity, the rates of vesicle recycling into multiple pools and their calcium-dependence, the recovery from postsynaptic receptor desensitization, recovery from ion channel inactivation, and the varied binding kinetics of all the proteins involved in the release process all regulate recovery. Models of transmitter release are not well constrained by considering the time course of depression alone, but require knowledge of the rate of recovery across multiple time scales of activation. Such models have predictive value even for arbitrary patterns of afferent activity (Dittman et al., 2000; Hermann et al., 2009). Thus, understanding the recovery of release after different stimulus challenges is critical to understanding the dynamics of the synapses and how they might be affected in pathological states. Some of the mechanisms that participate in recovery from depression have been studied experimentally, and are discussed next.
Recovery from Ca2+-channel inactivation
Calcium channels can be inactivated by high concentrations of calcium near the cytoplasmic face of the channel (Cens et al., 2006; Lee et al., 2000), and calcium-dependent inactivation appears to play a role in sculpting transmitter release from inner hair cells (Grant and Fuchs, 2008). At the endbulb synapse, indirect evidence also suggests that P/Q type Ca2+ channel inactivation may play an important role in synaptic depression, especially during low afferent firing rates (≤100 Hz) (Wang et al., 2010), similar to what has been observed in the calyceal terminals (Forsythe et al., 1998; Xu and Wu, 2005). Recovery from synaptic depression at low rates should largely depend on the rate at which the presynaptic Ca2+ channels recover from inactivation. Although no direct measurements of the time course of endbulb P/Q Ca2+ channel recovery has been made, measurements in the MNTB calyx have found that the recovery time constant is a few seconds (Forsythe et al., 1998). This time constant parallels the recovery of postsynaptic excitatory postsynaptic current (EPSC) amplitudes after depression (Forsythe et al., 1998; Xu and Wu, 2005). Full recovery of EPSC amplitude at the endbulb also takes a few seconds (τrec = ~2 sec) at 100 Hz (Wang and Manis, 2008), consistent with the hypothesis that the time course of P/Q type Ca2+ channel recovery from inactivation at least partially contributes to recovery from depression.
Recovery from desensitization
Desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors can be quite prominent in younger animals and cyclothiazide, an allosteric regulator of AMPA receptor desensitization (Patneau et al., 1993; Trussell et al., 1993; Yamada and Tang, 1993), is very effective in reducing desensitization in juvenile animals (Bellingham and Walmsley, 1999; Raman and Trussell, 1995; Yang and Xu-Friedman, 2008). Desensitization can also be detected in older, mature animals (Chanda and Xu-Friedman, 2010b), but both receptor desensitization and the effectiveness of cyclothiazide are reduced at older ages (Wang and Manis, 2008; Wang et al., 2010). Cyclothiazide may also have other actions on presynaptic channels that affect release (Barnes-Davies and Forsythe, 1995; Ishikawa and Takahashi, 2001), so it is not clear that all of the effects of cyclothiazide can be exclusively attributed to reduction of AMPA receptor desensitization. The decrease in desensitization across ages however is thought to reflect ongoing changes in AMPA receptor subunit composition during development (Lu and Trussell, 2007; Lu et al., 2007; Wang and Kaczmarek, 1998; Wang et al., 1998). Recovery from desensitization at the endbulb AMPA receptors has a time constant of approximately 10 msec (Chanda and Xu-Friedman, 2010b; Raman and Trussell, 1995). Surprisingly, desensitization during high-frequency stimulus trains appears to be greatest only during the initial rapid phase of depression (Chanda and Xu-Friedman, 2010b), and may be less important in determining the final steady-state response. However, desensitization appears to play a role in the steady-state response in chick nucleus magnocellularis, although the effects of desensitization become attenuated with increasing age (Brenowitz and Trussell, 2001).
Recovery from vesicle depletion
Recovery from short-term synaptic depression at the endbulb terminal is facilitated by residual terminal Ca2+ accumulation at high activation rates (>100 Hz) (Chanda and Xu-Friedman, 2010b; Wang and Manis, 2008; Yang and Xu-Friedman, 2008). This Ca2+-facilitated replenishment of depleted vesicles was first described at the MNTB calyx (Wang and Kaczmarek, 1998; Wang and Manis, 2008). Following high rates of afferent firing, an additional fast component of recovery becomes evident, with a time constant of 25 msec (Wang and Manis, 2008). Using a simple model incorporating a Ca2+-dependent replenishment of a single releasable vesicle pool, Yang and Xu-Friedman (2008b) showed that this Ca2+-dependent recovery is critical to counteract the effect of depletion-based synaptic depression at the endbulb. It appears that the fast recovery mechanism requires intracellular Ca2+ accumulation to reach a certain “threshold” which is achieved only athi gh firing rates (>100 Hz in 1.5 or 2 mM external Ca2+) or by certain firing patterns that include short high-frequency bursts, such as those during Poisson-like spike trains (Wang and Manis, 2008). It is not yet clear what the concentration or duration of elevated calcium must be reached to trigger the mechanisms that drive the fast vesicle replenishment. The rapid recovery may involve activation of Ca2+/calmodulin signaling pathways, since pharmacologically blocking these pathways significantly attenuates the fast recovery from depression at the endbulb synapse (Wang and Manis, 2008) and at the MNTB calyx (Sakaba and Neher, 2001).
Recovery with Poisson-like stimulus trains
In vivo, spontaneous and some driven activity in the ANF has Poisson distributed interspike intervals (Kiang, 1965; Taberner and Liberman, 2005). The instantaneous firing rate of individual fibers thus varies widely. Because of the rapid rise time and relatively slow decay of intraterminal Ca2+ (Bollmann and Sakmann, 2005), the presence of short intervals in a Poisson train should generate a higher internal peak Ca2+ levels than a regular spike train with the same mean rate. Indeed, evidence for the fast recovery has been observed with Poisson trains at moderate rates (100 Hz) where regular trains are followed by only a slow recovery in both the MNTB and at the endbulb synapse (Hermann et al., 2007; Wang and Manis, 2008). The calcium levels during the train will be determined by interactions between the activity pattern, calcium-dependent inactivation of calcium channels, changes in action potential width or falling rate for closely spaced spikes (Habets and Borst, 2006), a non-linear calcium dependence of the membrane calcium pumps (Scheuss et al., 2006), and the potential saturation of calcium buffering proteins (Blatow et al., 2003). Thus, it is possible that a 100 Hz Poisson train may result in both a higher average and peak terminal [Ca2+]i than a regularly-spaced 100 Hz train. While evidence for all of these mechanisms exists, a direct demonstration of such non-linear effects at the level of intraterminal Ca2+ at endbulb synapses is lacking. Interestingly, the faster recovery at high rates, which also must occur within the Poisson train itself, does not seem to affect the final depression levels (Hermann et al., 2007; Wang and Manis, 2008). One possibility is that depletion of the readily releasable vesicle pool is the dominant phenomenon during the train (Wang and Manis, 2008; Yang and Xu-Friedman, 2008), and there is little opportunity for substantial recovery to occur except during the brief lulls in activity. This might be expected because the fast recovery time constant, which at its fastest is ~25 msec, is longer than the mean interevent interval of 10 msec at 100 Hz.
3. Synaptic plasticity and auditory processing at the endbulb
The sustained sound-driven discharge of ANFs can reach 400 Hz (Liberman, 1978; Ohlemiller et al., 1991; Sachs and Abbas, 1974; Taberner and Liberman, 2005; Winter et al., 1990), while the spontaneous firing rates in silence can range from less than 1 to over 100 spike/sec. In vitro and in vivo recordings have revealed that the response entrainment (the ratio of spikes generated per presynaptic stimulus) of bushy cells to ANF stimulation falls below 50% when trains of shock stimuli are delivered at 200 Hz and higher (Englitz et al., 2009; Kopp-Scheinpflug et al., 2002; Wang and Manis, 2006). Thus recovery from severe synaptic depression during high rates of ANF activity can significantly shape the functional relationship between the ANF and the bushy neuron. The strength and fluctuations of the synaptic input can affect the precision of postsynaptic spike timing, and spike timing in this pathway is critical for fundamental auditory tasks such as sound localization. Consequently, the short term dynamics may affect the central representation of critical features of the acoustic environment. In this section, we explore the relationship between afferent rate and postsynaptic action potential generation across the endbulb synapses.
3.1. Impact of synaptic depression on postsynaptic spike timing
The endbulb synapse is generally considered to be strong relative to many CNS synapses because of the large number of release sites associated with each auditory nerve fiber. However, activation of the endbulb still often fails to drive postsynaptic action potentials during high rates of activity in vitro (Chanda and Xu-Friedman, 2010b; Typlt et al., 2010; Wang and Manis, 2006; Wang et al., 2010; Yang and Xu-Friedman, 2009) and in vivo (Englitz et al., 2009; Kopp-Scheinpflug et al., 2002; Kopp-Scheinpflug et al., 2003). Under most conditions, increasing depression leads to lower probability of bushy neuron spiking. However, the effects of depression on spike timing are highly context dependent because of convergent inputs bushy neurons receive, which can be revealed in in vitro recordings (Cao and Oertel, 2010; Oertel, 1985; Wang et al., 2010; Yang and Xu-Friedman, 2009). When all convergent sibling inputs carry similar timing information, depression can indirectly affect bushy neuron spike precision by determining the number of inputs required to reach firing threshold in concert with the temporal distribution of inputs (Xu-Friedman and Regehr, 2005; Yang and Xu-Friedman, 2009). On the other hand, when sibling inputs convey different timing information, endbulb synaptic depression can improve bushy neuron spike timing by suppressing highly active inputs that carry little temporal information (Fukui et al., 2006; Yang and Xu-Friedman, 2009). These in vitro results suggest possible mechanisms for the enhancement of temporal coding observed in the bushy neurons from in vivo recordings (Joris et al., 1994), in addition to postsynaptic channels that regulate coincidence detection (Rothman and Young, 1996; Rothman et al., 1993).
3.2. Impact of synaptic depression on postsynaptic spike reliability
Even though synaptic depression appears to be similar between endbulbs at the anteroventral cochlear nucleus (AVCN) (Oleskevich and Walmsley, 2002; Wang and Manis, 2008; Xu-Friedman and Regehr, 2005; Yang and Xu-Friedman, 2008) and the calyces at the MNTB (Wang and Kaczmarek, 1998; Wong et al., 2003), individual endbulb synapses are surprisingly unreliable for suprathreshold transmission both in vivo and in vitro (Kopp-Scheinpflug et al., 2002; Typlt et al., 2010; Wang et al., 2010) compared to the calyx (Lorteije et al., 2009). This is likely due to two factors. First, evoked EPSCs at endbulbs are smaller, because endbulbs have fewer release sites than the calyx (although the quantal content is slightly larger) (Oleskevich and Walmsley, 2002; Oleskevich et al., 2000; Wang and Manis, 2005). Second the higher resting release probability at the endbulb (Pr ~ 0.5) produces a larger trial-to-trial variance, as compared to the lower release probability (Pr ~ 0.2) at the calyx. Nevertheless, some of the postsynaptic unreliability can be overcome by the presence of convergent endings onto bushy neurons. When multiple endbulb synapses are activated simultaneously, the postsynaptic response reliability improves significantly (Wang et al., 2010; Xu-Friedman and Regehr, 2005). Convergent activation of a bushy neuron thus provides another means to enhance postsynaptic reliability. It has been shown that isolated individual endbulb terminals operate independently, i.e., synaptic depression of a given terminal is not influenced by activitiesin adjacent sibling terminals on the same bushy neuron (Xu-Friedman and Regehr, 2005; Yang and Xu-Friedman, 2009). Thus, high response reliability can also be achieved through the shared contributions of independently firing but only modestly depressed presynaptic fibers.
3.3. Modulation by GABA
Presynaptic modulation of release can have significant consequences for the functional synaptic relationship between neurons. Endbulb presynaptic release probability is modulated in an activity dependent fashion in bushy neurons of the nucleus magnocellularis in chickens as well as in the AVCN in mice (Brenowitz and Trussell, 2001; Brenowitz et al., 1998; Chanda and Xu-Friedman, 2010a). Activation of presynaptic GABAB receptors greatly reduces the presynaptic release probability, which in turn reduces the postsynaptic EPSC amplitude. In mice, this essentially silences postsynaptic bushy neuron firing. Only when converging ANFs are co-activated within a narrow time window of <0.5 msec are postsynaptic spikes generated. Thus GABA can regulate temporal integration in bushy neurons by suppressing individual inputs while favoring coincident activation of multiple inputs (Chanda and Xu-Friedman, 2010a). In chickens, activation of GABAB receptors paradoxically enhances the reliability of bushy neuron responses during high frequency trains, due to reduced transmitter depletion as well as a decrease in postsynaptic AMPA receptor desensitization (Brenowitz and Trussell, 2001).
4. Effects of hearing loss on synaptic plasticity at the endbulb
Since the main excitatory input to the principal neurons of the cochlear nucleus comes directly from the auditory nerve fibers, it may be expected that hearing loss could modify synaptic transmission at ANF synapses. Many aspects of the synaptic transmission in the cochlear nucleus have been studied in different models of hearing loss, with a primary emphasis on the endbulb synapse. From the perspective of the cochlear nucleus, there are three categories of hearing loss can be broadly defined based on whether or not the cochlear nucleus neurons still receive either physical or functional input from the periphery. In the first category, the cells receive no synaptic connections from the cochlea. This category includes models using cochlear ablation and some forms of congenital deafness. The second category is defined by reduced activity of synaptic inputs, and includes studies with conductive, on-going age-related, and noise-induced hearing loss at the stages when spiral ganglion cells are still present. A third category includes hearing loss produced by partial cochlear ablation or chemical means that causes subtotal spiral ganglion cell death and degeneration of central auditory terminals, and thus affects the number of residual fibers innervating the cochlear nucleus. In this section, we compare and contrast effects of different modes of hearing loss on synaptic property and transmission in cochlear nucleus neurons.
4.1. Hearing loss and presynaptic release probability
The effects of hearing loss on synaptic transmission at the endbulb have been studied most systematically in congenitally deaf (dn/dn) mutant mice (Oleskevich and Walmsley, 2002) and DBA mice which exhibit early onset age-related hearing loss (Wang and Manis, 2005; Wang and Manis, 2006). Mice with dn/dn mutation are deaf, whereas DBA mice develop nearly normal thresholds, but then rapidly develop a progressive high-frequency hearing loss. In both cases, auditory nerve fibers survive and can be electrically stimulated, even though their numbers may be reduced. For the most part, neurons in the VCN survive so long as deprivation occurs after the onset of hearing (Mostafapour et al., 2000; Zirpel et al., 2000). Moreover, the intrinsic membrane properties of VCN neurons seem to be resistant to sensory deprivation, and exhibit only minor changes which do not qualitatively alter their characteristic firing properties both in vitro (Cao et al., 2008; Francis and Manis, 2000; Lu et al., 2007; Wang and Manis, 2005; Wang and Manis, 2006) and in vivo (Cai et al., 2009).
In dn/dn mutants, evoked EPSCs are significantly larger (~170%) than normal, and this is caused by an increased probability of release. Using mean-variance analysis, Oleskevich and Walmsley (2002) show that the probability of transmitter release at the endbulb is extremely high (Pr ~ 0.8) in mutant mice compared to that (Pr~ 0.5) in control animals. Following high frequency stimulation, dn/dn mice show a greater depression of evoked EPSCs, and a significant increase in the occurrence of delayed-release (asynchronous) mEPSCs, which would likely reduce postsynaptic response reliability and precision (Yang and Xu-Friedman, 2010). The increase in Pr and delayed-release suggest that endogenous calcium buffering may be impaired or undeveloped in the presynaptic terminals of the auditory nerve in dn/dn mice. Similarly, in congenitally deaf jerker mice, mEPSC events in octopus cells are more frequent and synaptic depression is more severe in the mutant mice, again indicating that presynaptic release probability is likely high (Cao et al., 2008).
In contrast, in DBA/2J mice, which develop with normal hearing thresholds, but begin to show elevated hearing thresholds by P30, Wang and Manis (2005) showed that the spontaneous mEPSC frequency is greatly reduced (~60%) and release probability, measured by mean-variance analysis, is 30% lower in endbulb synapses after the onset of hearing loss. However, synaptic depression at different stimulation rates (100–300 Hz) is similar in both normal and hearing-impaired DBA mice, indicating that other compensatory changes may be occurring in these hearing-impaired animals. Even though entrainment to high frequency train stimulation (100 Hz) is reduced in hearing impaired DBA2/J mice, no extra jitter in synaptic delay is detected (Wang and Manis, 2006). Additionally, some postsynaptic changes are also observed in hearing impaired DBA2/J mice (discussed below).
Recently, synaptic properties at the auditory nerve to T-stellate cell synapses have been examined in both congenitally deaf (Cao et al., 2008) and noise deafened (Rich et al., 2010) mice. In both cases, a dramatic increase (3 to >5 fold) in mEPSC frequency is observed with no major changes in mEPSC kinetics. An increase in mEPSC amplitude is seen 2 weeks after intense noise exposure that produces a permanent threshold shift (Rich et al., 2010), which together with a modest increase in mEPSC decay time constants may suggest remodeling of postsynaptic receptors, although the increase in amplitude could also be explained by changes in quantal size (e.g., packaging of transmitter into synaptic vesicles). Interestingly, an in vivo study examining VCN neuron responses to acoustic stimuli after focal noise lesions (created by exposure to intense narrow band noise) reveals a steeper rate-level function only in “chopper” (stellate) neurons which may contribute to loudness recruitment (Cai et al., 2009). Loudness recruitment is a phenomenon in which hearing impaired subjects perceive sounds just above their threshold levels as being louder than expected (or, alternatively, loudness grows more rapidly above threshold than for normal hearing listeners). Whether loudness recruitment can be fully explained by an altered strength of ANF synaptic transmission onto stellate cells remains to be explored.
4.2. Hearing loss and postsynaptic receptors
One clear consequence of hearing loss is an altered distribution and/or number of neurotransmitter receptors in the VCN. However, the details how receptor distribution or numbers change clearly depend on the type and duration of hearing loss. In this section, we provide a brief review of some of the consequences of different kinds of hearing loss for transmitter release and postsynaptic receptor expression.
Unilateral cochlear ablation produces time-dependent changes in the calcium-dependent release of D-[3H]-aspartate (as a surrogate for glutamate release) in the guinea pig cochlear nucleus (Potashner et al., 1997). Although this release is arises from many different kinds of synapses in the slice, it includes a partial, but significant, contribution from auditory nerve terminals (Wenthold, 1979; Wenthold and Gulley, 1977). In the VCN, evoked release is initially decreased, but eventually recovers by 145 days post-ablation to near control levels. At the same time, AMPA receptors, as assessed by specific [3H]AMPA binding to tissue sections, shows an initial slight decrease, followed by an increase to near control levels by ~150 days post-lesion (Suneja et al., 2000). In the adult rat VCN, N-methyl-D-aspartate receptor (NMDAR) R1 subunit mRNA decreases transiently in spherical bushy cells after cochlear ablation in adult rat VCN (Sato et al., 2000), but the mRNA levels recover by 20 days post-ablation. These significance of these results is unclear because NMDA receptors do not appear to contribute significantly to the endbulb EPSC in rats after P21 (Isaacson and Walmsley, 1995), although they are present in young adult mice at the same synapses (Cao and Oertel, 2010). Using ototoxic antibiotics to destroy hair cells (and reduce spiral ganglion cell density) starting at P7 in rats, (Marianowski et al., 2000) demonstrated decreases in mRNA for NMDAR1, NMDAR2A and NMDAR2B, and the flop isoforms of AMPA receptors, relative to age-matched controls, at ages up to P16. Overall, cochlear ablation at later ages appears to cause an initial decrease in AMPA and NMDA receptor expression, followed by a return to near-control levels later in life. The sources of non-cochlear glutamatergic inputs that participate in these remaining circuits have not been identified, but could include intrinsic connections from glutamatergic cells within the cochlear nucleus (for example, from granule cells or T stellate cells), or connections from descending inputs.
Noise-induced hearing loss can produce effects that differ from cochlear ablation, for several reasons. First, noise exposure drives the auditory system with high firing rates for a sustained period, which might be expected to engage a variety of pre- and postsynaptic mechanisms, including the induction of long-term changes in synaptic strength and neuronal excitability. In addition, noise exposure does not immediately result in degeneration of spiral ganglion cells, and the duration of the hearing loss can be temporary (with lower intensity or brief exposures) or permanent (Wang et al., 2002).
Peripheral damage from noise exposure produces some changes in calcium- dependent D-[3H]-asparate release that are similar to those seen with cochlear ablations. One difference is that shortly following the noise exposure there is enhanced transmitter release in the days before release drops presumably due to spiral ganglion cell degeneration (Muly et al., 2004). Interestingly, there was a late increase in AMPA receptor density following noise exposure (Muly et al., 2004). In T-stellate cells, mEPSC amplitude is larger after intense noise exposure both at 2 days and 2 weeks (Rich et al., 2010), which may be due to changes in postsynaptic receptor composition, although this could also reflect changes in quantal content or more frequent multiquantal release. It is difficult however to compare spontaneous quantal release at specific synapses with global depolarization-evoked release at all synapses in a slice, since these types of release are mediated by very different mechanisms and may be reflect the activation of different populations of synapses.
Genetic hearing loss
In some congenitally deaf mice, many aspects of synaptic transmission are remarkably similar to their control cohorts (Cao et al., 2008), especially on the postsynaptic side. On the other hand, in a different genetic model, dn/dn mice, significant changes in quantal content and release probability have been seen (Oleskevich and Walmsley, 2002). In contrast, in age-related hearing loss, endbulb receptor current properties can change over time (Wang and Manis, 2005). Miniature EPSCs are significantly slower (~115%) and smaller (~70%) in high frequency regions of hearing impaired DBA/2J mice. Moreover, evoked EPSCs show less rectification, suggesting recruitment of GluR2 subunits into the AMPA receptor complex, consistent with a redistribution of AMPA receptor subunits (GluR2–4) following cochlear damage (Rubio 2006, Potashner 1999). In comparison to most of the above-cited studies, in these DBA/2J mice hearing develops relatively normally before the hearing loss begins, and the changes in synaptic currents are seen while there is still some functional hearing in the high-frequency regions of the cochlear nucleus. It is likely that different forms of genetic deafness may lead to different patterns of changes in central synaptic transmission that depend on the history of activity in the afferent fibers (including, and perhaps critically, during development prior to the onset of hearing)
Conductive hearing loss
Monaural conductive hearing loss results in a decrease in metabolic activity of the ipsilateral cochlear nucleus and a compensatory increase in activity of the contralateral cochlear nucleus (Sumner et al., 2005; Tucci et al., 2002; Tucci et al., 2001). Some of these changes appear to be associated with synaptic inputs from the contralateral cochlear nucleus (Sumner et al., 2005). Compensatory changes have also been seen following even brief periods of hearing deficit which induced rapid reversible changes in the ipsilateral and contralateral expression of glycine and glutamate receptor subunits in the cochlear nuclei (Whiting et al., 2009). In this study, postembedding immunogold labeling showed a bilateral upregulation of GluR3 on fusiform and bushy cells, while rapid compensatory mechanisms resulted in lower GluR4 subunit expression in fusiform cells of the contralateral cochlear nucleus.
New synapses after hearing loss
One of the later consequences of cochlear ablation is an increase in GAP-43 expression in the VCN (Illing et al., 1997). GAP-43 is a prominent protein in axonal growth cones but not mature synapses (Benowitz and Routtenberg, 1997), and thus can be a marker for the generation of new synapses. GAP-43 in the cochlear nucleus may be associated with new terminals of collaterals of olivocochlear neurons both after cochlear ablation (Kraus and Illing, 2004) and noise damage (Michler and Illing, 2002). Consistent with new or additional innervation by cholinergic fibers, there is also an increase in choline acetyltransferase activity in the VCN after cochlear ablation (Jin et al., 2005). One interpretation of these observations is that inactive or degenerating glutamatergic synapses are replaced by cholinergic synapses, which are one of the few other excitatory inputs to the VCN (for example, see Schofield et al., this volume). Decreases in functional glutamatergic transmission have also been shown to lead to a compensatory increase in nicotinic acethylcholine (Ach) receptorexpression in the hypothalamus and cerebellum (Belousov et al., 2001). However, it is entirely unclear whether this innervation serves only a slow, modulatory role (see Oertel et al., this volume), or whether it mediates a fast, excitatory transmission. Fujino and Oertel (2001) found that T-stellate cells in the VCN could be excited by bath-applied ACh through nicotinic receptors, while only about 25% of bushy cells were responsive to ACh (Oertel and Fujino, 2001). Even though ACh can modulate the responses of VCN neurons to sound (Caspary et al., 1983), no direct evidence exists for synaptically-evoked release of ACh. Thus, it is unclear what role remodeling of cholinergic synapses might play in regulating the activity of cochlear nucleus neurons following cochlear ablation.
Taken together, it is clear that auditory nerve activity plays a critical and active role in maintaining normal synaptic function at the endbulb of Held and other synapses in the cochlear nucleus. Auditory nerve activity regulates both presynaptic (release probability) and postsynaptic (receptor composition and kinetics) functions at these synapses. Depending on the etiology of hearing loss and how it affects activities in the auditory nerve, different kinds of hearing loss may affect distinct components of synaptic transmission and cochlear nucleus neurons. The changes in synaptic complement that follow cochlear ablation, including the potential increase in cholinergic synapses, indicates that it will be important to carefully identify the sources of synaptic inputs in the future. Spontaneous release from cholinergic terminals in the cochlear nucleus seems rare, or at least is difficult to detect, as in normal hearing mice, antagonists of glutamatergic receptors nearly completely eliminate miniature EPSCs (Gardner et al., 1999).
5. Conclusions and perspectives
Synaptic input from the auditory nerve to the cochlear nucleus shows short-term temporal dynamics that undoubtedly play a role in sculpting information processing within the nucleus, and contribute to the parallel processing of auditory information that is established by neurons of the cochlear nucleus. While phenomena such as synaptic depression may contribute to sensory adaptation, rate-dependent recovery from depression may help ensure continued precision in spike timing during intense acoustic stimulation. There are two major areas that seem fruitful to pursue along these lines. First, as we have mentioned, much of the data on synaptic transmission has been derived from brain slice studies, where extracellular calcium has often been elevated relative to expected levels in intact brain. This leads to high initial release probabilities and enhanced depression. Furthermore only recently have the effects of ongoing evoked release using stimulus patterns that mimic spontaneous activity at the synapses begun to be analyzed. Studies which emphasize the use of normal extracellular calcium and magnesium, in conjunction with the addition of evoked activity based on spontaneous or driven discharge patterns may give further insight into the dynamics of release under standard conditions. To a first order, such manipulations are likely to partially linearize synaptic dynamics (or decrease the non-linearities). Second, most computational models of ANF input to the cochlear nucleus have utilized strictly linear synapses with no temporal dynamics (Pressnitzer et al., 2001; Rothman and Young, 1996; Rothman et al., 1993; Spirou et al., 2005). While these models have been successful in explaining specific features of the initial stages of central integration, it will be interesting to see how the inclusion of temporal dynamics influences the predictions of processing by specific cell types. The field is at a point where fairly realistic single cell computational models can be proposed based on our knowledge of synaptic function and dynamics, the intrinsic membrane excitability of different classes of CN neurons, and the details of auditory nerve responses to even complex stimuli.
The adjustments in central synaptic function that occur consequent to hearing loss pose a number of interesting problems. Changes in synaptic dynamics, through altered release probabilities or different synaptic “biases” resulting from altered basal spontaneous activity, are expected to influence how dynamic acoustic stimuli are represented centrally, and in turn may influence both detection and discrimination of acoustic features. While a number of studies have examined the effects of hearing loss on basal synaptic transmission, spontaneous release, and metabolic activity, few have so far examined the consequences of hearing loss on synaptic dynamics of the residual synapses. Furthermore, the difference between synaptic depression at different sets of excitatory synapses as produced by regular trains and Poisson trains suggests that high-rate periodic stimulation, as used in some cochlear implants, may not optimally take advantage of the temporal nonlinearities of central synapses in terms of presenting time-varying features of the acoustic environment to the central auditory system.
Acknowledgments
This work was supported by NIH grants R03DC008190 to YW amd R01DC004551 (PBM, HAO). We would also like to thank an anonymous reviewer for some very constructive suggestions.
Glossary
- ACh
acetylcholine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANF
auditory nerve fiber
- AVCN
anteroventral cochlear nucleus
- EPSC
excitatory postsynaptic potential
- MNTB
medial nucleus of the trapezoid body
- NMDA
N-methyl-D-aspartate
- Pr
probability of release
- VCN
ventral cochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–70. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Belousov AB, O’Hara BF, Denisova JV. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J Neurosci. 2001;21:2015–27. doi: 10.1523/JNEUROSCI.21-06-02015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–34. doi: 10.1038/nn1417. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci. 2001;21:9487–98. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABA(B) receptors. Neuron. 1998;20:135–41. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- Cai S, Ma WL, Young ED. Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. J Assoc Res Otolaryngol. 2009;10:5–22. doi: 10.1007/s10162-008-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–74. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cao XJ, Oertel D. Auditory nerve fibers excite targets through synapses that vary in convergence, strength and short-term plasticity. J Neurophysiol. 2010 doi: 10.1152/jn.00451.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XJ, Shatadal S, Oertel D. Voltage-sensitive conductances of bushy cells of the Mammalian ventral cochlear nucleus. Journal of Neurophysiology. 2007;97:3961–75. doi: 10.1152/jn.00052.2007. [DOI] [PubMed] [Google Scholar]
- Cao XJ, McGinley MJ, Oertel D. Connections and synaptic function in the posteroventral cochlear nucleus of deaf jerker mice. J Comp Neurol. 2008;510:297–308. doi: 10.1002/cne.21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Havey DC, Faingold CL. Effects of acetylcholine on cochlear nucleus neurons. Exp Neurol. 1983;82:491–8. doi: 10.1016/0014-4886(83)90419-3. [DOI] [PubMed] [Google Scholar]
- Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. Voltage- and calcium-dependent inactivation in high voltage-gated Ca(2+) channels. Prog Biophys Mol Biol. 2006;90:104–17. doi: 10.1016/j.pbiomolbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Chanda S, Xu-Friedman MA. Neuromodulation by GABA converts a relay into a coincidence detector. J Neurophysiol. 2010a;104:2063–74. doi: 10.1152/jn.00474.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S, Xu-Friedman MA. A Low-affinity Antagonist Reveals Saturation and Desensitization in Mature Synapses in the Auditory Brainstem. J Neurophysiol. 2010b;103:1915–1926. doi: 10.1152/jn.00751.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature. 2003;421:66–70. doi: 10.1038/nature01248. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–85. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englitz B, Tolnai S, Typlt M, Jost J, Rubsamen R. Reliability of synaptic transmission at the synapses of Held in vivo under acoustic stimulation. PLoS One. 2009;4:e7014. doi: 10.1371/journal.pone.0007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons. Hear Res. 2000;149:91–105. doi: 10.1016/s0378-5955(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Segev I, Markram H, Tsodyks M. Coding of temporal information by activity-dependent synapses. J Neurophysiol. 2002;87:140–8. doi: 10.1152/jn.00258.2001. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci. 2001;21:7372–83. doi: 10.1523/JNEUROSCI.21-18-07372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I, Sato T, Ohmori H. Improvement of phase information at low sound frequency in nucleus magnocellularis of the chicken. Journal of Neurophysiology. 2006;96:633–41. doi: 10.1152/jn.00916.2005. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Time course and permeation of synaptic AMPA receptors in cochlear nuclear neurons correlate with input. J Neurosci. 1999;19:8721–9. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande LA, Spain WJ. Synaptic depression as a timing device. Physiology (Bethesda) 2005;20:201–10. doi: 10.1152/physiol.00006.2005. [DOI] [PubMed] [Google Scholar]
- Grant L, Fuchs P. Calcium- and calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99:2183–93. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- Habets RL, Borst JG. An increase in calcium influx contributes to post-tetanic potentiation at the rat calyx of Held synapse. J Neurophysiol. 2006;96:2868–76. doi: 10.1152/jn.00427.2006. [DOI] [PubMed] [Google Scholar]
- Hermann J, Grothe B, Klug A. Modeling short-term synaptic plasticity at the calyx of held using in vivo-like stimulation patterns. J Neurophysiol. 2009;101:20–30. doi: 10.1152/jn.90243.2008. [DOI] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol. 2007;98:807–20. doi: 10.1152/jn.00355.2007. [DOI] [PubMed] [Google Scholar]
- Illing RB, Horvath M, Laszig R. Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43 immunoreactivity in the rat. J Comp Neurol. 1997;382:116–38. doi: 10.1002/(sici)1096-9861(19970526)382:1<116::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol. 1995;73:964–73. doi: 10.1152/jn.1995.73.3.964. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol. 2001;533:423–31. doi: 10.1111/j.1469-7793.2001.0423a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Sun Y. Effects of cochlear ablation on choline acetyltransferase activity in the rat cochlear nucleus and superior olive. J Neurosci Res. 2005;81:91–101. doi: 10.1002/jnr.20536. [DOI] [PubMed] [Google Scholar]
- Jones HC, Keep RF. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol. 1988;402:579–93. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Carney LH, Smith PH, Yin TC. Enhancement of neural synchronization in the anteroventral cochlear nucleus. I. Responses to tones at the characteristic frequency. J Neurophysiol. 1994;71:1022–36. doi: 10.1152/jn.1994.71.3.1022. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S. Discharge patterns of single fibers in the cat’s auditory nerve. Massachussetts Institute of Technology Press; Cambridge: 1965. [Google Scholar]
- Kopp-Scheinpflug C, Dehmel S, Dorrscheidt GJ, Rubsamen R. Interaction of excitation and inhibition in anteroventral cochlear nucleus neurons that receive large endbulb synaptic endings. J Neurosci. 2002;22:11004–18. doi: 10.1523/JNEUROSCI.22-24-11004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2003;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Illing RB. Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J Comp Neurol. 2004;475:374–90. doi: 10.1002/cne.20180. [DOI] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–8. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–43. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–55. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Central projections of auditory-nerve fibers of differing spontaneous rate. I. Anteroventral cochlear nucleus. The Journal of comparative neurology. 1991;313:240–58. doi: 10.1002/cne.903130205. [DOI] [PubMed] [Google Scholar]
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci. 2009;29:13770–84. doi: 10.1523/JNEUROSCI.3285-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Trussell LO. Development and elimination of endbulb synapses in the chick cochlear nucleus. J Neurosci. 2007;27:808–17. doi: 10.1523/JNEUROSCI.4871-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Harris JA, Rubel EW. Development of spontaneous miniature EPSCs in mouse AVCN neurons during a critical period of afferent-dependent neuron survival. J Neurophysiol. 2007;97:635–46. doi: 10.1152/jn.00915.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod KM, Horiuchi TK, Carr CE. A role for short-term synaptic facilitation and depression in the processing of intensity information in the auditory brain stem. J Neurophysiol. 2007;97:2863–74. doi: 10.1152/jn.01030.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Manis PB. Two distinct types of inhibition mediated by cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 2009;102:1287–95. doi: 10.1152/jn.91272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianowski R, Liao WH, Van Den Abbeele T, Fillit P, Herman P, Frachet B, Huy PT. Expression of NMDA, AMPA and GABA(A) receptor subunit mRNAs in the rat auditory brainstem. I. Influence of early auditory deprivation. Hear Res. 2000;150:1–11. doi: 10.1016/s0378-5955(00)00166-0. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci U S A. 1998;95:5323–8. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler SA, Illing RB. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. J Comp Neurol. 2002;451:250–66. doi: 10.1002/cne.10348. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Cochran SL, Del Puerto NM, Rubel EW. Patterns of cell death in mouse anteroventral cochlear nucleus neurons after unilateral cochlea removal. J Comp Neurol. 2000;426:561–71. doi: 10.1002/1096-9861(20001030)426:4<561::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Muly SM, Gross JS, Potashner SJ. Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res. 2004;75:585–96. doi: 10.1002/jnr.20011. [DOI] [PubMed] [Google Scholar]
- Oertel D. Use of brain slices in the study of the auditory system: spatial and temporal summation of synaptic inputs in cells in the anteroventral cochlear nucleus of the mouse. J Acoust Soc Am. 1985;78:328–33. doi: 10.1121/1.392494. [DOI] [PubMed] [Google Scholar]
- Oertel D, Fujino K. Role of biophysical specialization in cholinergic modulation in neurons of the ventral cochlear nuclei. Audiol Neurootol. 2001;6:161–6. doi: 10.1159/000046825. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Echteler SM, Siegel JH. Factors that influence rate-versus-intensity relations in single cochlear nerve fibers of the gerbil. J Acoust Soc Am. 1991;90:274–87. doi: 10.1121/1.401298. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol. 2002;540:447–55. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Clements J, Walmsley B. Release probability modulates short-term plasticity at a rat giant terminal. J Physiol. 2000;524(Pt 2):513–23. doi: 10.1111/j.1469-7793.2000.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Vyklicky L, Jr, Mayer ML. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993;13:3496–509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1997;148:222–35. doi: 10.1006/exnr.1997.6641. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Meddis R, Delahaye R, Winter IM. Physiological correlates of comodulation masking release in the mammalian ventral cochlear nucleus. J Neurosci. 2001;21:6377–86. doi: 10.1523/JNEUROSCI.21-16-06377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Trussell LO. The mechanism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor desensitization after removal of glutamate. Biophys J. 1995;68:137–46. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich AW, Xie R, Manis PB. Hearing loss alters quantal release at cochlear nucleus stellate cells. Laryngoscope. 2010;120:2047–2053. doi: 10.1002/lary.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JS, Young ED. Enhancement of neural synchornization in computational models of ventral cochlear nucleus bushy cells. Auditory Neuroscience. 1996;2:47–62. [Google Scholar]
- Rothman JS, Manis PB. The roles potassium currents play in regulating the electrical activity of ventral cochlear nucleus neurons. Journal of Neurophysiology. 2003;89:3097–113. doi: 10.1152/jn.00127.2002. [DOI] [PubMed] [Google Scholar]
- Rothman JS, Young ED, Manis PB. Convergence of auditory nerve fibers onto bushy cells in the ventral cochlear nucleus: implications of a computational model. J Neurophysiol. 1993;70:2562–83. doi: 10.1152/jn.1993.70.6.2562. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J Comp Neurol. 1997;385:230–44. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J Acoust Soc Am. 1974;56:1835–47. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–31. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Shiraishi S, Nakagawa H, Kuriyama H, Altschuler RA. Diversity and plasticity in amino acid receptor subunits in the rat auditory brain stem. Hear Res. 2000;147:137–44. doi: 10.1016/s0378-5955(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Yasuda R, Sobczyk A, Svoboda K. Nonlinear [Ca2+] signaling in dendrites and spines caused by activity-dependent depression of Ca2+ extrusion. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8183–94. doi: 10.1523/JNEUROSCI.1962-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirou GA, Brownell WE, Zidanic M. Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. Journal of Neurophysiology. 1990;63:1169–90. doi: 10.1152/jn.1990.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Rager J, Manis PB. Convergence of auditory-nerve fiber projections onto globular bushy cells. Neuroscience. 2005;136:843–63. doi: 10.1016/j.neuroscience.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol. 2005;94:4234–43. doi: 10.1152/jn.00401.2005. [DOI] [PubMed] [Google Scholar]
- Sun L, Kosugi Y, Kawakami E, Piao YS, Hashimoto T, Oyanagi K. Magnesium concentration in the cerebrospinal fluid of mice and its response to changes in serum magnesium concentration. Magnes Res. 2009;22:266–72. doi: 10.1684/mrh.2009.0186. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. AMPA receptor binding in adult guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Exp Neurol. 2000;165:355–69. doi: 10.1006/exnr.2000.7471. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–69. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989;3:209–18. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–96. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Tucci D, Cant NB, Durham D. Conductive hearing loss results in changes in cytochrome oxidase activity in gerbil central auditory system. J Assoc Res Otolaryngol. 2002;3:89–106. doi: 10.1007/s101620010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hear Res. 2001;155:124–32. doi: 10.1016/s0378-5955(01)00256-8. [DOI] [PubMed] [Google Scholar]
- Typlt M, Haustein MD, Dietz B, Steinert JR, Witte M, Englitz B, Milenkovic I, Kopp-Scheinpflug C, Forsythe ID, Rubsamen R. Presynaptic and postsynaptic origin of multicomponent extracellular waveforms at the endbulb of Held-spherical bushy cell synapse. Eur J Neurosci. 2010;31:1574–81. doi: 10.1111/j.1460-9568.2010.07188.x. [DOI] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394:384–8. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol. 2005;94:1814–24. doi: 10.1152/jn.00374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol. 2006;7:412–24. doi: 10.1007/s10162-006-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol. 2008;100:1255–64. doi: 10.1152/jn.90715.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren C, Manis PB. Endbulb synaptic depression within the range of presynaptic spontaneous firing and its impact on the firing reliability of cochlear nucleus bushy neurons. Hear Res. 2010;270:101–9. doi: 10.1016/j.heares.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. J Neurosci. 1998;18:1148–60. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ. Release of endogenous glutamic acid, aspartic acid and GABA from cochlear nucleus slices. Brain research. 1979;162:338–43. doi: 10.1016/0006-8993(79)90294-4. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Gulley RL. Aspartic acid and glutamic acid levels in the cochlear nucleus after auditory nerve lesion. Brain research. 1977;138:111–23. doi: 10.1016/0006-8993(77)90787-9. [DOI] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience. 2009;163:1264–76. doi: 10.1016/j.neuroscience.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibres. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci. 2003;23:4868–77. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–45. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. II. Few synaptic inputs. J Neurophysiol. 2005;94:2526–34. doi: 10.1152/jn.01308.2004. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13:3904–15. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol. 2008;99:2510–21. doi: 10.1152/jn.01293.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Impact of synaptic depression on spike timing at the endbulb of Held. J Neurophysiol. 2009;102:1699–710. doi: 10.1152/jn.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Developmental mechanisms for suppressing the effects of delayed release at the endbulb of Held. J Neurosci. 2010;30:11466–75. doi: 10.1523/JNEUROSCI.2300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel L, Janowiak MA, Veltri CA, Parks TN. AMPA receptor-mediated, calcium-dependent CREB phosphorylation in a subpopulation of auditory neurons surviving activity deprivation. J Neurosci. 2000;20:6267–75. doi: 10.1523/JNEUROSCI.20-16-06267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]