Abstract
Telomeres are the DNA–protein complexes that protect the ends of eukaryotic chromosomes. The cellular enzyme telomerase counteracts telomere shortening by adding telomeric DNA. A growing body of literature links shorter telomere length and lower telomerase activity with various age-related diseases and earlier mortality. Thus, leukocyte telomere length (LTL) and telomerase activity are emerging both as biomarkers and contributing factors for age-related diseases. However, no clinical study has directly examined telomerase activity and telomere length in different lymphocyte subtypes isolated from the same donors, which could offer insight into the summary measure of leukocyte telomere maintenance.
We report the first quantitative data in humans examining both levels of telomerase activity and telomere length in four lymphocyte subpopulations from the same donors—CD4+, CD8+CD28+ and CD8+CD28− T cells and B cells, as well as total PBMCs—in a cohort of healthy women. We found that B cells had the highest telomerase activity and longest telomere length; CD4+ T cells had slightly higher telomerase activity than CD8+CD28+ T cells, and similar telomere length. Consistent with earlier reports that CD8+CD28−T cells are replicatively senescent cells, they had the lowest telomerase activity and shortest telomere length. In addition, a higher percentage of CD8+CD28− T cells correlated with shorter total PBMC TL (r = −0.26, p = 0.05). Interestingly, telomerase activities of CD4+ and CD8+CD28+ T cells from the same individual were strongly correlated (r = 0.55, r < 0.001), indicating possible common mechanisms for telomerase activity regulation in these two cell subtypes. These data will facilitate the understanding of leukocyte aging and its relationship to human health.
Keywords: Telomere, Telomerase, Immune cells, Aging, Aging-related diseases
1. Introduction
Telomeres, DNA–protein complexes at the end of eukaryotic chromosomes, shorten with each cell division. Cells with critically short telomeres can enter replicative senescence, a state of irreversible cell growth arrest. Thus, telomere shortening is viewed as a mitotic clock that counts down the number of cell divisions (Harley et al., 1990). The cellular enzyme telomerase counteracts telomere shortening by RNA-templated addition of DNA nucleotides onto telomeres, thereby lengthening the telomeric DNA (Greider and Blackburn, 1989, 1987, 1985). In a cohort of otherwise healthy premenopausal women, lower telomerase activity, although not shorter telomere length, in resting peripheral mononuclear cells (PBMC) was associated with the major risk factors for cardiovascular disease (CVD) (Epel et al., 2006) and animal studies show that low telomerase is linked to diseased tissues (Serrano and Andres, 2004). Numerous clinical studies link shorter telomere length—and inferred accelerated telomere shortening rate—in leukocytes to aging and aging-related diseases (Cawthon et al., 2003; Samani et al., 2001; Brouilette et al., 2003; Benetos et al., 2004; von Zglinicki, 2000; Kurz et al., 2004; Jeanclos et al., 2000; Aviv et al., 2006; Valdes et al., 2005; Panossian et al., 2003; Zhang et al., 2003; Gardner et al., 2005; Zhai et al., 2006; Fitzpatrick et al., 2007; Collerton et al., 2007; Adaikalakoteswari et al., 2007; Starr et al., 2007; Harris et al., 2006). Furthermore, subsequent studies showed shorter LTL and more rapid leukocyte telomere shortening (Epel, 2009) predicts earlier mortality (Cawthon et al., 2003; Epel, 2009; Carrero et al., 2008; Honig et al., 2006; Kimura et al., 2008). Thus, low telomerase and short telomeres appear to be clinically important in human health and LTL is increasingly emerging as a biomarker for cellular aging in epidemiology studies. A better understanding ofwhat telomerase activity and telomere length in immune subpopulations may reflect will provide mechanistic insights into how cellular aging contributes to poor health.
PBMCs are composed of subsets of lymphocytes (including T cells, B cells, and natural killer cells) and monocytes. Of these, only T and B cells have been reported to have detectable, although low, telomerase activity (Weng, 2008). Total PBMC telomerase activity would thus be predicted to be determined by the percentage of T and B cells as well as the activity per cell for each cell type. PBMC telomere length is determined by the percentage of all the cell types in the PBMC cell preparation as well as the telomere length of each cell type. Several factors contribute to telomere length: telomeres that were inherited (genetic), the level of telomerase activity, environmental and cellular factors that influence the rate of telomere attrition and telomerase activity, and number of cell divisions (history of division). While previous work has shown a decline in telomerase activity and shortening of telomere length as lymphocytes progress from naïve to memory cells, and in general with chronological age, no clinical study has directly examined telomerase activity and telomere length in lymphocyte subpopulations from the same donor.
Telomerase activity is highly regulated in the development and differentiation of T and B cells (Weng, 2008). When stimulated by their specific antigens, T and B cells are activated and undergo multiple rounds of cell division. While most of the activated cells die at the end of the immune response, memory cells remain in the body during the whole lifespan and become reactivated upon encounters with the same antigen. Telomere maintenance and telomerase regulation are closely linked to the activation and differentiation of T and B cells: telomerase activity in resting T and B cells decreases as they progress from naïve to memory cells, and is upregulated upon antigen stimulation(Weng, 2008). Concomitantly, telomeres are longer in naïve T lymphocytes than memory cells, supporting the notion that net telomere shortening occurs, despite the increased telomerase activity upon activation. Patients with an autosomal dominant form of the genetic disease dyskeratosis congenita, caused by mutations in the telomerase components leading to lower telomerase activity, die of bone marrow failure, demonstrating that sufficient telomerase activity is essential for proper immune function. Ectopic expression of the telomerase gene hTERT in human CD4+ and CD8+T cells extends their lifespan in culture, underlining the importance of telomerase in T cell function (Luiten et al., 2003; Rufer et al., 2001; Roth et al., 2003; Dagarag et al., 2004). While telomere length reflects the cumulative effects of genetic and environmental factors, telomerase activity is dynamic and is likely an essential modifiable factor in mediating environmental and lifestyle factors and telomere length changes. Thus measuring both telomere length and telomerase activity in lymphocytes may provide key readouts for the effects of environmental factors and interventions on aging.
We describe here methods for simultaneously sorting total PBMCs into three T cell subtypes: CD4+, CD8+CD28+ and CD8+CD28− cells as well as B cells, and for quantitatively measuring telomere length and telomerase activity in these cell types directly ex vivo. We also report findings from our methodology studies testing effects of collection methods (time of day, latency from draw to assay, and tube type). We found that, on a per cell basis, B cells have the highest telomerase activity and longest mean telomere length; CD4+ T cells have slightly higher telomerase activity than CD8+ CD28+ T cells, but similar telomere length. Consistent with earlier reports that CD8+CD28− T cells are the most differentiated cell type and hence predicted to have undergone the most cell divisions, they had the shortest telomere length among the four groups and, interestingly, the lowest telomerase activity. In addition, having a high percentage of CD8+CD28− T cells correlated with shorter mean telomere length measured in total PBMCs (r = −0.26, p = 0.05), consistent with the notion that both measurements serve as indicators of cellular aging. A novel observation was that telomerase activity in all four cell types from the same individual was correlated, with CD4+ and CD8+CD28+ T cell telomerase activity levels most strongly correlated (r = 0.55, r < 0.001). These findings indicate that telomerase activity in these cell types might be controlled by common pathways. However, we did not find correlations between telomerase activity level and telomere length for any specific cell type, reflecting the fact that telomere length is determined not only by the level of telomerase activity, but also other factors including genetic, cellular and environmental factors.
These are the first quantitative data we are aware of in humans examining telomere length and basal levels of telomerase in subsets of PBMCs from the same individuals. This work will facilitate further understanding of leukocyte aging and human health.
2. Materials and methods
2.1. Blood draw and staining protocol
Fasting blood (50 ml) was drawn from healthy participants of an ongoing clinical study. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque (Sigma-Aldrich, St. Louis, MO) gradient centrifugation, washed in PBS (Dulbecco's Phosphate Buffered Saline, PBS without Mg++ and Ca++, Invitrogen-Biosource, Carlsbad, CA) and then incubated for 15 min at 4 °C with fluorescent-conjugated monoclonal antibodies: anti-CD3-PB, anti-CD4-FITC, anti-CD28-PE, anti-CD19 APC-Cy7 (all from BD biosciences, San Jose, CA) and anti-CD8-ECD (Beckman Coulter, Miami, FL). Stained cells were sorted on a FACS Vantage DiVa II (BD Biosciences, San Jose, CA) into the following fractions: CD4+T cells (CD3+CD4+), CD8+CD28+T cells (CD3+CD8+CD28+), CD8+CD28− T cells (CD3+CD8+ CD28−) and B cells (CD3−CD19+). The cell sorter was configured with 3 lasers, and argon-ion at 488 nm, a krypton at 647 nm, and a violet enhanced krypton at 407 nm; each laser outputting 200mW. An aerosol management system (Cytek Development, Inc., Fremont, CA) was used for operator protection. Cells were collected into AIM V serum-free media (Invitrogen, Carlsbad, CA). Sorted cells were pelleted by centrifugation at 7000 rpm at 4°C for 5min in an Eppendorf refrigerated microcentrifuge (Model 5417R, Eppendorf, Westbury, NY) and washed twice with cold PBS. Two aliquots of cell pellets, each containing approximately 0.5 million cells, were saved to prepare DNA for telomere length measurement and telomerase activity assay respectively. DNA was prepared using Gentra Puregene Cell kit (QIAGEN, Valencia, CA). Extracts for measuring telomerase activity were prepared from cell pellets according to the TRAPeze kit (Upstate/CHEMICON, Temecula, CA). Due to the low percentages of CD8+CD28− and B cells, not enough cells were obtained for telomere length measurement and/or telomerase activity assay in some participants, leading to various sample sizes.
2.2. Assay of telomere length
The telomere length measurement assay was adapted from the published original method (Cawthon, 2002). The primers for the telomere PCR were tel1b [5′-CGGTTT (GTTTGG)5GTT-3′], used at a final concentration of 100 nM, and tel2b [5′-GGCTTG(CCTTAC)5CCT-3′], used at a final concentration of 900 nM. The primers for the single-copy gene (human beta-globin) PCR were hbg1 [5′ GCTTCTGACACAACTGTGTTCACTAGC-3′], used at a final concentration of 300 nM, and hbg2 [5′-CACCAACTTCATCCACGTTCACC-3′], used at a final concentration of 700 nM. The final reaction mix contained 20 mM Tris–HCl, pH 8.4; 50 mM KCl; 200 µM each dNTP; 1% DMSO; 0.4× Syber Green I (Invitrogen, Carlsbad, CA); 22 ng E. coli DNA (MP Biomedicals, Solon, OH); 0.4 Units of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) and 0.5–10 ng of genomic DNA per 11 µl reaction. Tubes containing 26, 8.75, 2.9, 0.97, 0.324 and 0.108 ng of a reference DNA (from Hela cancer cells) were included in each PCR run so that the quantity of targeted templates in each research sample could be determined relative to the reference DNA sample by the standard curve method. The same reference DNA was used for all PCR runs.
All PCRs were carried out on a Roche Lightcycler 480 real-time PCR machine with 384-tube capacity (Roche Diagnostics Corporation, Indianapolis, IN). The telomere thermal cycling profile consisted of: cycling for T (telomeric) PCR: denature at 96 °C for 1 s, anneal/extend at 54 °C for 60 s, with fluorescence data collection, 30 cycles; cycling for S (single-copy gene) PCR: denature at 95 °C for 15 s, anneal at 58 °C for 1 s, extend at 72 °C for 20 s, 8 cycles; followed by denature at 96 °C for 1 s, anneal at 58 °C for 1 s, extend at 72 °C for 20 s, hold at 83 °C for 5 s with data collection, 35 cycles.
To control for inter-assay variability, 8 control DNA samples were included in each run. In each batch, the T/S ratio of each control DNA was divided by the average T/S for the same DNA from 10 runs to obtain a normalizing factor. This was done for all 8 control samples and the average normalizing factor for all 8 samples was used to correct the participant DNA samples to obtain the final T/S ratio. The T/S ratio for each sample was measured twice. If the duplicate T/S value and the initial value varied by more than 7%, the sample was run a third time and the average of the two closest values was reported. Typically, about 5% of samples needed to be assayed the third time. Using this method, the inter-assay coefficient of variation (CV) for telomere length measurement was 4%.
2.3. Gel-TRAP assay
Gel-TRAP assays were performed by the Telomerase Repeat Amplification Protocol (TRAP) using a commercial kit (TRAPeze Telomerase Detection Kit, Upstate/CHEMICON, Temecula, CA). Cells sorted by a FACS Vantage DiVa II were spun down, washed with DPBS and stained by Trypan blue, live cells were counted using a hemocytometer. 5 × 105 – 1 × 106 cells per sample were pelleted and lysed with 1 × CHAPS buffer as directed by the manual for the TRAPeze kit. An extract corresponding to 5000 cells/µl was made and between 2000 and 10,000 cells were used for TRAP reactions. The reaction was carried out according to the TRAPeze kit manual and run on a 10% polyacrylamide–8 M urea sequencing gel. The gel was exposed to a phosphorimager plate overnight and scanned on a STORM 860 (GE Healthcare, Piscataway, NJ). The 293T cell line was used as a positive telomerase activity control and standard. Telomerase activity is expressed as the equivalent number of 293T cells. Telomerase activity was quantified using the software ImageQuant 5.2 (GE Healthcare, Piscataway, NJ). Briefly, signals from the product ladders on the gels were added and normalized against the signal from internal control band for the same lane to get the product/internal control value. For each telomerase activity assay reaction, the product/internal value is divided by the product/internal control value from twenty 293T cells and then multiplied by 20 to obtain the final telomerase activity units, defined as 1 unit = the amount of product from one 293T cell/10,000 immune cells.
2.4. Participant description
The study participants were recruited from the San Francisco Bay Area, using flyers, advertisements, and notices in dementia clinics and senior daycare centers. The advertisement for volunteers requested healthy non-smoking women. Since data indicate that stress affects cell aging, we examined a sample with a range of stress levels. The sample included women who were caregiving for a family member, and control women, who were not caregiving. There were 34 dementia caregivers, 3 mothers of children with autism, and 29 control women, who were not caregiving. All participants filled the written consent to this study. They study was approved by UCSF Institutional Review Board for Human Research. Analyses by stress levels are beyond the scope of this paper, and will be reported elsewhere. The sample was examined as a whole, since the questions addressed here were independent of age and stress level. The sample was mostly white, and completed high school, with a mean age of 61, and mean BMI of 26.4 (See Table 1 for demographics).
Table 1.
| Nb | 66 |
| Age (years, range 36–79) | 61 (8.3) a |
| Race (%) | |
| Caucasian | 83.1 |
| Black | 4.6 |
| Hispanic/Latina | 1.5 |
| Asian/Pacific Islander, or Native American | 10.8 |
| BMI (kg/m2) | 26.4 (5.3) |
| Education (years) | 15.6 (2.1) |
| Income ($1000) | 87.3 (55.0) |
Mean (SD) or frequency.
The number of participants ranges from 58 (income) to 66.
2.5. Statistical analysis
Means and standard deviations or frequencies for several demographic variables and BMI were computed across the sample (see Table 1).
Because our data included multiple values per person for percent cell type, telomere length, and telomerase activity, mixed linear models (SAS PROC MIXED) with an unstructured covariance matrix were used to compute within-person correlations. We evaluated raw means and standard deviations for telomere length, telomerase activity, and percent cell types (SAS PROC MEANS) but used mixed models to compare within-person means for these variables, using F-tests of fixed effects to evaluate whether group values differed as a whole and contrast statements to test whether values for specific cell types differed from other groups (Tables 2–4). We also performed post-hoc pairwise tests of least-square means for the cell types, using the Tukey method to account for multiple comparisons. We used Spearman correlations to evaluate associations between age and cell variables.
Table 2.
Telomerase activity (per 10,000 cells) by cell type.
| N | Cell type | Mean value | SD |
|---|---|---|---|
| 57 | PBMC | 5.36 | 3.60 |
| 66 | B | 4.27 | 2.97 |
| 67 | CD4+ | 3.12 | 1.60 |
| 67 | CD8+CD28+ | 2.59 | 1.47 |
| 61 | CD8+CD28− | 1.66 | 1.13 |
F value = 25.8, for any group differences, p < 0.0001.
All pairwise differences were significant (p < 0.02) (see supplemental Table 1).
Table 4.
Percentage of each cell type.
| N | Cell type | Mean value | SD |
|---|---|---|---|
| 66 | B | 13.12 | 6.27% |
| 66 | CD4+ | 48.35 | 9.91% |
| 66 | CD8+CD28+ | 9.76 | 3.61% |
| 66 | CD8+CD28− | 5.45 | 6.15% |
F-value = 289.4, p < 0.0001.
All pairwise differences were significant (p < 0.01) (see supplemental Table 3).
Scatter plots of telomerase activity comparing EDTA and heparin collection tubes, and comparing morning and afternoon blood draws were created using Microsoft Excel. Box plots for telomerase and telomere length were generated using GraphPad Prism®.
3. Results
3.1. Modified gel-TRAP assay to quantitatively measure telomerase activity in resting immune cells
The telomere repeat amplification protocol (TRAP), developed by Kim and Wu (1997), is the most commonly used method to measure human cell telomerase activity. In this method, an oligonucleotide (“oligo”) is used as the substrate for telomere addition by telomerase. This oligo, called TS primer, is first end-labeled by T4 polynucleotide kinase (PNK) to add radioactive 32P onto its 5′ end for signal detection. The labeled primer is then incubated with the extract and dNTPs to allow addition of nucleotides unto the TS primer. In the final step of the protocol, the products are amplified by PCR to increase detection sensitivity with TS and a reverse primer (ACX or RP) that anneals to TS, generating a ladder of products with 6 basepair (bp) increments, visualized by gel-electrophoresis and phosphorimager detection of the signal bands.
While the robust telomerase activity in cancer cells can be readily detected by the above method, very few studies have systematically attempted to quantify the extremely low telomerase activity in resting immune cells. Telomerase activity in blood cell samples was first reported in 1995 by Broccoli et al. (1995). Hiyama et al. (1995) further examined telomerase activity in resting PBMCs from124 Japanese individuals ranging from 0 to 86 years. Using extracts derived from 3 × 104 PBMCs, most, but not all individuals had detectable telomerase activity (100 out of 124);when 10,000 cells were used, only 55 showed positive results; with 3000 cells per reaction, only one adult showed positive telomerase activity. In that study, telomerase activity was scored as positive or negative, using an immortal human cell line as positive control, where at least 10 immortalized cells were needed to get a positive signal.
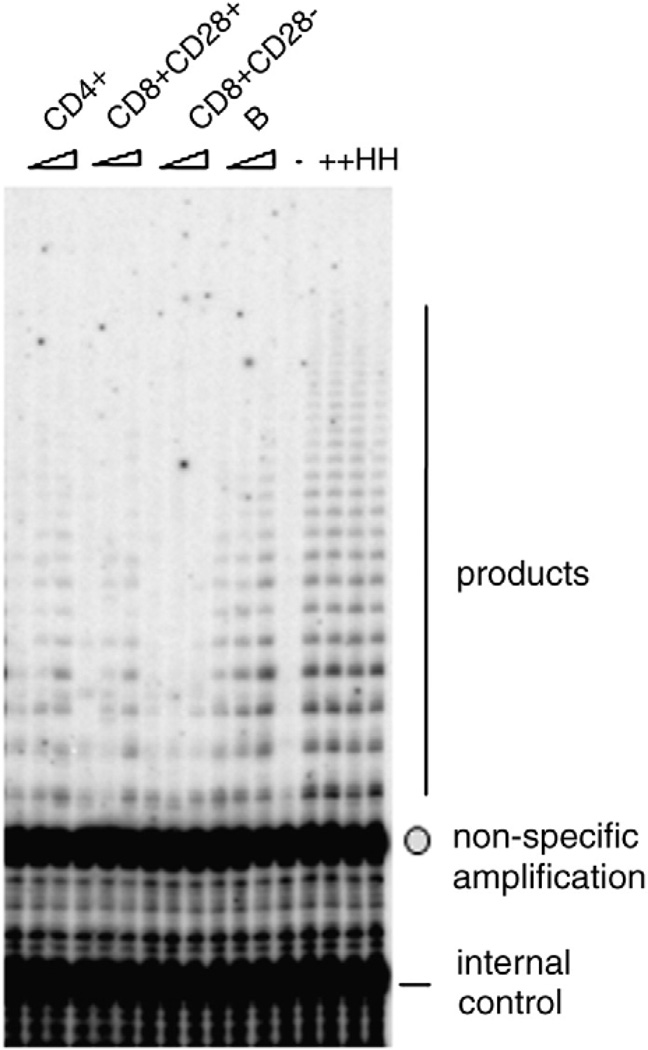
Lymphocytes typically have 0.01–0.3% of the TRAP activity seen in cancer cells on a per cell basis, demanding high sensitivity for the assay. Therefore, for the results reported here, we made some modifications in order to quantify telomerase in resting lymphocytes. First, we found that the PCR cycle numbers needed to be increased to 29 cycles to reliably detect products. However, under this PCR protocol, the bottom-most (smallest size DNA product fragment, 50 bps) band of the PCR product ladder generated by amplification of the telomerase reaction products overlaps non-specific signals that are present even in the no-extract control (Fig. 1). The quantity of this background is negligible when 25 cycles of PCR are used, as in the case of high telomerase activity in cancer cells. However, with the low telomerase activity seen in resting lymphocytes, this creates a significant background signal. Therefore, this band is excluded from our quantification. Second, because PBMCs contain different amounts of contaminating red blood cells and platelets, which will contribute to total protein in the extract, we counted cells and used viable cell numbers to normalize among different samples, instead of normalizing to total protein. Third, for every sample, we tested 2–3 different cell numbers to ensure the assay was in the linear range with respect to cell number. Typically, resting immune cells have a narrow range—2000–10,000 cells per reaction—over which telomerase activity remains linear with cell sample input. Fourth, to standardize the measurements, we used 293T cells, a transformed immortal cell line, as the positive control and the standard. We defined telomerase activity unit as 1 unit = the amount of product from one 293T cell or from 10,000 immune cells. Extracts were made every 6 months from freshly grown 293T cells, aliquoted and stored at−80 °C. For each batch of assays, we used two aliquots of 293T cells. In addition, two aliquots of extract from Hela cells, another cancer cell line, were assayed in each batch. A direct phosphorimager image of a typical telomerase activity gel is shown in Fig. 1. With the above precautions and modifications, we determined that the inter-assay variability for this method for the same sample assayed on different days is 6.7%.
Fig. 1.
A typical gel phosphorimager image showing TRAP activity using the TRAPeze kit, from the four cell types from one study subject. +: Standard sample containing extracts from 20 293T cells. 2000, 5000 and 10,000 cells were used for each sample. The arrow points to the non-specific band and the circle points to the internal control band (see Materials and methods).
3.2. Effects of variations in collection and preparation of PBMCs
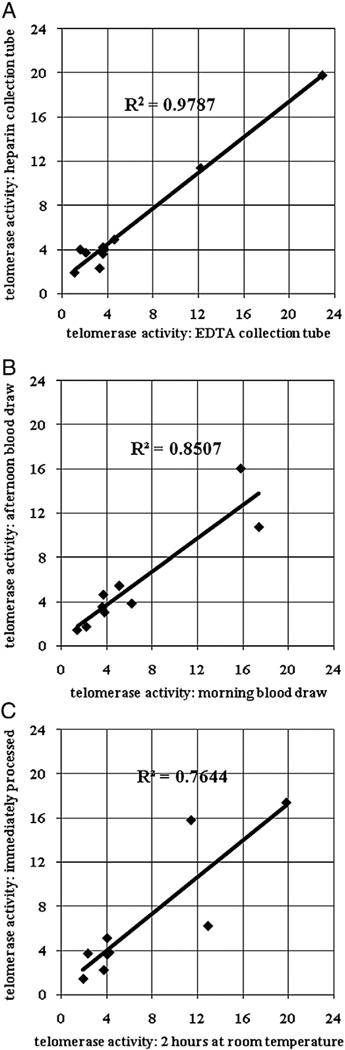
To systematically establish a lab protocol for clinical studies involving examining telomerase activity in unstimulated PBMCs, we compared the effects of several parameters on telomerase activity: two types of blood collection tubes (heparin vs. EDTA), blood draw in the morning vs. afternoon from the same individuals, and immediately processing the blood vs. leaving the blood at room temperature for 2 h. PBMCs were prepared as described in Materials and methods and telomerase activity was measured using the modified gel-TRAP assay. Fig. 2A shows that telomerase activity obtained from heparin vs. EDTA collection tubes from the same individuals was highly correlated. However, telomerase activity from PBMCs prepared from morning vs. afternoon blood draw had some variation within an individual (Fig. 2B). Likewise, leaving blood at room temperature for 2 h before proceeding with the Ficoll purification procedure caused telomerase activity to vary in some individuals (Fig. 2C). Therefore, in all the study participants reported here, we drew blood from morning fasting subjects who had been resting seated in a dimmed room in the clinic for at least 30 min, and purified PBMCs with in 1 h after blood draw.
Fig. 2.
Comparison of telomerase activity with various parameters during blood draw. A) heparin (green top) vs. EDTA (lavender top) collection tubes from the same individual (n = 9). B) blood draw in the morning vs. in the afternoon from the same individual (n = 9). C) Room temperature for 2 h vs. immediate processing of blood from the same individual (n = 9).
3.3. Examination of telomerase activity from CD4+, CD8+CD28+, CD8+CD28− T cells and B cells
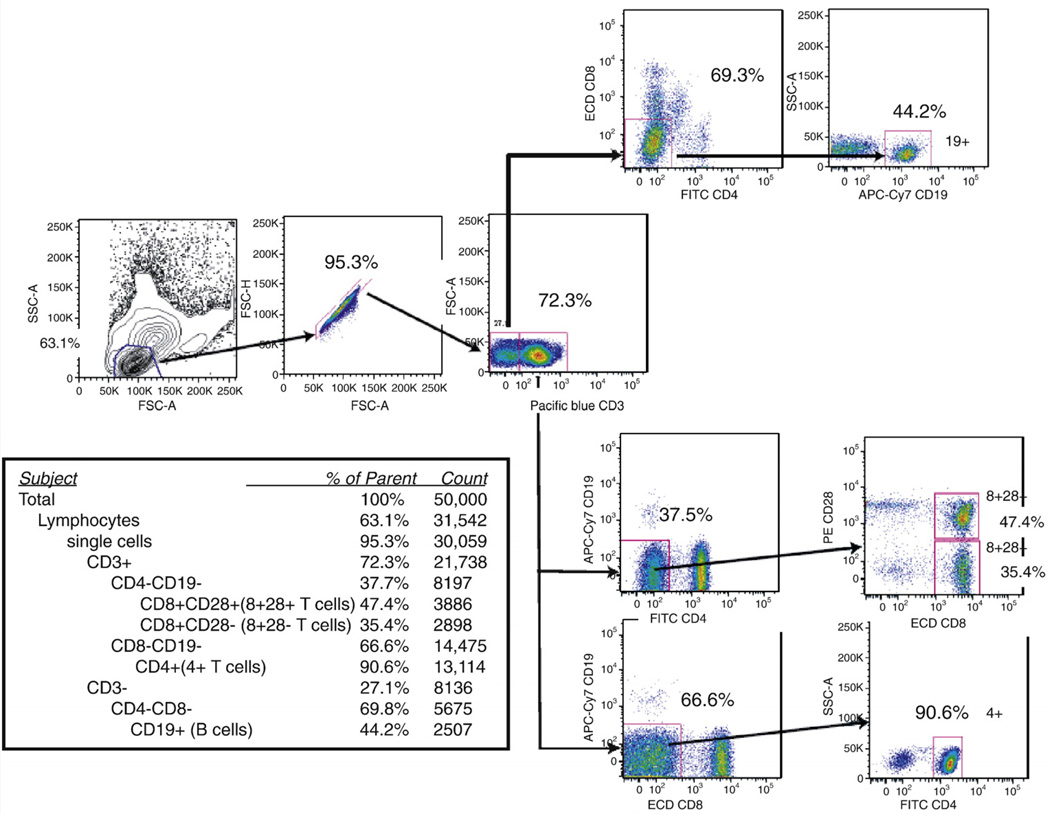
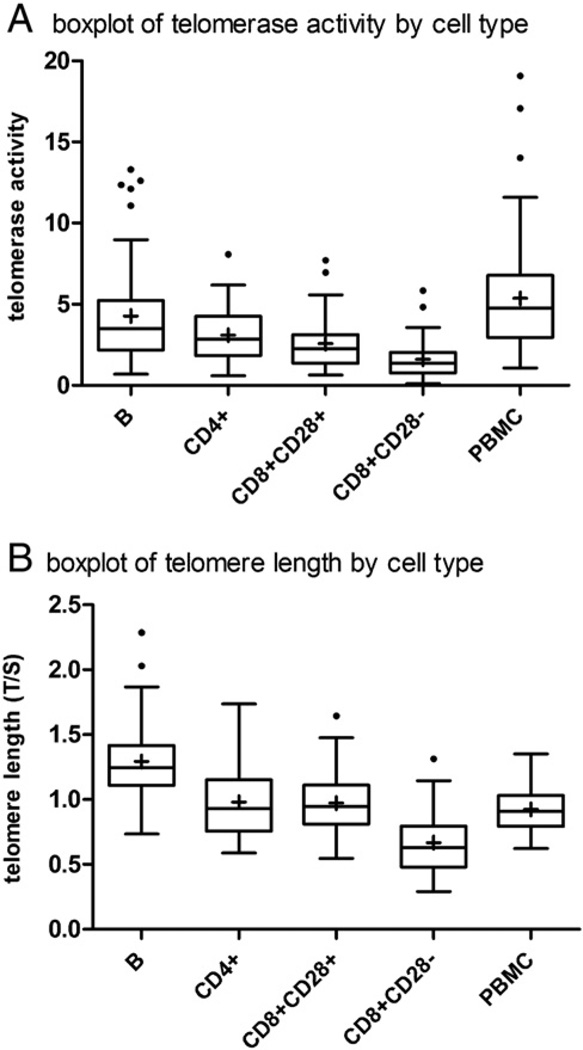
PBMCs were stained with a multicolor panel that allowed simultaneous sorting of CD4+, CD8+CD28+, CD8+CD28−T cells and B cells. An example is shown in Fig. 3. For each of the four sorted cell types and for total PBMCs, telomerase activity levels across the sample fell into a log-normal distribution (data not shown). The means and ranges of values differed for each sorted cell type. We found significant differences between cell types for telomerase activity (F = 25.8, p < 0.0001, Table 2 and Fig. 4). B cells had the highest telomerase activity per cell among the four groups (F test for contrast = 25.8, p < 0.0001). This result is consistent with previous reports (Weng et al., 1997) as well as our current finding that B cells have longer telomeres than T cells (Table 3 and Fig. 4). CD4+ T cells had slightly higher telomerase activity than CD8+CD28+ T cells, although the difference was not statistically significant (Table 2 and Fig. 4). Consistent with early reports that CD8+CD28−T cells are end-stage CD8+ T cells, they had the lowest telomerase activity among the four cell types (Table 2 and Fig. 4). Interestingly, within an individual, telomerase activity was most strongly correlated between CD8+CD28+ and CD4+ T cell types (r = 0.55, p < 0.001) (Table 5). This strong correlation may reflect common regulatory pathways for telomerase in these two cell types, which are closely related to each other in hematopoietic lineage. We found other positive, though smaller correlations between telomerase of different cell types within the same individual (Table 5).
Fig. 3.
FACS sorting panel.
Fig. 4.
Box plots for telomerase activity and telomere length.
Table 3.
Mean telomere length (t/s ratio) by cell type.
| Group | N | Cell type | Mean value | SD |
|---|---|---|---|---|
| 1 | 60 | B | 1.29 | 0.30 |
| 2 | 61 | CD4+ | 0.99 | 0.28 |
| 3 | 55 | CD8+CD28+ | 0.97 | 0.24 |
| 4 | 33 | CD8+CD28− | 0.67 | 0.24 |
| 5 | 60 | PBMC | 0.92 | 0.17 |
F-value = 57.7, for any group differences, p < 0.0001.
All pairwise differences were significant (p < 0.0001) except CD8+CD28+ and CD4+ (p = 0.99) (see supplemental Table 2).
Table 5.
Within-person correlations for telomere length and telomerase activity by cell type.
| Telomere length | Telomerase activity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | CD4+ | CD8+CD28+ | CD8+CD28− | PBMC | B | CD4+ | CD8+CD28+ | CD8+CD28− | PBMC | |
| Telomere length | ||||||||||
| B | 1 | 0.36 ** | 0.50 ** | 0.33 * | 0.35 * | 0.12 | ||||
| CD4+ | 1 | 0.46 ** | 0.67 *** | 0.25 | 0.14 | |||||
| CD8+CD28+ | 1 | 0.54 ** | 0.63 *** | −0.00 | ||||||
| CD8+CD28− | 1 | 0.40 ** | 0.13 | |||||||
| PBMC | 1 | 0.06 | ||||||||
| Telomerase activity | ||||||||||
| B | 1 | 0.35 * | 0.23 | 0.16 | 0.20 | |||||
| CD4+ | 1 | 0.55 *** | 0.44 ** | 0.02 | ||||||
| CD8+CD28+ | 1 | 0.34 * | 0.16 | |||||||
| CD8+CD28− | 1 | 0.09 | ||||||||
| PBMC | 1 | |||||||||
N varies due to missing data points, N = 30 to 61.
p < 0.05.
p < 0.01.
p < 0.001.
3.4. Telomere length in CD4+, CD8+CD28+, CD8+CD28− T cells and B cells
We also measured telomere length in CD4+, CD8+CD28+ and CD8+CD28− T cells and in B cells in samples in which enough cells were still left after removing 0.5 million cells to measure telomerase activity (Table 3). Again, significant differences were observed between cell types for telomere length (Table 3). Consistent with earlier reports that B cells have longer telomeres than T cells, B cells had the longest telomeres among the four cell subtypes we examined (F test for contrast = 57.7, p < 0.0001). Telomere lengths in CD4+ and CD8+CD28+ T cells were similar on average across the entire sample, and indeed highly correlated with each other with in an individual (Tables 3 and 5). Also consistent with the TRAP measurements, CD8+CD28− T cells had the shortest mean telomere length of the four groups (F test for contrast = 57.7, p < 0.0001). In addition, we found significant within-person correlations for telomere length in all cell types including total PBMCs (Table 5), suggesting that common determinants, which likely include both genetic and environmental factors, exist for leukocyte telomere length for the different cell types.
Most relevant to the epidemiological question of what is reflected by the commonly used measure of LTL, we found that PBMC telomere length was correlated differentially with the cell types. It was correlated somewhat weakly with B cell and CD4+ T cell telomere length (r = 0.35 and r = 0.25 respectively), and more strongly with CD8+ T cell telomere length (r = 0.63 for CD8+CD28+ T cells and r = 0.40 for CD8+CD28− T cells). Age, which is commonly related to LTL in reported studies, was unrelated to telomere length in B cells (r = 0.08, p = 0.54), CD4+ T cells (− 0.07, p = 0.60), or CD8+CD28+ T cells (r = −0.16, p = 0.24). Age appeared to be related to shorter telomere length in both CD8+CD28− T cells (r = −0.27, p = 0.12) and PBMCs (r = −0.23, p = 0.07). However, given the small sample size these correlations were not statistically significant, especially for CD8+CD28− T cells (N = 33).
We were also interested in whether percent cell type might be an informative parameter related to LTL. Thus, we examined whether presence of each cell type (as % of lymphocytes from flow cytometry) was associated with bulk mean TL in PBMCs. As shown in Table 6, the only cell type in which its frequency was weakly associated with PBMC TL was CD8+CD28− T (r = −0.26, p = 0.05; See Table 6). Therefore, percentage of each cell type was a weak indicator of PBMC TL. Lastly, we were interested in whether chronological age was associated with percentage of cell type. Age was not related to any cell type except percent CD8+CD28+ T cells, showing a modest negative correlation (r = −0.36, p = 0.003).
Table 6.
Within-person correlations for percent cell type and PBMC telomere length, N = 66.
| PBMC TL | |
|---|---|
| Percent | |
| B | 0.16 |
| CD4+ | −.06 |
| CD8+CD28+ | 0.14 |
| CD8+CD28− | −0.26* |
p = 0.05.
We found no significant intra-individual correlations between telomere length and telomerase activity in any of the four cell types or PBMCs. This was not unexpected since telomere length in a cell is determined by multiple factors including genetic inheritance, levels of telomerase previously operating in the lineage of the cell, environmental factors that influence the rate of telomere attrition and telomerase activity, and number of cell divisions (history of division). In future work, examination of the correlation between the rate of change in telomere length vs. telomerase activity level will be informative.
In summary, this is the first report that describes the quantitative measurement of both telomerase activity and telomere length simultaneously in unstimulated CD4+, CD8+CD28+, CD8+CD28−T cells and B cells as well as PBMCs from the same participant. This establishes the methodology as well as the normal range of telomerase activity values in this cohort population.
4. Discussion
The study described here is a systematic examination of telomere length and telomerase activity in the four cell types from the adaptive immune system from the same donor, analyzing natural (unstimulated) cells. While a rich body of evidence has linked leukocyte telomere length to aging and aging-related diseases, nearly all studies have used PBMCs or leukocytes for the measurement. A limitation of such studies is therefore that the measurements from PBMCs do not offer any mechanistic explanation about what types of immune cells are driving the findings. Since PBMCs and leukocytes are composed of many cell types, and the percentage of each cell type varies from individual to individual, it is difficult to discern mechanistically the role of telomere maintenance in mediating environmental and lifestyle factors and aging-related deterioration of immune function using measurement solely from PBMCs and leukocytes.
Furthermore, whether telomerase activity in unstimulated immune cells may be related to telomere length change has not been examined in humans. It has previously been reported that a lifestyle factor (chronic stress) was associated with shorter TL in PBMCs and in T cells and monocytes, suggesting a general effect of stress across two different immune cell types (Damjanovic et al., 2007). Consistent with this idea, we found significant within-person correlations between the four cell types examined here for telomerase activity. The strongest correlation was between CD4+ and CD8+CD28+ T cells, suggesting that telomerase activity in CD4+ and CD8+ T cells might be subject to the same regulatory mechanisms. Although developmental regulation of telomerase activity in T and B cells has been studied, there has been no report on whether or how telomerase activity in unstimulated T or B cells is regulated. In the future, examination of the mechanisms of how environmental and biochemical factors might regulate telomerase activity in unstimulated immune cells will provide insight on how telomerase insufficiency contributes to aging-related diseases.
One of the prevailing theories of immunosenescence is that it reflects early aging of CD8+ T cells, which can then release pro-inflammatory cytokines (Effros, 2009). Previous reports suggest that CD8+CD28−T cells, a subset of CD8+ T cells that have lost the CD28 marker (the T cell co-stimulation marker for antigen recognition (Dagarag et al., 2004)), bear hallmarks of replicative senescence (Dagarag et al., 2004). CD8+CD28− T cells show minimal replicative capacity in response to antigen stimulation and their telomeres are shorter than their CD28+ counterparts. The percentage of CD8+CD28− T cells is much higher in the elderly and in people with chronic infection (such as HIV infection). A high percentage of CD8+CD28− T cells is related to impaired response to vaccination and anti-viral function (Saurwein-Teissl et al., 2002; Goronzy et al., 2001; Effros et al., 2003), and predicts early all-cause mortality in the elderly (Wikby et al., 2002). Therefore it is of particular interest that these cells had the lowest telomerase activity, as well as the shortest telomeres, among the different cell types assayed in this study. These characteristics would likely make these CD8+ CD28− T cells the most vulnerable to dysfunctional telomere-induced cellular senescence. Further, it is of relevance to population studies of LTL and health to know what cell types LTL reflects best. Within the cell types studied here (B, CD4+, CD8+), we found that PBMC TL was correlated with shorter TL across cell types, but most weakly among CD4+ T cells, and most strongly among CD8+cells. This is despite the fact that CD4+ T cells were the most prevalent cell type in our samples (on average, 48.35% of PBMCs). In fact, when direct percentages of cells were examined, it was the percentage of senescent CD8+ T cells (CD8+CD28−) that were most correlated with PBMC TL, although the correlation was small (r = −0.26). Nevertheless, these results suggest that when blood TL is studied in large population studies, it may most reflect CD8+ T cells, the cells most indicative of immunosenescent states. Chronological age is usually weakly correlated with telomere length in large cross sectional studies. Within the narrow age range studied here, we found age was weakly and nonsignificantly related to shorter TL in PBMCs, and was significantly and inversely related to frequency of CD8+CD28+ T cells. This finding supports the theory of early immunosenescence of CD8+ T cells with aging.
One caveat to this study is the possibility of ongoing immune response in some of the subjects that could alter the telomerase activity of certain cell populations at the time of the blood draw. This is addressed in our study by excluding participants with elevated white blood cell counts or core body temperature at the time of visit. These participants were rescheduled for blood draw. In addition, we exclude major chronic disease, autoimmune disorders, severe asthma, history of stroke, epilepsy, or brain injury; conditions that might complicate telomerase and telomere length measurement. However, it still remains possible after these precautions that some subjects may have ongoing immune response.
Another limitation of the current study is that it is unclear whether the low level of telomerase detected in these lymphocytes reflects overall low level of telomerase activity in each cell or rather a different number of activated cells which have high levels of telomerase activity mixed in the mostly resting lymphocyte population. To definitively address this issue requires telomerase activity measurement in single cells, which is not possible with the current technique. We aimed to address this question by examining the correlation between activation marker and telomerase activity. CD69 marker was measured in T cells and B cells of 25 participants in this study, we found no correlation between the percentage of CD69+ cells and telomerase activity in total PBMC, CD8+CD28+, CD8+CD28− and B cells. A negative correlation was found between percentage of CD69+ cells and telomerase activity in CD4+ T cell (r = −0.39, p = 0.05). While this correlation may be interesting, it does not support the hypothesis that the overall telomerase activity in each cell type is the net result of some activated cells with high telomerase activity and majority of cells with extremely low or no telomerase activity.
Clearly, more work needs to be done examining other cell types and senescent markers to understand more fully what LTL represents. The current study provides new and relevant methodological information and a foundation for future studies on the mechanisms by which genetic and environmental factors and lifestyle changes contribute to telomere-based cellular aging in humans.
Supplementary Material
Acknowledgements
We thank the study participants for their support and generous contribution of time. We thank Dr. Richard Cawthon for his technical advice on the telomere length measurement method. Jue Lin and Joshua Cheon were supported by the Bernard and Barbro Foundation. The Gladstone flow core and the Core Immunology Lab were supported by The UCSF-GIVI Center for AIDS Research P30AI027763.
The Core Immunology Lab was also supported by NIH/NCRR UCSF-CTSI grant number UL1 RR024131-01. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jim.2009.09.012.
Contributor Information
Jue Lin, Email: jue.lin@ucsf.edu.
Elissa Epel, Email: EEpel@lppi.ucsf.edu.
Joshua Cheon, Email: Joshua.cheon@ucsf.edu.
Candyce Kroenke, Email: candyce.kroenke@ucsf.edu.
Elizabeth Sinclair, Email: esinclair@sfgh.ucsf.edu.
Marty Bigos, Email: bigos@stanford.edu.
Owen Wolkowitz, Email: OwenW@lppi.ucsf.edu.
Synthia Mellon, Email: mellon@cgl.ucsf.edu.
Elizabeth Blackburn, Email: elizabeth.blackburn@ucsf.edu.
References
- Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195:83. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J. Clin. Endocrinol. Metab. 2006;91:635. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl. Acad. Sci U. S. A. 1995;92:9082. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2003;23:842. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Carrero JJ, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, Barany P, Radhakrishnan K, Lindholm B, Soveri I, Nordfors L, Shiels PG. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J. Intern. Med. 2008;263:302. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Collerton J, Martin-Ruiz C, Kenny A, Barrass K, von Zglinicki T, Kirkwood T, Keavney B. Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur. Heart J. 2007;28:172. doi: 10.1093/eurheartj/ehl437. [DOI] [PubMed] [Google Scholar]
- Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J. Immunol. 2004;173:6303. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J. Immunol. 2007;179:4249. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Kleemeier Award Lecture 2008—the canary in the coal mine: telomeres and human healthspan. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:511. doi: 10.1093/gerona/glp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit. Rev. Immunol. 2003;23:45. doi: 10.1615/critrevimmunol.v23.i12.30. [DOI] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007;165:14. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001;75:12182. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci. Lett. 2006;406:260. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, Shay JW, Ishioka S, Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 1995;155:3711. [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann. Neurol. 2006;60:181. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Kim NW, Wu F. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP) Nucleic Acids Res. 1997;25:2595. doi: 10.1093/nar/25.13.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am. J. Epidemiol. 2008;167:799. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004;117:2417. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- Luiten RM, Pene J, Yssel H, Spits H. Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T-cell clones and confers resistance to oxidative stress-induced apoptosis. Blood. 2003;101:4512. doi: 10.1182/blood-2002-07-2018. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Roth A, Yssel H, Pene J, Chavez EA, Schertzer M, Lansdorp PM, Spits H, Luiten RM. Telomerase levels control the lifespan of human T lymphocytes. Blood. 2003;102:849. doi: 10.1182/blood-2002-07-2015. [DOI] [PubMed] [Google Scholar]
- Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 2002;168:5893. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ. Res. 2004;94:575. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- Starr JM, McGurn B, Harris SE, Whalley LJ, Deary IJ, Shiels PG. Association between telomere length and heart disease in a narrow age cohort of older people. Exp. Gerontol. 2007;42:571. doi: 10.1016/j.exger.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann. N. Y. Acad. Sci. 2000;908:99. doi: 10.1111/j.1749-6632.2000.tb06639.x. [DOI] [PubMed] [Google Scholar]
- Weng NP. Telomere and adaptive immunity. Mech. Ageing Dev. 2008;129:60. doi: 10.1016/j.mad.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng NP, Granger L, Hodes RJ. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci U. S. A. 1997;94:10827. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 2002;37:445. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- Zhai G, Aviv A, Hunter DJ, Hart DJ, Gardner JP, Kimura M, Lu X, Valdes AM, Spector TD. Reduction of leucocyte telomere length in radiographic hand osteoarthritis: a population-based study. Ann. Rheum. Dis. 2006;65:1444. doi: 10.1136/ard.2006.056903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kong Q, Zhang Z, Ge P, Ba D, He W. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cogn. Behav. Neurol. 2003;16:170. doi: 10.1097/00146965-200309000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.