Abstract
Collaborative work between experimentalists and computational chemists have demonstrated a stong synergy which allowed the rationalization of allenyl azide chemistry and permited the development of an efficient synthetic tool aimed at the preparation of several alkaloids. Saturated allenyl azides undergo a reaction cascade involving key diradical intermediates that follow the Curtin-Hammett model whereas unsaturated allenyl azides form indolidene intermediates that furnish the final indole products via electrocyclic ring closure events taking place out of the Curtin-Hammett regime. The regiochemistry of the reaction cascade with the latter substrates can be manipulated by Cu(I) addition to the reaction mixture.
Keywords: Allenyl azide, azatrimethylenemethane, indole, DFT
1. INTRODUCTION
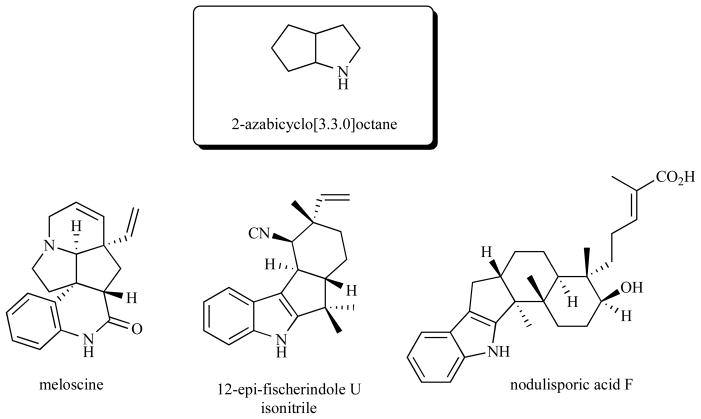
The total chemical synthesis of structurally complex natural products remains a vital component of the organic chemistry practice. Synthesis campaigns can provide a practical source of potential lead compounds for pharmaceutical advances, promote the development of new chemistry, and, in the most favorable cases, provide an intellectually satisfying bridge between science and artistic creativity. Within this framework, biologically active alkaloids comprise a broad class of challenging targets for total synthesis, and any emerging methodology that can address the synthesis demands of some subset of structurally related members of this family can be of great value. One such structural component characteristic of different classes of alkaloids is the 2- azabicyclo[3.3.0]octane core (Fig. 1). Alkaloid families that feature this bicyclic moiety are exemplified by the representative members meloscine (1) from the Melodinus alkaloids, 12-epi-fischerindole U isonitrile (2) from the fisherindole alkaloids, and nodulisporic acid F (3) from the indole diterpenoid alkaloids.
Fig. 1.
Representative 2-azabicyclo[3.3.0]octane-containing natural alkaloids.
In our labs, we set out to develop an efficient and potentially general method for the assembly of suitably functionalized versions of the 2-azabicyclo[3.3.0]octane core that could serve as centerpieces of synthesis strategies for the targets illustrated in Fig. (1). Due to the high structural complexity surrounding the 2-azabicyclo[3.3.0]octane core in most alkaloids, any synthetic methodology aiming to build this motif needs to be very forgiving of sterically congested environments. It is with this focus in mind that we decided to explore the chemistry of diradicals.
Diradical-mediated reactions have received only episodic interest in organic synthesis. Both a paucity of general diradical generating methods and a concern for the possibly indiscriminate reactivity of these high-energy species may have contributed to this lack of attention. It is, however, their high reactivity that allows radicals to perform complex reactions under uniquely mild conditions. In fact, the intramolecular collapse of diradical intermediates seemed like an ideal candidate reaction to forge C—C bonds in challenging environments. By this line of thinking, reactions that proceed through intramolecular diradical closures offer the promise of facile bond formation in congested locations as a consequence of the typically minimal activation barriers for diyl union compared with heteropolar and/or closed-shell alternatives.
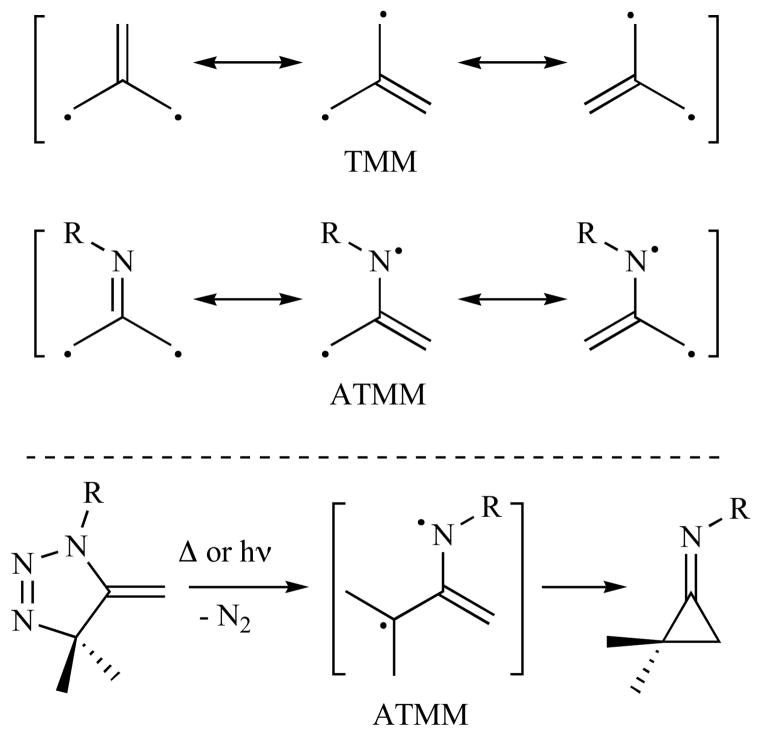
One of the most notable motifs among the diradical species employed in C—C bond forming reactions is the trimethylenemethane (TMM) diyl, which has been a synthetic cornerstone in numerous cyclization- and cycloaddition-based approaches to cyclopentanoid natural products [1–3]. A single-atom substitution within this motif that has not received much attention is N-for-C, which affords the azatrimethylenemethane (ATMM) diyl (Fig. 2). By analogy with TMM chemistry, the ATMM variant might provide ready access to polycyclic nitrogen-containing cyclopentanoid alkaloid-like frameworks. The development of ATMM chemistry has lagged far behind its all-carbon relative, but early reports suggested its intermediacy in “triaza” decomposition chemistry [4–6], and seminal investigations by Quast on triazoline decomposition chemistry in the 1970’s--1980’s provided the first systematic and robust evidence for the existence of this elusive species [7–12]. Futhermore, this earlier work also led to the expectation that (1) intermolecular allene/azide cycloaddition would not be a viable route to precursor triazolines due to incompatible regiochemistry, and (2) facile ATMM closure to the iminocyclopropane might render diyl capture problematic (Fig. 2).
Fig. 2.
Trimethylenemethane (TMM) and azatrimethylenemethane (ATMM) diyl structures.
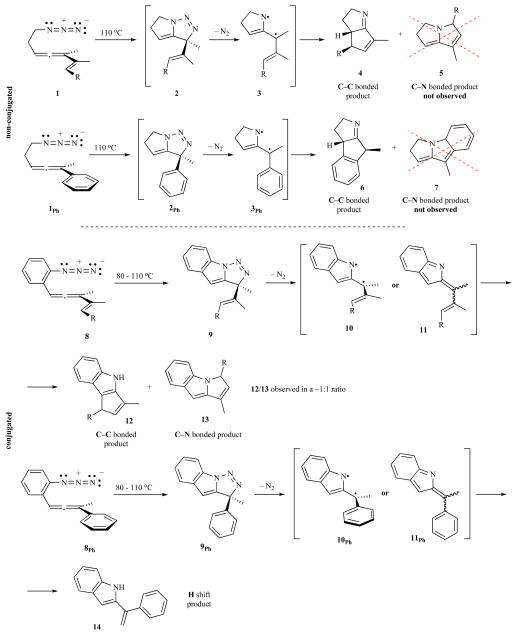
The intramolecular version of the allene/azide cycloaddition could, in principle, alleviate part of the aforementioned concerns associated with ATMM generation and subsequent chemistry. Preliminary experimental work on the chemistry and the scope of this reaction provided promising results, but also left a number of questions unanswered (Fig. 3) [13, 14]. In the first exploratory campaign [13] the thermochemistry of 5-azidoallenes, exemplified by 1 and its aryl analogue 1Ph provided indirect evidence for the intermediacy of an ATMM species in an apparent cycloaddition-extrusion-collapse reaction cascade (see Fig. 3). The complete regioselectivity of this reaction towards the C—C bonded cores 4 and 6 renders this protocol quite useful for the preparation of several polycyclic natural alkaloids (see Fig. 1). This reaction not only proceeds with complete regioselectivity but, additionally, it also furnishes a single diasteromer of the bicyclic product. This degree of control is unexpected, taking into account the modest levels of diastereoselectivity typically reported for diradical collpase in other systems [15]. In a second series of experiments [14], o-azido phenylallene derivatives 8 also were tested and, surprisingly, their thermal transformations did not exhibit the complete regioselectivity of the former non-conjugated species. Mixtures of C—N and C—C bonded adducts were obtained in similar amounts (C—C/C—N ratios varying from 1:1.5 to 2.7:1). Moreover, its aryl analogue 8Ph afforded neither the corresponding C—C nor the C—N bonded adduct. Instead, a sigmatropic H-shift product was obtained.
Fig. 3.
Preliminary results and mechanistic speculation for the azide-allene cyclization cascade sequence.
All of these results together provide a very concise route to the complex structural motif 2-azabicyclo[3.3.0]octane, which can be readily prepared in a single pot from the corresponding allenyl azide. They also, however, illustrate the limitations of the methodology in terms of regiocontrol, moderate yields, and the need for a model that explains the observed stereochemistry and can thus result in predictability. The key to overcoming these limitations lies in responding the following mechanistic questions:
Why does the unconjugated substrate 1 yield only the C—C bonded product 4 upon thermolysis, whereas the conjugated analogue 8 provides both C—C 12 and C—N 13 bonded products in roughly equal amounts?
Which intermediate, the diyl 10 or the closed shell alternative 11, is formed first from 9, and which of these species is the direct precursor to the tetracyclic products 12/13?
What is the basis for the strictly cis stereochemical outcome upon cyclization of diyl 3 to form 4?
Why do the alkenyl-bearing substrates 8 yield tricycles 12/13 through participation of the alkene, whereas the aryl-bearing analogue 8Ph does not engage the aryl ring in C—C bond formation, but rather forms a simple 2-styrylindole product 14?
It is at this point in the development of this new synthetic methodology that molecular simulations present unequaled opportunities to complement the experiments and illuminate the direction that should be followed to improve the methodology.
2. RESULTS AND DISCUSSION
Given the clear differences in regioselectivity for the different substitution patterns, we decided to perform separate mechanistic explorations in silico. First, we studied the behaviour of non-conjugated allenyl azides and then we explored the changes in this mechanism induced by the introduction of conjugation using an aromatic group as a tether between the reacting allenyl and azide groups. Structurally simplified models for conjugated and non-conjugated allenyl azides were employed throughout the computational work in order to minimize cost (see below).
2.1. Non Conjugated Allenyl Azides
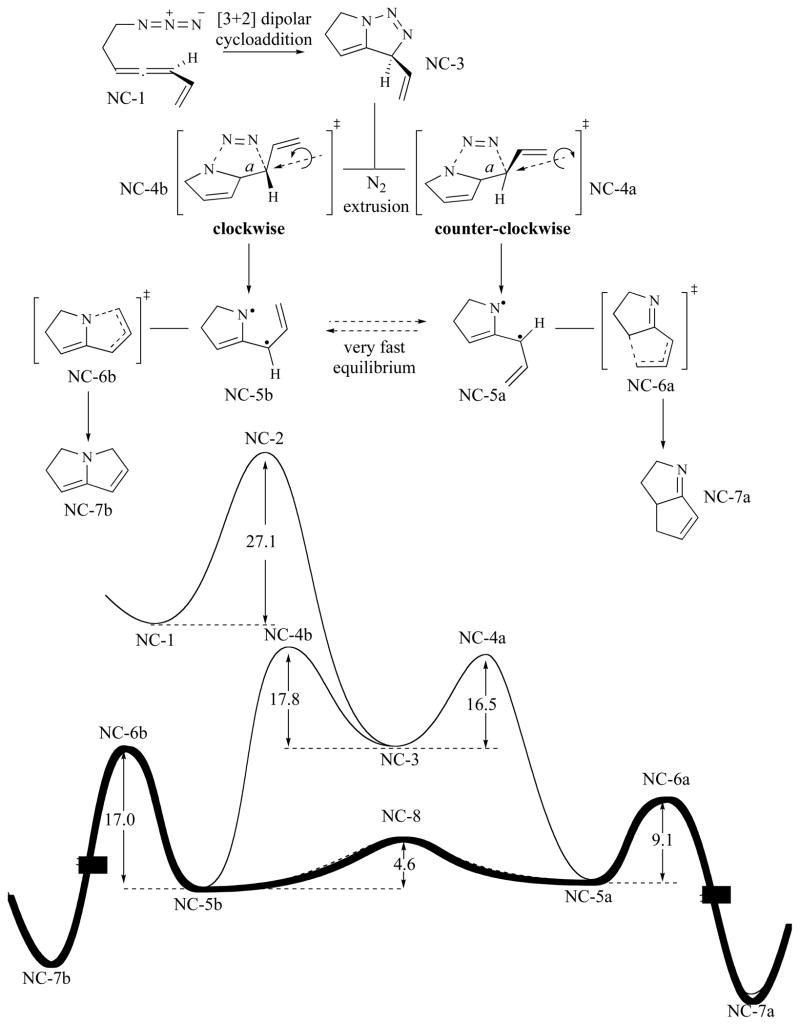
The reaction cascade of non-conjugated allenyl azides leads to C—C bond formation and yields the imines 4 and 6 as single products when starting from allyl and aryl derivatives 1 and 1Ph, respectively [16]. Calculations on the model system NC-1 (Fig. 4) provide support for the preliminary mechanistic picture proposed along with the experimental work [13]. The computed reaction pathway for the model system illustrates the diradical nature of key intermediates and also provide theoretical support for the regio-selectivity of the cascade process. The cascade of reactions begins with a [3+2] dipolar cycloaddition between the azide and the allene moieties to form triazoline NC-3. This process is the rate limiting step (27.2 kcal/mol) and all the remaining steps show considerably lower activation barriers. The triazoline intermediate readily loses N2 according to the relatively low reaction barriers obtained for NC-4a and NC-4b. This N2 extrusion occurs in an off-plane motion and, depending on the facial direction of this loss, the allyl substituent turns either clockwise (NC-4b) or counterclockwise (NC-4a) via rotation about bond a (see Fig. 4). These alternative rotation modes afford two isomeric diyls, NC-5a and NC-5b. These two diyls have the potential to collapse and yield NC-7a and NC-7b, respectively. However, these transient intermediates react under Curtin-Hammett conditions: a very fast interconversion pathway between diyls NC-5a and NC-5b is available (via a very low energy transition state NC-8). Following the expectations of the Curtin-Hammett model, the ratio of final products NC-7a and NC-7b depends only on the free energy difference between product-determining transition states NC-6a and NC-6b. The considerable energetic cost associated with C—N bond formation ( 17 kcal/mol) compared to the activation barrier for the C—C bond forming transition state NC-6a (9.1 kcal/mol) (see the profile highlighted in bold in Fig. 4) then guarantees formation of NC-7a as single product.
Fig. 4.
Mechanistic profile for the reaction cascade of non conjugated allenyl azides computed at the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d,p) level.
The cascade reaction sequence occurring under thermal activation yields a single product (4 or 6, depending on the starting material) when the azide and allene moieties are not connected through conjugation (cf. reactants 1 and 1Ph in Fig. 3). Moreover, the reaction proceeds with complete stereocontrol to furnish a single diastereomer of bicyclic product 4.
2.1.1. Regio- and Stereochemistry
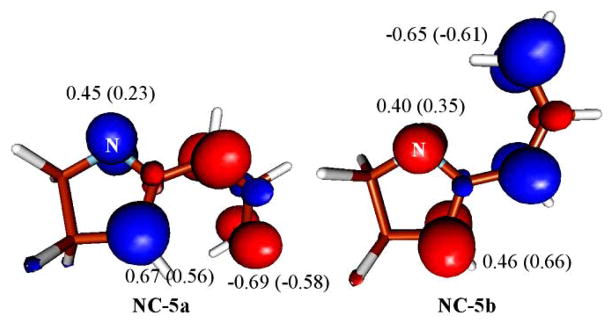
The key to regioslectivity with the non-conjugated substrate lies in the activation barrier difference for the formation of the final products under Curtin-Hammett control (9.1 vs. 17.0 kcal/mol for NC-7a and NC-7b, respectively). In terms of electronic and molecular structure, this difference can be traced to two main features: (1) the greater stability of a C—C bond vs. a C—N bond and (2) the diradical being localized predominantly on the carbon atoms (see Fig. 5). These two features directly translate to NC-7a being both thermodynamically and kinetically favoured with respect to the alternative C—N bonded product NC-7b. It is worth mentioning that the spin density argument parallels a frontier orbital analysis since carbon exhibits a larger orbital coefficient than nitrogen [17].
Fig. 5.
Isosurface of the spin density (at a 0.02 e value) on the diyl intermediates NC-5a and NC-5b. The numerical values printed are the Mulliken spin density at the intermediate geometry and at the corresponding transition state geometry NC-6a and NC-6b (in parentheses) in atomic units.
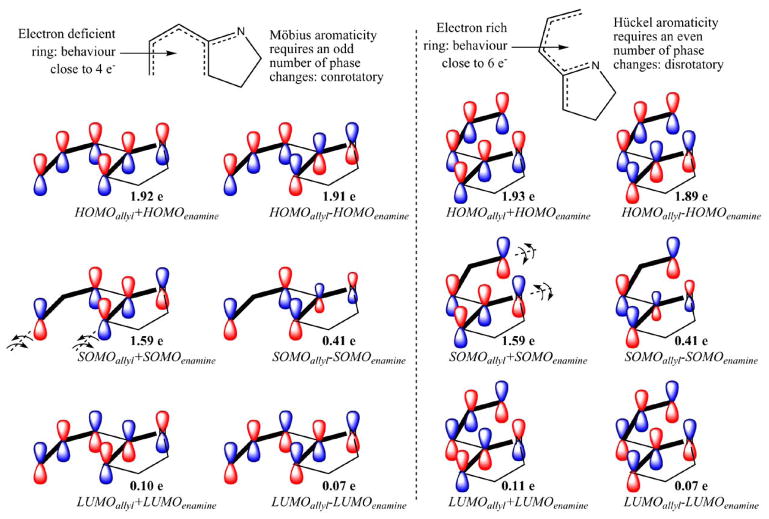
The stereoselectivity observed in this cascade reaction is also defined at the very last step, in which the final C---C bond is formed. Interestingly, the C—C bond forming transition state NC-6a is predicted to conrotate in its way towards the final product. On the other hand, the C—N bond forming alternate transition state NC-6b is predicted to undergo a disrotatory motion towards the corresponding final product NC-7b. A detailed orbital analysis of the transition states of the final cyclization step suggests that the reacting diyls can be visualized as an orbital combination of allyl and enamine moieties joined by a tether (see Fig. 6). Furthermore, this analysis also indicates that control of stereoselectivity actually lies with the frontier orbitals. The cyclizing system NC-5a contains, in principle, five π electrons. However, the electron withdrawing nitrogen atom involved in the diradical polarizes the electron density of this species (see Fig. 6). As a result, the all-carbon system involved in C—C bond formation within NC-6a is electron depleted and behaves, formally, as a four electron electrocyclic ring closure. On the other hand, the alternate nitrogen-included ring in transition state NC-6b is electron density enriched and undergoes cyclization in disrotatory fashion, according to a six electron electrocyclic closure model. The experimental observation that the single stereoisomer 4 is formed from 1 is consistent with the conrotatory motion suggested by the NC-6a analysis. Since 5 is not formed, and the nitrogen does not bear a substituent in any event, it is not possible to test the disrotatory cyclization prediction of the NC-6b analysis with this system. This detailed orbital analysis could be best described as an extension of the Woodward–Hoffmann rules to diyl systems and is illustrated in detail in Fig. (6).
Fig. 6.
CASSCF natural orbitals of the active space of transition states for the C—C bond forming cyclization NC-6a (left) and C—N bond forming cyclization NC-6b (right). Bold lines indicate the disconnected ally and enamine radicals and the proposed allyl and enamine orbital combinations leading to each natural molecular orbital. Natural orbital populations are also provided to help quantify the diradical character inherent to these species (41% for both NC-6a and NC-6b, DR = [2 - n(HOMO)] · 100). The active space in these calculations includes the entire π system of the diyl intermediate resulting in a CASSCF(6,6)/6-31G(d) level of theory.
2.2. Electronic Effects
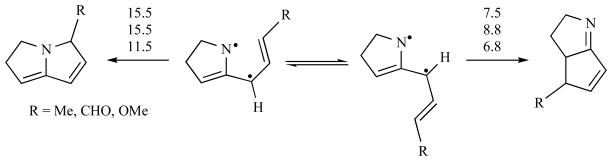
Given the proposed parallel between these radical collapse reactions and electrocyclic processes, it is reasonable to explore whether electronic effects operate on the diyl reactivity in line with the effects typically found in pericyclic reactions. As illustrated in Fig. (7), an electron withdrawing group attached to the carbon terminus of the cyclizing diyl implies a mild increase in the energy barrier for the C—C bond forming transition state NC-6a (~1 kcal/mol) and no effect on the C—N bond forming counterpart (maintaining an activation energy of 15.5 kcal/mol). On the other hand, the electron rich methoxy substituent produces a significant reduction of activation energy at both transition states (6.8 and 11.5 kcal/mol for NC-6a and NC-6b, respectively). Despite these electronic effects, the difference in energies of activation between pathway a and pathway b are such that the only expected product in all cases is NC-7a. Thus, these calculations predict that no variation in terms of product distributions should be expected for the cyclization cascade of substituted non-conjugated allenyl azides.
Fig. 7.
Electronic effects imposed by electron deficient and electron rich groups on the diradical collapse of diyls NC-5a and NC-5b. Activation free energies relative to the most stable diradical conformation are shown (kcal/mol).
2.2.1. Further Experimental-Computational Synergy
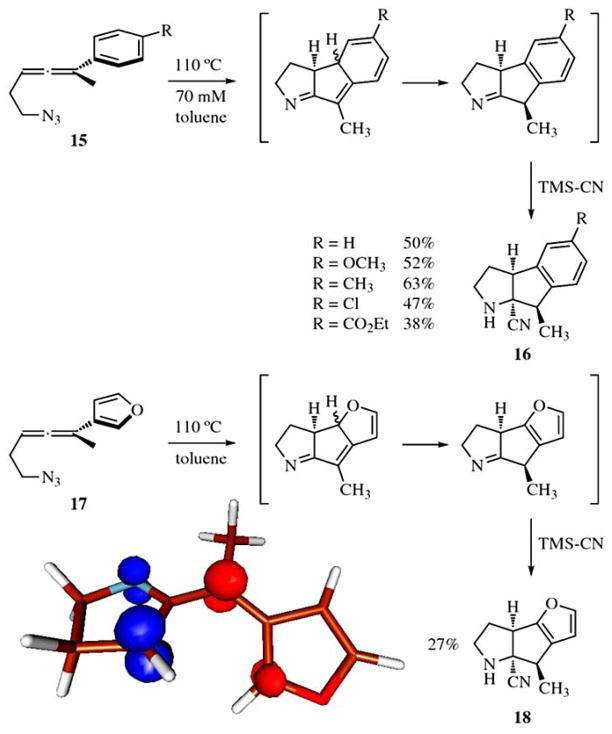
The ability of aryl and furyl substituted allenyl azides to undergo this reaction cascade was experimentally explored in some detail due to the interesting fusion of rings that could be readily obtained via this methodology (see Fig. 8) [18]. Both allenyl azide derivatives afforded the expected products (after imine capture with TMS-CN) in moderate yields. The regioselectivity observed for the capture of the diradical in the 3-furyl substituted derivative also was easily rationalized once the spin density of the diradical intermediate was computed. The cyclization occurs regioselectively at C(2) of the furan nucleus as the spin density of the diyl system is localized at this position (see Fig. 8).
Fig. 8.
Experimental results in the allenyl azide cascade of aryl and furyl derivatives. The spin density of the diradical intermediate for the furyl rationalizes the observed regioselectivity.
2.3. Conjugated Allenyl Azides
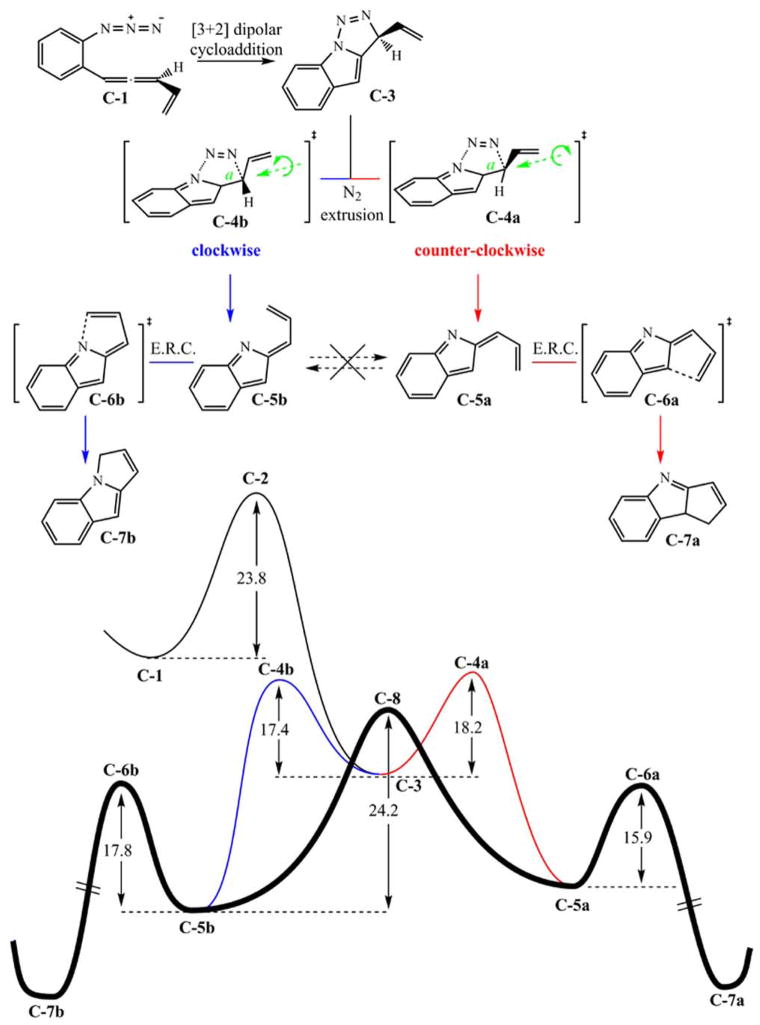
2.3.1. Mechanism of the Reaction Cascade
The inclusion of an ortho-disubstituted phenyl system tethering the allenyl and azide reactive centers broadens the scope of this chemistry to the synthesis of valuable indole products (like, for instance, the fischerindole family of compounds) [19–21]. In preliminary experimental work, however, this structural modification leads to an erosion of the remarkable regioselectivity obtained with the saturated derivatives. For example, a mixture of an approximately 1:1 ratio of regioisomers C-7a and C-7b is obtained consistently [13]. The inclusion of an unsaturated tether, allowing conjugation between the allenyl and azide moieties, dramatically changes key features of the potential energy surface of these substrates with respect to the non-conjugated parent system. The initial stages of the cascade process are very similar to the chemistry illustrated above. In a first step, the allenyl azide undergoes facile [3+2] dipolar cycloaddition to afford a triazoline intermediate. The following nitrogen loss occurs in an out-of-plane motion, coupled to vinyl rotation, which depends on the facial direction of the extrusion (Fig. 9). The activation free energy for both processes, the dipolar cycloaddition and the nitrogen loss, are comparable with those of the non-conjugated system, reinforcing the similarity between these profiles in the initial steps of the reaction cascade. The aryl group comes into play upon nitrogen extrusion by enabling an electronic rearrangement that avoids the formation of a diradical species. In turn, a pair of isomeric indolidene intermediates C-5a and C-5b is formed (see Fig. 9). Since these intermediates are closed-shell in nature, the interconvertion between C-5a and C-5b implies rotation about a formal double bond and is, therefore, a very costly isomerization (24.2 kcal/mol). Because of the now unfavoured interconversion of C-5a and C-5b, this conjugated substrate operates in the final step of the cascade out of the Curtin--Hammett regime and each indolidene isomer undergoes an electrocyclic ring closure reaction without interconversion to afford the final products C-7a and C-7b, respectively.
Fig. 9.
Mechanistic profile for the reaction cascade of conjugated allenyl azides computed at the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d,p) level.
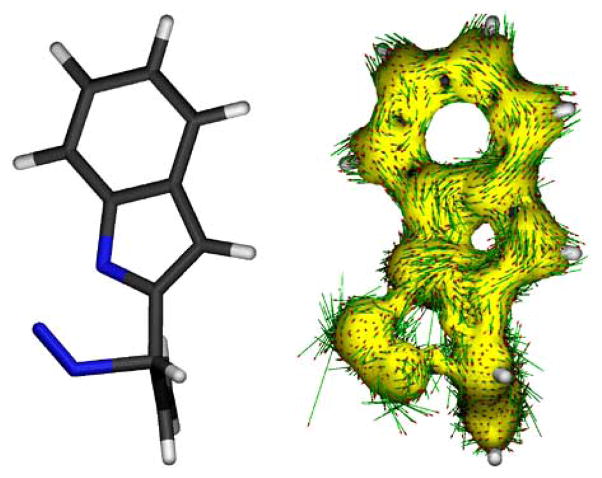
The concerted nitrogen loss step within these conjugated substrates was unexpected since it formally corresponds to a symmetry forbidden [10π + 2π] thermal, suprafacial, pericyclic reaction. These results triggered further exploration into the characteristics of this transition state. The anisotropy of the current induced density (ACID) provides a visual description of the delocalization of the electron density at the transition state. Moreover, the associated vector field gives clear indications of regions in which ring currents operate. The calculation of the ACID for the transition state corresponding to the nitrogen loss provides a clear picture of the limited applicability of the Woordward–Hoffmann rules in this case (Fig. 10) [22, 23]. The electron delocalization is severed at the scissile C—N and N—N bonds, thus indicating that this is not a pericyclic type transition state, bound to satisfy the W-H rules, but an accidentally simultaneous cleavage of two bonds requiring no participation of the conjugated system. Further calculations aimed at quantifying the level of aromaticity at the cleaving bonds in this transition state also confirm the lack of aromaticity at the triazoline ring and the absence of ring currents typically associated with pericyclic transition states [24].
Fig. 10.
ACID isosurface (0.05 au) for the transition state involved in nitrogen extrusion (C-4a).
2.3.2. Regio- and Stereochemistry
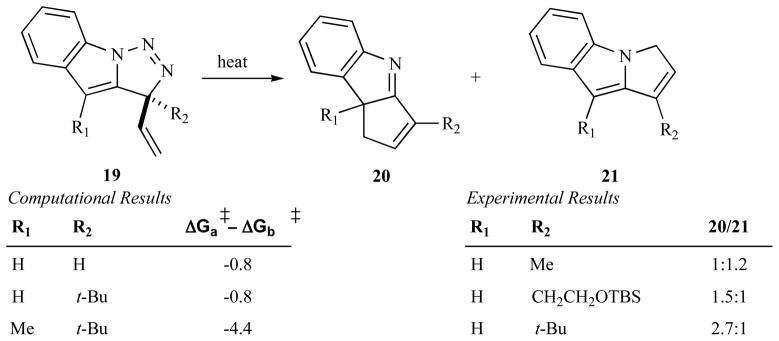
The regiochemistry of product formation with the conjugated substrates is defined during the nitrogen extrusion step, where geometrically distinct E and Z indolidenes are formed, since Curtin--Hammet kinetics are not applicable. Each of the two alternate facial directions for nitrogen loss is coupled to a specific bond a rotation (see Fig. 9), which leads to alkene isomers C-5a and C-5b. These isomers undergo electrocyclic ring closure to furnish the final products. Thus, the ratio of C-5a-to-C-5b determines the final product regioisomer ratio. Regiocontrol in the transformation of these conjugated substrates would be a highly desired quality. Computational efforts aimed at providing ways to improve the level of regioselectivity concluded with the hypothesis that facial discrimination at the nitrogen extrusion step could be achieved via steric interactions. Two relevant sites where chemical modifications are synthetically viable were identified and the effects of increrasing steric bulk at these sites were tested both computationally and experimentally (see Fig. 11). This approach, while, offering improved regioselectivity [24], presents a number of disadvantages: the bulky substituents are not easily manipulated, yields are lower in some cases and the improved regioselectivity obtained is still very far from complete.
Fig. 11.
Computational and previous experimental work on the effect of substitution with bulky groups on regioisomer production.
The stereochemistry of the reaction cascade for the conjugated substrates is determined, in parallel with the parent non-conjugated analogues, in the final cyclization step. Calculations suggest that Woodward--Hoffmann rules do genuinely apply to these substrates and the indolidene intermediates undergo electrocyclic ring closure via the expected conrotatory transition states. The stereochemistry for the conjugated substrates bearing a proton at C(3) is, however, irrelevant for practical purposes since enamine-imine tautomerization favors the formation of indole structures where the stereochemical information is lost (compare C-7a in Fig. 9 and 12 in Fig. 3).
Fig. 12.
Cyclization pathways for aryl-substituted conjugation connected allenyl azides.
2.3.3. Aryl Analogues
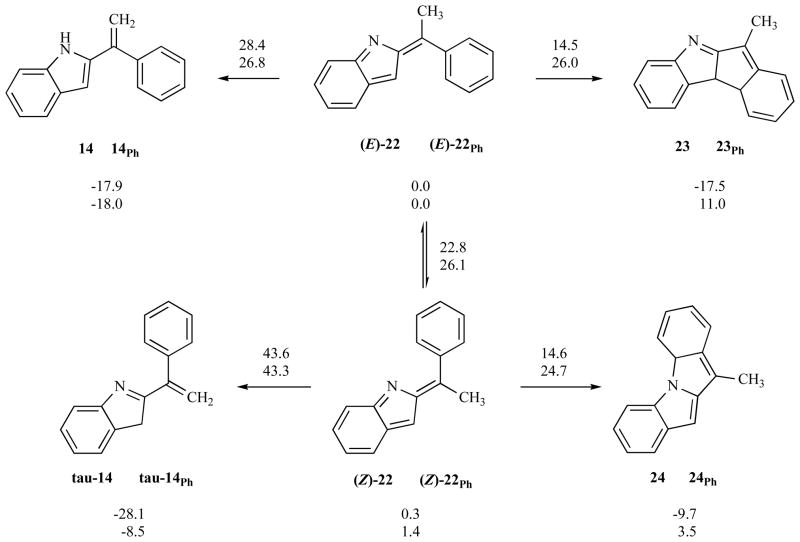
The last mechanistic question raised in the introduction asked why do the alkenyl-bearing substrates 8 yield tetracycle through participation of the alkene, whereas the aryl-bearing analogue 8Ph does not engage the aryl ring in C—C bond formation, but rather forms a simple 2-styrylindole product 14. A computational exploration of the energy profiles for vinyl and aryl substituted intermediates provides insight on this point (Fig. 12).
The introduction of a phenyl group at the allene terminus leads to an intermediate ((E)-22 or (Z)-22) whose cyclization involves dearomatization of this substituent when it participates in the electrocyclic ring closure. The energetic penalty associated with this loss of aromaticity is high enough to drastically disfavour the associated transitions states with respect to the vinyl analogue (25 kcal/mol vs 14.5 kcal/mol, respectively). Additionally, the product of the cyclization sustains the dearomatization penalty and, as a consequence, the tetracyclic products are also thermodynamically unstable with respect to the indolidene intermediates. In this scenario, an alternative process that does not require dearomatization of the phenyl substituent is energetically feasible. The [1,7] hydrogen shift from the methyl group of indolidene (E)- occurs through a competitive transition state (26.8 kcal/mol) 22Ph and furnishes a thermodynamically stable indole 14Ph. Given the high energetic cost of the electrocyclic ring closure transition states, the geometric isomer (Z )-22 Ph undergoes double bond isomerization prior to the hydrogen shift to yield 14Ph. Thus, reaction through (Z)-22 to form either tau-14 or 24 is predicted to be unlikely by these calculations.
2.3.4. Further Experimental-Computational Synergy
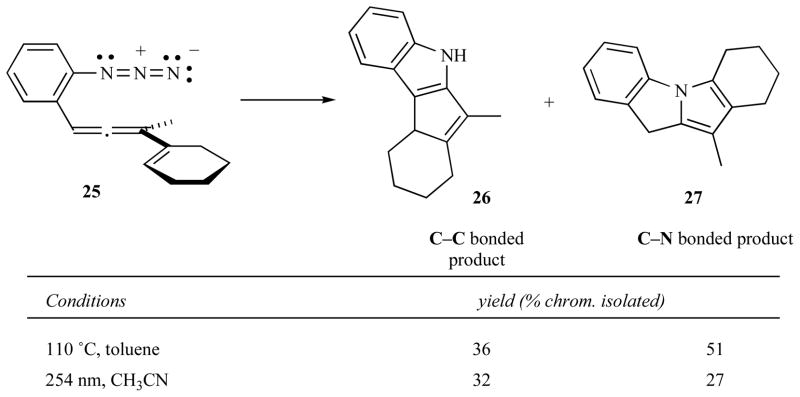
Further work on the improvement of regioselectivity in this rearrangement led to the exploration of photoactivation. The first experiments on the photochemically activated cascade were rather discouraging since the formation of roughly equal amounts of the C—C and C—N bonded products persisted (see Fig. 13). These experiments, however, confirmed that this reaction could be initiated under low temperature conditions [25].
Fig. 13.
Initial thermal and photochemical results.
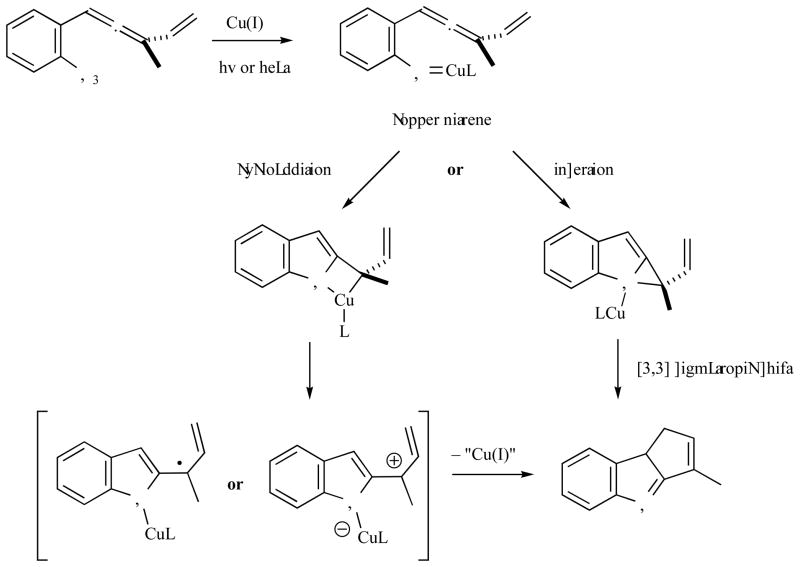
Extended work on photoactivation of conjugated allenyl azides (e.g., 25, Fig. 13) provided irreproducible mixtures of regioisomers in ratios ranging from 1:1 to 6:1. Due to difficulties in experimentally identifying the source of this variable regioselectivity, we resorted to simulation for insight and direction. A number of computational experiments were performed in order to explore whether light could induce preferential C—C bond formation in the reaction cascade. Unfortunately, all the simulations led to inconclusive results and the reason for the occasionally observed improved regioselectivity could not be determined. With the computational data suggesting no preference for any of the alternate pathways under light activation, more experimental examination was undertaken. Eventually, the irreproducible regioselectivity was linked to the presence of traces of copper in the reaction flask surviving purification from the cuprate-mediated allenyl azide synthesis [25]. Copper was speculated to promote the release of nitrogen from the azide group and furnish a nitrene intermediate that would undergo cyclization to the C—C bonded product. Under this hypothesis, catalytic ammounts of copper would suffice to favor the desired product formation (Fig. 14).
Fig. 14.
Initial working hypotheses for the mechanism of the copper mediated rearrangement of conjugated allenyl azides.
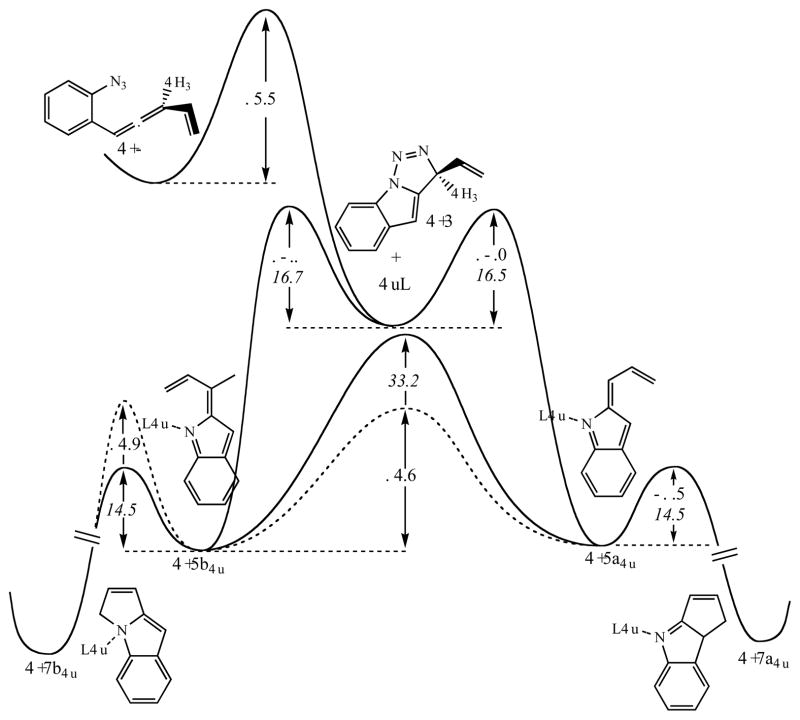
Computational exploration of this mechanistic proposal, however, provided a different energy landscape (Fig. 15). The nitrene formation step was as kinetically feasible as the previously observed [3+2] dipolar cycloaddition, but it also was predicted to provide mixtures of regioisomeric indolidene intermediates in a 50:50 ratio. However, inclusion of Cu(I) in this mechanistic picture led to the expectation that copper complexation to the nitrogen atom of key intermediates played a decisive role in determining the regiochemical outcome of the reaction. Interestingly, copper complexation specifically to the indolidene nitrogen atom had two key consequences: 1) the activation barrier for double bond isomerization was lowered by 10 kcal/mol, and 2) since the lone pair of the nitrogen atom was compromised by complex formation, the C—N bond forming electrocyclic ring closure was penalized by another 10 kcal/mol compared to the free indolidene (Fig. 15). This energy profile suggested that copper complexation impeded the cyclization of the regioisomeric indolidene C-5b Cu, and therefore larger-than-catalytic amounts of copper should provide better regioselectivity ratios [26]. In this computational scenario, isomerization of C-5b Cu into C-5a Cu now competes with cyclization to C-7b Cu and so a partial Curtin-Hammet scheme is restored.
Fig. 15.
Comparison of the free energy profiles for the reaction cascade of free and copper complexed conjugated allenyl azides. Plain numbers and dashed lines indicate the effect of copper, numbers in italics indicate activation barriers for the mechanism without copper participation. In these simulations HCN was used as the copper ligand L. For a detailed discussion on the copper complexation for these simulations, see reference.
Resuming the experimental work with this information in hand proved very satisfactory. Not only was copper confirmed as the source of improved regioselectivity for the CC bonded product, but also the predicted non-catalytic role of this ion was observed. Thermally activated rearrangement of allenyl azides in the presence of copper also showed greater regioselectivity for the C-C-bonded indole product than in the non-Cu thermal reactions. Additionally, both systematic increases in Cu concentration within the reaction mixture and systematic increases in the Cu loads with respect to the substrate showed clear trends of improved regioselectivity, observations consistent with a non-catalytic role for copper in the reaction mechanism (See Table 1). More detailed analysis of the results summarized in Table 1 suggests that, despite copper being the ultimate source of regioselectivity, photochemical activation provided better results than thermolysis. This observation can be easily explained due to the milder thermal conditions of photoactivation (~30°C), leading to better discrimination between alternate reaction pathways.
Table 1.
Discovery and optimization of the Cu mediated photochemical cascade cyclization of 28 into 29
| Entry | hv (nm) | Δ°C | conditionsa | yieldb | 29/30 ratioc |
|---|---|---|---|---|---|
| a | 110 | no additives | 80 | 1:1.6 | |
| b | 254 | no additives | 67 | 1:1 | |
| c | 254 | CuI (0.5 equiv) | 67 | 4:1 | |
| d | 254 | CuI (0.8 equiv) | 72 | 6:1 | |
| e | 254 | CuI (1.2 equiv) | 67 | 9:1 | |
| f | 254 | CuI (1.5 equiv) | 70 | 10:1 | |
| g | 110 | CuI (1.5 equiv), 5 mM | 75 | 1:1.6 | |
| h | 110 | CuI (1.5 equiv), 35 mM | 66 | 3.5:1 | |
| i | 110 | CuI (1.5 equiv), 51 mM | 65 | 5:1 |
3. CONCLUSION
The confluence of theory and experiment is responsible for guiding this research venture to its current frontier. The pathway of discovery for this organic synthesis methodology project is typical of many such efforts; in the experimentalist’s hands an idea (intramolecular allenyl azide cycloaddition for ATMM generation) led to initial proof-of-principle results that prompted more in-depth studies with more sophisticated substrates. Along the way, some observations were made that did not match expectations – something was amiss; the pencil-and-paper working hypothesis that evolved from the preliminary results did not lead to correct predictions of chemical outcome with more complex substrates. For example, the lack of regiochemical control with the conjugated allenyl azide substrates compared with the non-conjugated analogues was one such event. When hypothesis and experimental outcome do not match, it is time to modify the hypothesis. At this juncture, the pencil-and-paper chemist can use intuition, insight and guesswork to refine the hypothesis, but such exercises often take on an ad hoc character as the perplexing results are “fit” back into the now modified hypothesis. For the regioselectivity issue, this hypothesis modification took the form of invoking steric effects at the diyl termini of an indole diradical. Unfortunately, further experimental probing of this proposition did not lead to consistent results, and the question of what to do next became increasingly uncertain. Enter the computational chemist, who offers something that the experimentalist lacks; a deliberate and defensible methodology to guide hypothesis development/modification from a first-principles perspective. Gone is the ad hoc guesswork and arbitrariness that can creep into unanchored thinking. Best of all, this theoretical framework can be developed ab initio and checked against the experimental observations at every stage of the project. Returning to the regioselectivity puzzle with the conjugated allenyl azide substrate, the computationally derived mechanistic scenario (Fig. 9) highlighted the central role of a heretofore unappreciated intermediate indolidene (and not a diradical like originally supposed by analogy with the non-conjugated case), and the non-Curtin-Hammet kinetics that emerged from it. With this new model in hand, seemingly unconnected and puzzling experimental observations now were brought into focus. Predictions could be made with some measure of confidence, and the continuing checks against experimental results provided boundaries for the theoretical model and motivation for revisiting the theory, if necessary.
The synergy between experiment and theory advanced the research project in many productive directions. A satisfying rationale for the experimental observations was developed, and in addition, guidance for further experimentation emerged (Fig. 11). These further experiments will produce new observations, which may (or may not) lead to revisions in the theory. Whereas this cycle is characteristic of any application of the scientific method, the particular advantage that theory lends to the enterprise is efficiency; the broad framework for understanding offered by robust theory provides a solid launching point so that often time-consuming experimental inquiries can be focused on more productive thrusts, while at the same time minimizing unproductive diversions. A clear example of the steering value of computational input in the allenyl azide cyclization chemistry emerged from considering the copper-mediated results. Initial pencil-and-paper musings led to a mechanistic pathway involving copper-nitrene cycloaddition chemistry, as described in Fig. (14). Reaction through this pathway focused attention on the role that copper-carbon bonded intermediates might play, and further experiments were planned with these thoughts in mind. However, the timely input of theory in this case discouraged what would have been an unproductive experimental campaign, and focused attention on a more plausible series of optimization experiments based upon the Cu---N binding motif of Fig. (15). Satisfying optimization results were obtained. Thus, an open dialog between experimentalist and theorist demonstrated, at least within the context of this allenyl azide cyclization project, that scientific advances could be made which otherwise might not even have been envisioned, let alone experimentally realized. Both disciplines benefit, and the scientific perimeter of this research area is extended.
Acknowledgments
The authors are grateful to CESGA for allocation of supercomputer time. Funding from the Ministerio de Ciencia y Innovación (CTQ2009-13703) and the Xunta de Galicia (INCITE09 314 294 PR) to CSL is also gratefully acknowledged. KSF thanks the National Institutes of Health (GM 72572) for support of the experimenal aspects of this work.
References
- 1.Dowd P. Trimethylenemethane. Acc Chem Res. 1972;5:242–248. [Google Scholar]
- 2.Berson J. Diradicals. Wiley-VCH; New York: 1982. [Google Scholar]
- 3.Allan A, Carroll G, Little R. The versatile trimethylenemethane diyl; diyl trapping reactions-retrospective and new modes of reactivity. Eur J Org Chem. 1998;1:1–12. [Google Scholar]
- 4.Bleiholder R, Shechter H. Addition of electronegatively substituted azides to allenes. J Am Chem Soc. 1968;90:2131–2137. [Google Scholar]
- 5.Bingham E, Gilbert J. Reaction of carbethoxynitrene with allenes. J Org Chem. 1975;40:224–228. [Google Scholar]
- 6.Barker S, Storr R. Flash-pyrolysis of 1-vinylbenzotriazoles. J Chem Soc, Perkin Trans. 1990;1:485–488. [Google Scholar]
- 7.Quast H, Weise Vélez C. Enantioselective synthesis and thermal reorganization of an optically active methyleneaziridine. Angew Chem Int Ed Engl. 1978;17:213–214. [Google Scholar]
- 8.Quast H, Fuss A, Heublein A. Cyclopropanimines by photolysis of pyrazolinimines; thermal reaction of an excited state. Angew Chem Int Ed Engl. 1980;19:49–50. [Google Scholar]
- 9.Quast H, Meichsner G. Synthese, photolyse und thermolyse eines 1,4,4-trialkyl-4,5-dihydro-5-methylen-1h-1,2,3-triazols. hoch diastereoselektive bildung des (e)-2,2-dimethyl-n-neopentyl-1-cyclopropanimins. Chem Ber. 1987;120:1049–1058. [Google Scholar]
- 10.Quast H, Fuss A, Heublein A, Jakobi H, Seiferling B. Photochemical formation of heteromethylenecyclopropanes 24.retention of configuration in two photochemical reactions: formation of cyclopropanimines by extrusion of molecular nitrogen from tetraalkyl-4-imino-1-pyrazolines and [2+1]-cycloreversion of cyclopropanimines to isocyanides and alkenes. Chem Ber. 1991;124:2545–2554. [Google Scholar]
- 11.Quast H, Bieber L, Danen W. Tris(imino)methanes. an electron spin resonance identification of nitrogen analogues of trimethylenemethane [11] J Am Chem Soc. 1978;100:1306–1307. [Google Scholar]
- 12.Quast H, Fuss A, Nüdling W. Photoextrusion of molecular nitrogen from annulated 5-alkylidene-4,5-dihydro-1h-tetrazoles: Annulated iminoaziridines and the first triplet diazatrimethylenemethane. Eur J Org Chem. 1998:317–327. [Google Scholar]
- 13.Feldman KS, Iyer MR. Allenyl azide cycloaddition chemistry. synthesis of pyrrolidine-containing bicycles and tricycles via the possible intermediacy of azatrimethylenemethane species. J Am Chem Soc. 2006;127:4590–4591. doi: 10.1021/ja050757w. [DOI] [PubMed] [Google Scholar]
- 14.Feldman KS, Iyer MR, Hester DK., II Allenyl azide cycloaddition chemistry. synthesis of annelated indoles from 2-(allenyl)phenyl azide substrates. Org Lett. 2006;8:3113–3116. doi: 10.1021/ol061216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson ER, Gajewski JJ, Shook CA, Cohen T. Theory and Mechanism of the Allylidenecyclopropane to Methylenecyclopentene Thermal Isomerization. J Am Chem Soc. 1995;117:8495–8501. [Google Scholar]
- 16.Note that, due to these imines not being stable to SiO2 chromatographic purification, we experimentally isolate a CN adduct obtained via treatment with TMS-CN.
- 17.Cichra DA, Duncan CD, Berson JA. Mechanistic characterization of the intermediates in the thermal and photochemical deazetation of two isomeric methylenepyrazolines, 7-isopropylidene-2,3-diazabicyclo[2.2.1 lhept-2-ene and 2,2-dimethyl-3,4-diazabicyclo3[. 3.0]octa-3,8-diene. an approach to the generation and capture of protoplanar and protobisected trimethylene-methanes. J Am Chem Soc. 1980;102:6527–6533. [Google Scholar]
- 18.Feldman KS, Iyer MR, Lopez CS, Faza ON. Allenyl azide cycloaddition chemistry: exploration of the scope and mechanism of cyclopentennelated dihydropyrrole synthesis through azatrimethylenemethane intermediates. J Org Chem. 2008;73:5090–5099. doi: 10.1021/jo8008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baran PS, Richter JM. Enantioselective total syntheses of welwitindolinone A and fischerindoles I and G. J Am Chem Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. [DOI] [PubMed] [Google Scholar]
- 20.Baran PS, Maimone TJ, Richter JM. Total synthesis of marine natural products without using protecting groups. Nature. 2007;446:404–408. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 21.Richter JM, Ishihara Y, Masuda T, Whitefield BW, Llamas T, Pohjakallio A, Baran PS. Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J Am Chem Soc. 2008;130:17938–17954. doi: 10.1021/ja806981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herges R, Geuenich D. Delocalization of electrons in molecules. J Phys Chem A. 2001;105:3214–3220. [Google Scholar]
- 23.Geuenich D, Hess K, Kohler F, Herges R. Anisotropy of the induced current density (acid), a general method to quantify and visualize electronic delocalization. Chem Rev. 2005;105:3758–3772. doi: 10.1021/cr0300901. [DOI] [PubMed] [Google Scholar]
- 24.Lopez CS, Faza ON, Feldman KS, Iyer MR, II, DKH Cyclization cascade of allenyl azides: a dual mechanism. J Am Chem Soc. 2007;129:7638–7646. doi: 10.1021/ja070818l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman KS, Hester DK, Lopez CS, Faza ON. Allenyl azide cycloaddition chemistry. Photochemical initiation and cui mediation leads to improved regioselectivity. Org Lett. 2008;10:1665–1668. doi: 10.1021/ol800429j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman KS, Hester DK, Iyer MR, Munson PJ, Lopez CS, Faza ON. Allenyl azide cycloaddition chemistry. 2,3-cyclopentennelated indole synthesis through indolidene intermediates. J Org Chem. 2009;74:4958–4974. doi: 10.1021/jo900659w. [DOI] [PMC free article] [PubMed] [Google Scholar]