Abstract
Purpose of review
The Wnt/β-catenin signaling pathway plays a critical role in development and adult tissue homeostasis. Recent investigations implicate Wnt/β-catenin signaling in abnormal wound repair and fibrogenesis. The purpose of this review is to highlight recent key studies that support a role for Wnt/β-catenin signaling in fibrosis.
Recent findings
Studies of patients with fibrotic diseases have demonstrated changes in components of the Wnt/β-catenin pathway. In animal models, perturbations in Wnt/β-catenin signaling appear to aggravate or ameliorate markers of injury and fibrosis in a variety of different tissues. Studies also suggest that fibroblasts from different tissue sources may have markedly divergent responses to Wnt/β-catenin signaling. Cross-talk between Wnt/β-catenin and transforming growth factor-β pathways is complex and context-dependent, and may promote fibrogenesis through coregulation of fibrogenic gene targets. High throughput screening has identified several novel chemical inhibitors of Wnt/β-catenin signaling that may be of therapeutic potential.
Summary
Wnt/β-catenin signaling appears important in normal wound healing and its sustained activation is associated with fibrogenesis. The mechanism by which Wnt/β-catenin signaling may modify the response to injury is cell-type and context-dependent. Better understanding of this signaling pathway may provide a promising new therapeutic approach for human fibrotic diseases.
Keywords: β-catenin, fibrosis, Wnt, wound repair
Introduction
Fibrotic diseases may be attributable to a variety of causes, but it is generally thought that an initiating injury event activates repair processes that aim to restore the original tissue architecture, and a failure to finely tune the repair process leads to persistent fibroblast activation and tissue destruction. Thus, understanding the molecular events that drive fibroproliferation and matrix deposition has been a favored area of investigation. An emerging paradigm in the field of fibrosis is that persistent activation of signaling pathways required for normal embryonic development may drive abnormal wound healing and tissue repair. The Wnt/β-catenin signaling pathway is one of a core set of evolutionarily conserved signaling pathways that regulates diverse cellular outcomes. Although the roles of Wnt/β-catenin signaling in embryogenesis and adult tissue homeostasis are well known, the consequences of inappropriate Wnt/β-catenin signaling to fibrogenesis are just beginning to be understood. In this review, we discuss recent findings that support a contribution of Wnt/β-catenin signaling in abnormal wound healing and propose possible mechanisms by which this pathway may drive fibrogenesis. We also highlight recently identified small molecule inhibitors of Wnt/β-catenin signaling that are being examined in various animal models of tissue fibrosis to determine feasibility of targeting this pathway in human fibrotic diseases.
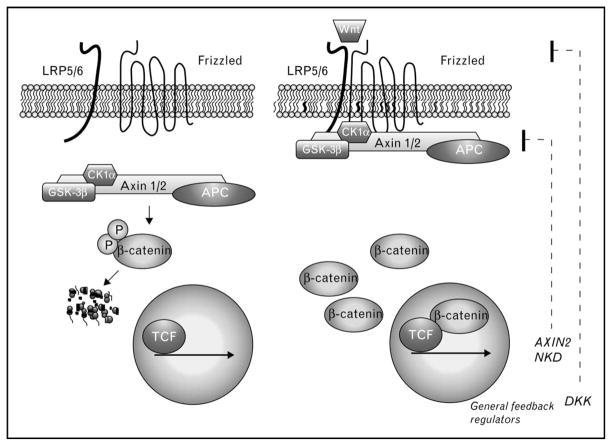
Canonical Wnts are secreted lipoglycoproteins that are used reiteratively throughout development and adult tissue homeostasis to instruct cells to adopt particular fates. Wnt-mediated cell fate specification is ultimately controlled by a transcription complex that contains the dual signaling/adhesion protein, β-catenin, and its DNA-binding partners known as lymphocyte enhancer factor (LEF)/T-cell factor (TCF) (Fig. 1, right). In this complex, β-catenin serves as an obligate coactivator through its ability to recruit components that promote chromatin remodeling and transcriptional initiation/elongation [1]. Wnts generate the nuclear signaling pool of β-catenin by inhibiting a multiprotein ‘destruction complex’ that continually phosphorylates β-catenin, flagging it for degradation by the ubiquitin/proteosome system (Fig. 1, left). Inhibition of this phosphorylation allows β-catenin to accumulate in the cytoplasm and enter the nucleus, in which it ultimately complexes with TCF-family proteins localized at gene promoters (Fig. 1, right).
Figure 1. Wnt pathway basics.
In the absence of Wnt (left), cytosolic β-catenin is continually phosphorylated by casein kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK-3β) within an Axin1 scaffold complex. This phosphorylation allows β-catenin to be recognized by a specific E3 ligase (βTrCP, not shown), which catalyzes the ubiquitylation and rapid degradation of β-catenin. The adenomatous polyposis coli (APC) tumor suppressor participates in the phosphodestruction of β-catenin by antagonizing β-catenin dephosphorylation by phosphatases. During Wnt activation (right), GSK3β activity is inhibited directly by LDL receptor related protein 5 and 6 (LRP5/6), which allows β-catenin to accumulate, enter the nucleus, interact with LEF/TCF family members and activate target genes. Fibrosis-relevant targets of Wnt/β-catenin signaling remain to be clarified. General targets of Wnt/β-catenin signaling include negative feedback regulators, Axin2, Naked (NKD) and Dickkopf (DKK).
Although β-catenin/TCF-mediated transcription occurs in all tissues, the genes activated by this transcriptional complex are remarkably cell-type and context-dependent (reviewed by [2]). For example, in self-renewing tissues such as intestine and blood, β-catenin/TCF transcription maintains the dedifferentiated, progenitor/stem cell fate [3–5]. In other tissue/cell types, β-catenin/TCF signaling promotes cellular differentiation, such as paneth cell specification within intestinal crypts [6], whereas a gradient of Wnt signaling exists along the portocentral axis in adult liver to express metabolic genes (e.g. glutamate synthetase) required for ammonia detoxification [7]. It is worth noting that some targets of β-catenin signaling appear to be universal, particularly, negative feedback components in the pathway such as Axin2, Dickkopf 1 and Naked (Fig. 1, right), which are typically followed to provide evidence for β-catenin signaling across many cell types. Nonetheless, the studies above indicate that β-catenin targets need to be assessed within individual cell types and conditions to understand the contribution of Wnt/β-catenin signaling to a particular tissue structure and function.
Evidence for Wnt signaling in fibrosis: lessons from skin
A critical role for Wnt/β-catenin signaling a fibroproliferative disorder was initially supported by the high rate of mutations detected in β-catenin (and its negative regulator, APC) in aggressive fibromatosis, which are desmoids tumors comprising a proliferation of cytologically benign fibroblasts [8,9]. Cutaneous wound healing studies in mice show that β-catenin signaling is indeed activated as a consequence of wounding [10••,11] and forced activation of β-catenin using a stabilized mutant that resists ubiquitin-mediated degradation (Catnbex3) is sufficient to drive hyperplastic wounds and exuberant collagen synthesis, mimicking aggressive fibromatoses in humans [12]. Conversely, removal of β-catenin using Cre-loxP technology resulted in smaller wounds [13]. These and other studies have led to a general model for how β-catenin signaling drives fibroproliferative disorders. In this model, activation of β-catenin signaling in fibroblasts imposes a healing phenotype by promoting fibroblast proliferation, migration and local invasion, and a failure to dampen this normal response results in hyperplastic wounds. This model predicts that in fibrotic diseases, Wnts and positive regulators of β-catenin signaling may be upregulated, whereas inhibitors of Wnt/β-catenin signaling are downregulated. Indeed, many recent studies support this prediction (see below) and suggest that the components of the Wnt/β-catenin pathway that ‘fine-tune’ the signal may be key drivers of β-catenin-mediated fibrosis.
Evidence for systemic elevation of Wnts in fibrosis?
Wnts are secreted glycoproteins typically viewed to diffuse over only a limited number of cell diameters in tissues [14]. However, recent studies suggest that Wnt activity can be detected in sera of aged or injured mice, in which it can promote skeletal muscle atrophy/fibrosis. For example, Brack et al. [15] used parasymbiosis of young and aged mice to demonstrate that the bloodstream of aged mice could drive muscle stem cells down a fibroblastic as opposed to myogenic lineage, resulting in fibrosis at the expense of muscle maintenance. Remarkably, this activity could be depleted by the Wnt inhibitor, secreted frizzled-related protein (SFRP) 3, suggesting that elevation of Wnts in the circulation can drive a form of fibrosis. Similar conclusions were drawn in mouse model of Duchenne Muscular Dystrophy, in which loss of dystrophin leads to upregulation of Wnt-activity in serum that promotes expansion of Sca1+ stromal cells and fibrosis [16•]. Evidence that Wnt activity can be detected in serum raises the intriguing possibility that Wnts or Wnt-based activity may serve as an accessible biomarker for fibrotic diseases.
Secreted frizzled-related proteins in tissue injury and fibrosis
SFRPs structurally resemble Wnt frizzled receptors and can inhibit β-catenin signaling by working as Wnt-decoy receptors [17]. Interestingly, recent studies show evidence of SFRP downregulation in fibroblasts recovered from fibrotic lesions, raising the possibility that fibroblasts from fibrotic lungs may be more sensitized to Wnt signals. For example, fibroblasts from both systemic sclerosis (SSc) fibrotic lungs and idiopathic pulmonary fibrosis (IPF) lungs express less SFRP1 compared with controls [18••], whereas a reduction in the level of SFRP1 was independently observed in fibroblasts derived from keloid lesions [19•]. In this latter study, SFRP1 silencing could be reversed in the presence of Trichostatin A, an inhibitor of histone deacetylation. Together, these data indicate that fibroblasts derived from fibrotic lesions can maintain genetic differences in cell cultures, and more important, suggest that epigenetic regulation of Wnt inhibitors like SFRP1 may contribute to persistent β-catenin signaling in these types of fibrosis.
Because SFRPs target the Wnt pathway in the extra-cellular space, there is a lot of interest in assessing whether recombinant SFRPs have therapeutic potential, and a number of studies show that recombinant SFRPs can limit collagen abundance and improve tissue structure/function in various injury models [20,21••,22•]. Although the mechanism through which SFRPs inhibit collagen synthesis is likely to be indirect and complex (see below), it is important to note that SFRPs can have Wnt-independent functions, as SFRP2 inhibits Bmp1/Tolloid-like metalloproteinases [23], which cleave the C-terminal peptides from procollagen [21••]. These data indicate that SFRP2 may be doubly relevant to fibrogenesis, serving to both antagonize Wnt receptor engagement and inhibit collagen processing/maturation.
What are the key targets of β-catenin signaling in fibrosis?
If the contribution of Wnt/β-catenin signaling to fibrogenesis is becoming increasingly clear, the cellular and molecular targets are less so. With regards to cellular targets, evidence for mutational activation of β-catenin signaling in fibroblasts from aggressive fibromatosis-associated desmoids tumors certainly suggests a key role for β-catenin signaling in fibroproliferation. Indeed a number of in-vitro studies support this idea, showing that Wnt/β-catenin signaling activation in fibroblast cultures enhances the proliferative, migratory and matrix producing aspects of these cells [10••,24]. However, some exceptions are noteworthy and serve to reiterate the heterogeneous nature of fibroblasts and the context-dependent nature of Wnt signals. For example, embryonic and postnatal fibroblasts derived from mouse skin respond to Wnt3a differently, despite similar inducibility of AXIN2 [10••]. Specifically, Wnt3a induces cell proliferation as well as TGFβ1 and collagen 1 expression in postnatal fibroblasts, but, in embryonic stage fibroblasts, Wnt3a does not promote proliferation and instead induces TGFβ3, which is typically associated with ‘scarless’ wound healing. In addition, although Wnt/β-catenin signaling promotes collagen gel contraction, αSMA expression, and cell migration in human dermal fibroblasts [24] and mouse embryonic fibroblasts [25•], our group found no significant contribution of β-catenin signaling to TGFβ1, collagen 1 (COL1) and alpha smooth muscle actin (aSMA) in three independent adult lung fibroblast lines [26•]. Altogether, these data indicate that the developmental stage and site of origin appear to impact the physiological targets of Wnt signaling in fibroblasts, which may be not surprising given evidence that fibroblasts show topographic diversity and positional memory through distinct Hox genes [27]. Nonetheless, although a shared set of Wnt/β-catenin regulated profibrotic genes would be expected to be found across the various fibroblast types, the limited studies available reveal few highly altered common targets (NIH373 [28], human dermal fibroblasts and CCL-186 fetal human lung fibroblasts [29]).
Given that Wnt signaling is known to control cell fate decisions throughout development and in adult stem cells, perhaps the relevant target of β-catenin-mediated fibrogenesis is a progenitor cell type that gives rise to fibroblasts, rather than a mature fibroblast. It is well known that β-catenin signaling antagonizes adipogenesis by inhibiting adipogenic transcription factors CCAAT/enhancer binding protein α (C/EBP-α) and peroxisome proliferator-activated receptor γ [30]. The observation that patients with systemic sclerosis seem to have a diminished adipose layer in their fibrotic skin suggests that fibrogenesis may occur at the expense of fat. Supporting this clinical finding, mice expressing Wnt10b under control of fatty acid binding protein 4 (FABP4) show progressive loss of subcutaneous and visceral adipose tissue with concomitant dermal fibrosis, as evidenced by increased collagen deposition, fibroblast activation, and myofibroblast accumulation [31••]. Moreover, explanted fibroblasts from these mice maintained increased Wnt signaling as well as elevated COL1 and aSMA message levels. Other studies also find that Wnt/β-catenin signaling antagonizes adipogenic gene expression and promotes dedifferentiation toward a myofibroblastic phenotype in both hepatic lipofibroblast [32] and 3T3-L1 cells [33•]. Altogether, it seems likely that a key target of Wnt/β-catenin signaling in fibrotic disease may be a multipotent adipogenic progenitor that can be diverted toward a fibroblastic fate. Future lineage tracing studies will be required to support this concept.
A discussion of potential key molecular targets of β-catenin signaling relevant to fibrosis would not be complete without mention of the most well known profibrotic cytokine, TGF-β, as work from a variety of cell types has shown evidence of cross-talk between the Wnt/β-catenin and TGF-β pathways. For example, Wnt/β-catenin signaling can upregulate the expression of TGF-β [10••,13], and TGF-β1 can promote β-catenin signaling [13,34–36]. Moreover, mice lacking SMAD3 show less β-catenin stabilization and activation during cutaneous wounding, whereas β-catenin null fibroblasts block the ability of TGF-β to promote proliferation in these cells [13]. In some cases, β-catenin and TGF-β synergize to coregulate the same gene through Smad and TCF-binding sites within the promoter [37]. However, cellular context likely matters, as other studies provide evidence for mutual antagonism between β-catenin and TGF-β signaling [38,39]. Altogether, these data indicate that β-catenin/TGFβ cross-regulation is complex. Whether the profibrotic effects of β-catenin signaling are largely mediated through TGF-β signaling is presently unclear.
Potential therapeutics
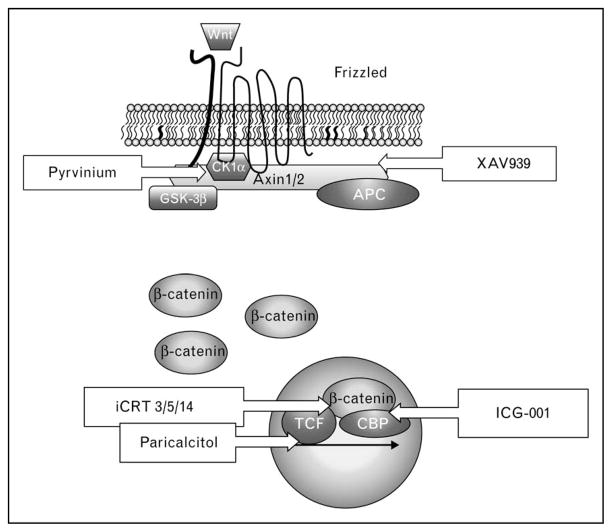
As suggested from the SFRP studies mentioned above, targeting the Wnt/β-catenin pathway may be a strategy for the treatment of fibrosis. A number of high-throughput screens have identified Wnt pathway inhibitors for potential therapeutic use (Fig. 2) [40••,41,42••]. ICG-001 interacts with cyclic AMP response element binding (CREB)-binding protein (CBP) and specifically blocks the β-catenin/CBP interface, which is required for the activation of a subset of β-catenin gene targets [43]. Recently, this compound was shown to reduce manifestations of lung fibrosis in the bleomycin mouse model [40••]. Other promising compounds include inhibitors of catenin-responsive transcription (iCRT) 3, 5 and 14, which appear to target the β-catenin/TCF interface but spare other critical functions of β-catenin (e.g. in cadherin-based adhesion) [42••]. XAV939 inhibits the stabilization and nuclear accumulation of β-catenin by targeting tankyrase 1 and 2, which negatively regulates Axin levels [41]. The antiparasitic drug, pyrvinium, inhibits β-catenin signaling at multiple levels in the pathway, activating CK1α, stabilizing Axin and inhibiting nuclear coactivators of β-catenin [44••]. Recent data indicate that pyrvinium may have therapeutic benefit in a coronary artery ligation model of myocardial infarct [45•,46]. Lastly, paricalcitol is an FDA-approved synthetic analog of vitamin D2, and early studies indicated that vitamin D analogs could promote the differentiation of colon carcinoma cells by inhibiting β-catenin signaling through competition between a ligand-activated vitamin D receptor and TCF-4 for β-catenin [46]. This inhibitor recently showed therapeutic benefit in an adriamycin-induced model of nephropathy and fibrosis, attenuating the expression of a number of Wnts, β-catenin signaling and markers of fibrotic repair, such as TGFβ1, connective tissue growth factor, fibronectin, collagen, and aSMA [47•].
Figure 2. Inhibitors of Wnt/β-catenin and their sites of action.
APC, adenomatous polyposis coli; CBP, CREB binding protein; CK1α, casein kinase 1α; GSK-3β, glycogen synthase kinase 3β; iCRT, inhibitor of catenin responsive transcription; TCF, T-cell factor. See text for details.
Conclusions
Over the past decade, significant progress has been made toward building a case that excessive Wnt/β-catenin signaling can promote aspects of fibrogenesis across a number of tissue types and cell systems. Less clear is the precise means through which this occurs. Does Wnt/β-catenin signaling simply collaborate with the classic profibrotic, TGFβ, to promote matrix synthesis and assembly or does it primarily control cell fate decisions that lead to an excessive number of fibroblasts at the expense of other cell types? Perhaps more importantly is whether Wnt/β-catenin signaling merely contributes to or is a key driver of fibrotic disease. Evidence for genetic or epigenetic alterations in Wnt pathway components that correlate with disease onset or severity would serve to answer this question. Lastly, regardless of whether β-catenin signaling emerges as a key driver or one of many signaling pathways promoting fibrosis, it is exciting to see a variety of Wnt pathway inhibitor compounds available for our community to determine therapeutic feasibility in models of fibrotic disease.
Key points.
Wnt/β-catenin signaling is implicated in fibrogenesis is a variety of tissues.
The effects of Wnt/β-catenin signaling are tissue-type and cell-context-dependent.
Novel small molecule inhibitors of Wnt/β-catenin signaling may provide therapeutic potential for fibrotic diseases.
Acknowledgments
The authors are supported by the following sources of funding: NIH-GM076561 and HL094643 to C.J.G. and NHLBI K08HL093216 and P30HL101292 to A.P.L.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 622–623).
- 1.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 2.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 4.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 6.van Es JH, Jay P, Gregorieff A, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 7.Sekine S, Lan BY, Bedolli M, et al. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 8.Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor) Oncogene. 1999;18:6615–6620. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- 9.Alman BA, Li C, Pajerski ME, et al. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- 10••.Carre AL, James AW, MacLeod L, et al. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plastic and reconstructive surgery. 2010;125:74–88. doi: 10.1097/PRS.0b013e3181c495d1. Embryonic and postnatal mice showed differences in wound healing and response to Wnt signal. [DOI] [PubMed] [Google Scholar]
- 11.Fathke C, Wilson L, Shah K, et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC cell biology. 2006;7:4. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheon SS, Cheah AY, Turley S, et al. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheon SS, Wei Q, Gurung A, et al. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 14.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 16•.Trensz F, Haroun S, Cloutier A, et al. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am J Physiol. 2010;299:C939–C947. doi: 10.1152/ajpcell.00253.2010. In a mouse model of muscular dystrophy, Wnt3a increases proliferation of the Sca1+ mesenchymal stem cells. [DOI] [PubMed] [Google Scholar]
- 17.Jones SE, Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 18••.Hsu E, Shi H, Jordan RM, et al. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–794. doi: 10.1002/art.30159. This is the first study comparing gene expression profiling of lungs and fibroblasts from both SSC and IPF lungs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Russell SB, Russell JD, Trupin KM, et al. Epigenetically altered wound healing in keloid fibroblasts. J Invest Dermatol. 2010;130:2489–2496. doi: 10.1038/jid.2010.162. Keloid fibroblasts show silencing of SFRP1 that may be epigenetically regulated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 21••.He W, Zhang L, Ni A, et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. SFRP2 negatively regulates BMP1 and is protective against myocardial injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Matsushima K, Suyama T, Takenaka C, et al. Secreted frizzled related protein 4 reduces fibrosis scar size and ameliorates cardiac function after ischemic injury. Tissue Eng Part A. 2010;16:3329–3341. doi: 10.1089/ten.tea.2009.0739. SFRP4 is increased in ischemic heart tissue and improves myocardial injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraoka O, Shimizu T, Yabe T, et al. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- 24.Poon R, Nik SA, Ahn J, et al. Beta-catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC cell biology. 2009;10:38. doi: 10.1186/1471-2121-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a Induces Myofibroblast Differentiation by Upregulating TGF-beta Signaling Through SMAD2 in a beta-Catenin-Dependent Manner. PLoS One. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. Mouse embryonic fibroblasts do not show increased proliferation in the presence of Wnt3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Lam AP, Flozak AS, Russell S, et al. Nuclear {beta}-catenin is increased in SSc pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0113OC. [Epub ahead of print] Human adult lung fibroblasts from three independent sources do not demonstrate Wnt-mediated activation of TGF-β and classic profibrotic genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, McLean S, Carter DE, Leask A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J Cell Commun Signal. 2007;1:175–183. doi: 10.1007/s12079-007-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klapholz-Brown Z, Walmsley GG, Nusse YM, et al. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2:e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 31••.Wei J, Melichian D, Komura K, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. Wnt10b expression driven by FABP4 in mice demonstrate progressive loss of subcuteneous and visceral adipose tissue with concomitant development of dermal fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng JH, She H, Han YP, et al. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G39–G49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- 33•.Gustafson B, Eliasson B, Smith U. Thiazolidinediones increase the wingless-type MMTV integration site family (WNT) inhibitor Dickkopf-1 in adipocytes: a link with osteogenesis. Diabetologia. 2010;53:536–540. doi: 10.1007/s00125-009-1615-1. Activation of Wnt signaling in human adipocyte and 3T3L1 fibrocytes shows transdifferentiation to a myofibroblastic phenotype. [DOI] [PubMed] [Google Scholar]
- 34.Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- 35.Satterwhite DJ, Neufeld KL. TGF-beta targets the Wnt pathway components, APC and beta-catenin, as Mv1Lu cells undergo cell cycle arrest. Cell Cycle. 2004;3:1069–1073. [PubMed] [Google Scholar]
- 36.Cheon SS, Nadesan P, Poon R, Alman BA. Growth factors regulate beta-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp Cell Res. 2004;293:267–274. doi: 10.1016/j.yexcr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci U S A. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y, Drissi H, Chen M, et al. Wnt-mediated regulation of chondrocyte maturation: modulation by TGF-beta. J Cell Biochem. 2005;95:1057–1068. doi: 10.1002/jcb.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang MH, Wendland JR, Chuang DM. Lithium inhibits Smad3/4 trans-activation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol Cell Neurosci. 2008;37:440–453. doi: 10.1016/j.mcn.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Henderson WR, Jr, Chi EY, Ye X, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. ICG-001 blocks β-catenin/CBP interaction and prevents bleomycin-induced pulmonary fibrosis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 42••.Gonsalves FC, Klein K, Carson BB, et al. Feature article: from the cover –an RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci U S A. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. The small molecule compounds iCRT3, 5, and 14 inhibit the β-catenin/TCF interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eguchi M, Nguyen C, Lee SC, Kahn M. ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem. 2005;1:467–472. doi: 10.2174/1573406054864098. [DOI] [PubMed] [Google Scholar]
- 44••.Thorne CA, Hanson AJ, Schneider J, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. Pyrvinium inhibits β-catenin signaling at the level of the destruction complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Saraswati S, Alfaro MP, Thorne CA, et al. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. This is the first in-vivo model demonstrating a protective effect of pyrvinium in cardiac injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2010;22:90–103. doi: 10.1681/ASN.2009121236. This is the first application of paricalcitol for the treatment of fibrosis in a kidney injury model. [DOI] [PMC free article] [PubMed] [Google Scholar]