Abstract
Apical SK/ROMK and BK channels mediate baseline and flow-induced K secretion (FIKS), respectively, in the cortical collecting duct (CCD). BK channels are detected in acid-base transporting intercalated (IC) and Na-absorbing principal (PC) cells. Although the density of BK channels is greater in IC than PC, Na-K-ATPase activity in IC is considered inadequate to sustain high rates of urinary K secretion. To test the hypothesis that basolateral NKCC in the CCD contributes to BK channel-mediated FIKS, we measured net K secretion (JK) and Na absorption (JNa) at slow (∼1) and fast (∼5 nl·min−1·mm−1) flow rates in rabbit CCDs microperfused in vitro in the absence and presence of bumetanide, an inhibitor of NKCC, added to the bath. Bumetanide inhibited FIKS but not basal JK, JNa, or the flow-induced [Ca2+]i transient necessary for BK channel activation. Addition of luminal iberiotoxin, a BK channel inhibitor, to bumetanide-treated CCDs did not further reduce JK. Basolateral Cl removal reversibly inhibited FIKS but not basal JK or JNa. Quantitative PCR performed on single CCD samples using NKCC1- and 18S-specific primers and probes and the TaqMan assay confirmed the presence of the transcript in this nephron segment. To identify the specific cell type to which basolateral NKCC is localized, we exploited the ability of NKCC to accept NH4+ at its K-binding site to monitor the rate of bumetanide-sensitive cytosolic acidification after NH4+ addition to the bath in CCDs loaded with the pH indicator dye BCECF. Both IC and PC were found to have a basolateral bumetanide-sensitive NH4+ entry step and NKCC1-specific antibodies labeled the basolateral surfaces of both cell types in CCDs. These results suggest that BK channel-mediated FIKS is dependent on a basolateral bumetanide-sensitive, Cl-dependent transport pathway, proposed to be NKCC1, in both IC and PC in the CCD.
Keywords: apical membrane voltage, tubular flow rates, bumetanide, intercalated cell, principal cell
the final renal regulation of K+ excretion occurs in the mammalian late distal and connecting tubules (CNT) and collecting ducts (12, 16, 18, 38, 41, 60). K+ secretion within these segments requires a favorable electrochemical gradient (a high cell K+ concentration and an apical membrane voltage that is depolarized with regard to the Nernst potential for K+), generated by apical Na+ entry through the epithelial Na+ channel (ENaC) and its electrogenic Na+-K+-ATPase-mediated basolateral extrusion in exchange for the uptake of K+, as well as an apical membrane permeability to K+. In animals fed a normal-K+ diet, K+ secretion in the cortical collecting duct (CCD) is completely dependent on Na+ absorption (14, 43, 68).
High tubular flow rates stimulate net K+ secretion in the distal nephron (18, 27, 39, 60, 77). This response reflects, at least in part, an increase in delivery to and reabsorption of Na+ by the distal nephron (27, 43, 64), which in turn increases the driving force for passive K+ efflux across the apical membrane. Two types of apical K+ channels have been functionally identified in the distal nephron: the inwardly rectifying ATP-sensitive secretory K+ (SK) channel encoded by ROMK (15, 16, 75), and a high-conductance Ca2+- and/or stretch-activated BK channel (32, 46, 63), considered to be comprised of a pore-forming α-subunit, a member of the slo family of K+ channels originally cloned from Drosophila, and a regulatory β-subunit (1, 10, 30). Using the rabbit CCD as a model of the distal nephron, we reported that an increase in luminal flow rate leads to a transient increase in [Ca2+]i and sustained increases in net Na+ absorption and K+ secretion, the latter sensitive to iberiotoxin (IbTX), a specific inhibitor of BK channels (33–35, 77). These data and studies by others (2) showing that mice lacking ROMK secrete K+ by a process that is, at least in the late distal tubule, IbTX-sensitive led to the conclusion that the BK channel mediates flow-induced K+ secretion (FIKS), a conclusion further supported by the observation that the fractional K+ excretion in BK β1-null mice subjected to acute volume expansion is lower than that measured in wild-type animals (48).
The CCD is a heterogeneous structure, comprised of two morphologically and functionally distinct cell populations. The principal cell has been traditionally considered to be responsible for Na+ absorption via ENaC and K+ secretion via the SK/ROMK channel. Intercalated cells secrete H+ via an apical vacuolar H+-ATPase (α-cells) or HCO3− via apical pendrin (β-cells) but can also, under certain conditions, reabsorb K+ via an apical H+-K+-ATPase (7, 36, 62, 66, 67); non-A non-B intercalated cells possess both apical H+-ATPase and pendrin (11, 29). Whereas ENaC and SK/ROMK channels are restricted to principal cells, apical conducting BK channels are detected in both principal and intercalated cells (32, 46).
A major question, as yet unresolved, is whether BK channels in intercalated or principal cells mediate FIKS. Multiple observations provide support of a role for intercalated cells in FIKS. 1) The density of immunodetectable apical BK channel α-subunit in rabbit (13, 45) and mouse (19, 20, 49) and functional (32, 46, 47, 63) BK channel activity are greater in intercalated than in principal cells. 2) Distal K+ secretion may occur via a Na+-independent pathway in the isolated rabbit CCD (44) and in rats fed a high-K+ diet for as little as 18 h, where a significant fraction of K+ excretion becomes amiloride-insensitive, potentially reflecting the recruitment of a nonprincipal cell-mediated alternate pathway for K+ secretion into the urine (14). 3) To the extent that the apical membrane voltage of the intercalated cell is depolarized relative to that of the principal cell (47), BK channel open probability (Po) would be high (due to the voltage sensitivity of Po), reducing the requirement for elevation of intracellular Ca2+ to open the channels (6, 47).
However, sustained transepithelial K+ secretion by intercalated cells would require a robust basolateral K+ uptake pathway, such as the Na+-K+-ATPase, to maintain a high steady-state [K]i for sustained luminal K+ secretion. Immunocytochemical studies in rat kidney performed using monoclonal antibodies directed against the Na+-K+-ATPase α-subunit and an antigen retrieval technique to reveal hidden epitopes revealed weak-to-moderate staining in all intercalated cells of the CCD (those with apical, basolateral, and diffuse anti-V-ATPase antibody labeling) and outer medullary collecting duct (OMCD) (57). However, functional measurements of Na+-K+-ATPase pump activity (3) and ouabain-sensitive currents were not detected in intercalated cells in rat CNT and CCD (47). In contrast, Na+-K+-ATPase pump activity was readily detected in principal cells in these same segments. In rabbit kidney, Na+-K+-ATPase antigenicity has been identified along the basolateral membrane of intercalated cells (54). However, electron microprobe analysis of intracellular electrolyte concentrations in individual cells in isolated, perfused rabbit CCDs revealed a significantly smaller increase in [Na]i in intercalated vs. principal cells exposed to basolateral ouabain (65). In sum, these data suggest that intercalated cells may not have sufficient Na+-K+-ATPase activity to sustain high rates of luminal K+ secretion.
In the colon, K+ is taken up at the basolateral membrane not only by the Na+-K+-ATPase but also by the Na-K-2Cl cotransporter NKCC1 (4). Immunodetectable Na-K-2Cl cotransporters have also been detected on the basolateral membrane of α-intercalated cells in the rat OMCD, using both a rabbit polyclonal NKCC1-specific antibody raised against a synthetic peptide (L232) as well a mouse monoclonal antibody that recognizes both absorptive (NKCC2) and secretory (NKCC1) isoforms of the cotransporter (17).
The purpose of this study was to test the hypotheses that basolateral NKCC is present in the CCD and contributes to BK channel-mediated FIKS. To test this, we examined in in vitro microperfused CCDs whether basolateral bumetanide inhibits IbTX-sensitive and thus BK channel-mediated FIKS and whether functional NKCC is present along the basolateral membranes of intercalated and/or principal cells, using fluorescent functional probes and immunolocalizations.
METHODS
Animals and isolation of single tubules.
Adult (>6 wk) female New Zealand white rabbits (Covance, Denver, PA) were housed in the Mount Sinai School of Medicine Center for Comparative Medicine and male Sprague-Dawley rats (180–250 g) at the University of Maryland School of Medicine. All animals were allowed free access to tap water and chow. Animals were euthanized in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee Committees at the Mount Sinai School of Medicine and the University of Maryland School of Medicine.
Rabbit kidneys were removed via a midline incision, and single tubules were dissected freehand in cold (4°C) Na+-Ringer solution containing (in mM) 135 NaCl, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 lactate, 6.0 l-alanine, 5.0 HEPES, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O, as previously described (35). A single tubule was studied from each animal, unless otherwise indicated.
Microperfusion of single tubules for measurement of cation transport.
Each isolated tubule was immediately transferred to a temperature- and O2-CO2-controlled specimen chamber set on the stage of a Nikon inverted epifluorescence microscope (Eclipse TE300), mounted on concentric glass pipettes, and perfused and bathed at 37°C with a HCO3−-containing solution (Burg's perfusate; in mM) 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 Na+ acetate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O (35). During the 45-min equilibration period and thereafter, the perfusion chamber was continuously suffused with a gas mixture of 95% O2-5% CO2 to maintain pH of the Burg's solution at a pH of 7.4 at 37°C. The bathing solution was continuously exchanged at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT).
Transport measurements were performed in the absence of transepithelial osmotic gradients and thus water transport was assumed to be zero. Three to four samples of tubular fluid were collected under water-saturated light mineral oil by timed filling of a calibrated 20-nl volumetric constriction pipette at slow (∼1) and fast (∼5 nl·min−1·mm−1) flow rates, as indicated (35, 78). To determine the concentrations of K+ and Na+ delivered to the tubular lumen, ouabain (200 μM) was added to the bath at the conclusion of each experiment to inhibit all active transport, and an additional three to four samples of tubular fluid were obtained for analysis. The cation concentrations of perfusate and collected tubular fluid were determined by helium glow photometry and the rates of net transport (Jx; in pmol·min−1·mm−1 tubular length) were calculated using standard flux equations, as previously described (13). The calculated ion fluxes were averaged to obtain a single mean rate of ion transport for the CCD at each flow rate under each condition. The flow rate was varied by adjusting the height of the perfusate reservoir. The sequence of flow rates was randomized within each group of tubules to minimize any bias induced by time-dependent changes in ion transport.
As indicated, some tubules were pretreated with bumetanide (10 or 100 μM) added to the bathing solution during the initial equilibration period. The inhibitor was present for at least 30 min before tubular fluid samples were first obtained. Samples of tubular fluid for measurement of net cation transport were collected in the continuous presence of the inhibitor. Note that bath bumetanide (100 μM) does not alter pHi in collecting ducts (73). Another set of CCDs was studied in the absence of bath Cl−; the Cl−-free bath solution (Cl−-free Burg's perfusate) contained (in mM) 110 Na+ gluconate, 25 NaHCO3, 2.5 K2HPO4, 4.0 Ca2+ acetate, 1.2 MgSO4, 4.0 Na+ lactate, 1.0 Na3 citrate, 6.0 alanine, 5.5 glucose, pH 7.4.
Microperfusion of single tubules for measurement of [Ca2+]i and bumetanide-sensitive basolateral NH4+ uptake.
Isolated CCDs were transferred to the specimen chamber assembled with a no. 1 coverslip (Corning) painted with a 1-μl drop of poly-d-lysine hydrobromide 0.01% (BD Biosciences, Bedford, MA), set on the stage of a Nikon inverted epifluorescence microscope (Eclipse TE300 or Diaphot) linked to a Cascade 512F camera (Photometrics) or a cooled Pentamax CCD camera (Princeton Instruments), interfaced with a digital imaging system (MetaFluor, Universal Imaging, Westchester, PA). The CCD was then mounted on concentric glass pipettes, cannulated, and the luminal flow rate was adjusted to either 1 or 5 nl·min−1·mm−1. Thereafter, the tubule was positioned directly on the poly-d-lysine to immobilize the segment for the duration of the experiment. Tubules were perfused and bathed at 37°C with Burg's perfusate (for Ca2+ measurements) or Na+-Ringer solution (for BCECF experiments) for the equilibration period and thereafter. Fura-2- or BCECF-loaded principal (exhibiting a dull appearance) and intercalated (bright) cells were visualized using a Nikon S Fluor ×40 objective (numeric aperture 0.9, working distance 0.3), as previously described (35). We did not attempt to distinguish acid-secreting α from base-secreting β intercalated cells in the present study. Images of the fluorescence emission at 510 nm (fura-2) or 530 nm (BCECF) overlying individually identified cells residing in the lateral wall of each perfused tubule (to capture the fluorescence signal from a single cell) were acquired using MetaFluor image acquisition software (Universal Imaging) and stored on a digital instruments computer. Autofluorescence was not detected at the camera gains utilized.
Measurement of [Ca2+]i.
Following equilibration, CCDs were loaded with 10 μM acetoxymethyl ester of fura-2 (Calbiochem, La Jolla, CA) added to the bath for 20 min. The tubule was then rinsed with perfusate, in some experiments containing bumetanide (10 μM), for 20 min. Fura 2-loaded CCDs were alternately excited at 340 and 380 nm and images, acquired every 2 s, were digitized for subsequent analysis. After stable baseline 340/380-nm fluorescence intensity ratios (FIRs) were obtained at a slow flow rate, the luminal flow rate was increased acutely, and FIRs were monitored using our commercially available digital image analysis system (MetaFluor).
At the conclusion of each experiment, an intracellular calibration was performed, using 10 μM EGTA-AM in a Ca2+-free bath and then a 2-mM Ca2+ bath containing ionomycin (10 μM) (35). Standard equations were used to calculate experimental values of [Ca2+]i. At least four randomly chosen cells were analyzed in the wall of each collecting duct. The mean [Ca2+]i values for principal and intercalated cells, distinguished by their differing fluorescence intensities (35), were calculated. As indicated, the effect of flow on [Ca2+]i was studied in some CCDs after pretreatment with and in the continued presence of bumetanide added to the bath.
Measurement of bumetanide-sensitive basolateral NH4+ uptake.
Following equilibration, CCDs were loaded with 10 μM acetoxymethyl ester of BCECF [2′7′-bis(-2-carboxyethyl)-5(and 6)-carboxyfluorescein; Invitrogen] added to the bath for 10 min. The tubule was then rinsed with Ringer solution, in some experiments containing bumetanide (10 μM), for 15 min.
BCECF-loaded cells were alternately excited at 490 and 440 nm using a DG-4 or LAMBDA 10–2 high-speed excitation wavelength switcher (Sutter); images of the fluorescence emission at 530 nm overlying individually identified BCECF-loaded cells residing in the lateral wall of each perfused tubule (to capture the fluorescence signal from a single cell) were acquired at 10-s intervals using MetaFluor image acquisition software (Universal Imaging) and were stored on a digital instruments computer. The 490/440-nm FIRs were subsequently calculated using our commercially available digital image analysis system (MetaFluor).
After stable baseline FIRs were obtained, the Ringer solution bathing the tubules was rapidly exchanged to one in which 20 mM NaCl was replaced by NH4Cl and 0.5 mM BaCl2 was added to block basolateral K+ channels (+ vehicle or 10 μM bumetanide) and FIRs were monitored for 3 min. Basolateral exposure of tubules to NH4Cl led to an initial rapid increase in FIR (i.e., cell alkalinization) reflecting NH3 uptake, followed by a fall in FIR (cell acidification) due to NH4+ uptake and activation of other pH-regulating transporters (e.g., Na+/H+ exchanger), and finally, on washout of the NH4Cl from the bath solution, cells acidify further as NH3 exits the cells (22, 55). The rate of NH4+ uptake was calculated by exponential curve fitting of the fall in FIR from the peak value during the first 60 s after addition of NH4Cl to the bath, as described by Heitzmann et al. (22), and expressed as change in FIR over 1,000 s. Analyses were performed using FIRs instead of pH values as BCECF calibrations are not expected to be linear at pH values above 7.8, a range that cells were expected to achieve in response to NH4Cl loading (5). At the end of the experiment, the tubule was perfused with rhodamine-DBA (Dolichos Biflorus agglutinin) to identify principal cells.
At least two randomly chosen principal and intercalated cells were analyzed in each CCD. Mean slopes of acidification (2nd phase after NH4Cl prepulse) for principal and intercalated cells were calculated for each CCD. In this series of experiments, the rates of acidification were compared in control and bumetanide-treated CCDs studied from the same animal, allowing us to perform a paired statistical analysis.
Relative quantitation of NKCC1 mRNA in CCD.
For molecular analysis of single rabbit CCDs, tubule microdissection was carried out in 1× PBS containing 10 mM ribonucleoside vanadyl complexes (New England Biolabs, Ipswich, MA) for no longer than 90 min after the death of the animal. Approximately 15-mm total length of CCDs was pooled for each sample. RNA was extracted from the single CCDs and cDNA was synthesized using random primers as described previously (45). NKCC1 transcript expression was quantitated via real-time PCR using primers and probes (forward primer: 5′-GAGGAGGAGGCGCATATTATTTAA-3′; reverse primer: 5′-ATCCCACCACATACATAGCAACTG-3′; probe: 5′-CGATTGGCCTGATCTTTGCTTTTGC-3′) designed using Primer Express software and synthesized by Applied Biosystems and the TaqMan Ribosomal RNA Control Reagent as the rabbit 18S internal positive reference control (Applied Biosystems, Foster City, CA).
Briefly, in a 384-well plate, 0.2-μl cDNA sample was added plus 8 μl of a cocktail mix containing 0.05 μl Platinum Taq DNA Polymerase, 1 μl of 10× PCR buffer, 1.1 μl of 50 mM MgCl2, 0.1 μl AmpErase uracil N-glycosylase (UNG), 0.2 μl Gene Amp dNTPs with dUTP, 0.2 μl passive reference ROX dye, 0.2 μl (20 pM) forward and reverse primers, and 0.04 μl TaqMan probe. Taq DNA Polymerase and ROX were purchased from Invitrogen (Carslbad, CA) and AmpErase UNG and dNTPs with dUTP from Applied Biosystems. Nuclease-free water was added for a total volume of 10 μl. Each plate was then covered with optical adhesive film and, after the initial steps of 50°C/2 min and 95°C/10 min, 40 cycles of 95°C/15 s (melt) and 60°C/1 min (anneal/extend), detection was performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Immunolocalization of NKCC1.
Kidneys of ketamine/xylazine-anesthetized rats were perfused for 2 min with PBS followed by 5-min perfusion with 2% paraformaldehyde in PBS. The kidneys were then removed and fixed (24 h at 4°C), rinsed in PBS, and embedded in paraffin. Cross-sections 3-μm thick, cut at the level of the papilla, were picked up on gelatin-coated coverslips and deparaffinized. Epitope retrieval was carried out as previously described (70) by placing coverslips in a pH 8 solution [Tris (1 mM), EDTA (0.5 mM), and SDS (0.1%)]. The retrieval solution and sections were heated to boiling in a microwave oven, transferred to a conventional boiling water bath (15 min), and then cooled to room temperature before the sections were thoroughly washed in distilled water to remove the SDS.
Sections were blocked for 30 min with 2% BSA, 0.2% fish gelatin, and 0.2% sodium azide in PBS. Previously described rabbit anti-NKCC1 antibody (17) and chicken anti-AQP2 IgY (71) were diluted in PBS containing 1% BSA, 0.2% fish gelatin, 0.1% Tween 20, and 0.2% sodium azide and a NaCl concentration of 0.5 M to reduce nonspecific antibody labeling. After overnight incubation in a humid chamber at 4°C and thorough washing in high-salt wash (incubation medium plus added NaCl at 0.5 M), the bound anti-NKCC1 antibody was detected with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Rockland) and enhanced with Alexa Fluor 488-conjugated donkey anti-goat IgG while anti-AQP2 was detected with Alexa Fluor 568-conjugated donkey anti-chick IgG (Jackson Laboratories).
Statistics.
Results are expressed as means ± SE; n is the number of animals or tubule samples used for in vitro microperfusion studies and PCR. Comparisons were made by paired and unpaired t-tests as appropriate, using commercially available statistical software for the calculations (SPSS, Chicago, IL). Significance was asserted if P < 0.05.
RESULTS
Effects of basolateral bumetanide on FIKS.
We previously reported that an increase in tubular fluid flow rate from 1 (slow) to 5 (fast) nl·min−1·mm−1 in rabbit CCDs microperfused in vitro is associated with a significant increase in net Na+ absorption (JNa) and net K+ secretion (JK; in pmol·min−1·mm−1) (33, 43, 61, 77). We confirmed these results in a group of untreated CCDs (n = 4; Fig. 1).
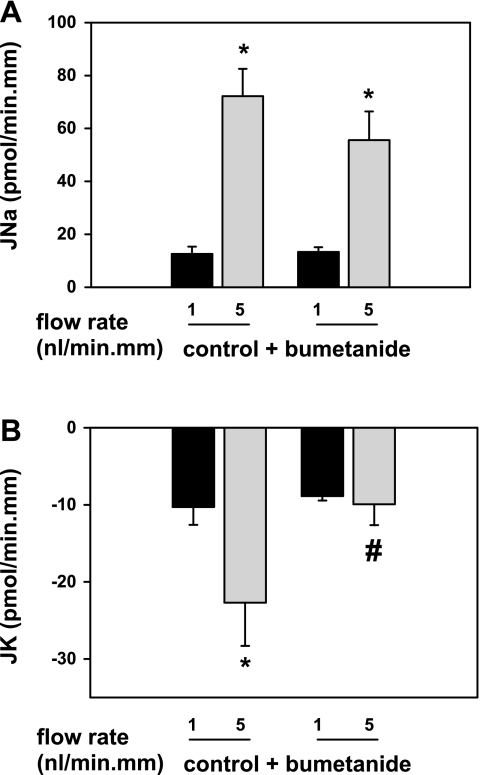
Fig. 1.
Effect of basolateral bumetanide (100 μM) on basal and flow-induced net cation transport (in pmol·min−1·mm−1) in isolated, perfused rabbit cortical collecting ducts (CCDs). In untreated CCDs (n = 4), an increase in tubular fluid flow rate from ∼1 (slow) to 5 (fast) nl·min−1·mm−1 was associated with a significant increase in Na absorption (JNa; A) and net K secretion (JK; B). Pretreatment of CCDs (n = 4) with 100 μM bumetanide added to the bath solution was without an effect on flow-stimulated JNa (A), but completely inhibited flow-induced K+ secretion (FIKS; B). These data provide compelling support for a critical role of a basolateral bumetanide-sensitive process in FIKS in the rabbit CCD. Means ± SE. *P < 0.05 compared with Jx at 1 nl·min−1·mm−1 in the same tubules. #P < 0.05 compared with Jx in control tubules studied at the same flow rate.
To examine whether transepithelial Na+ and K+ transport in the CCD is mediated by a basolateral NKCC, another group of CCDs (n = 4) was pretreated with 100 μM bumetanide, an inhibitor of NKCC, added to the bathing solution, and the rates of JK and JNa were measured at slow and fast flow rates in the continuous presence of the inhibitor. At a slow flow rate of 0.8 ± 0.1 nl·min−1·mm−1, JK and JNa in CCDs pretreated with basolateral bumetanide (n = 4) were identical to the transport rates measured in untreated control tubules perfused at 1.0 ± 0.1 nl·min−1·mm−1 [P = not significant (NS); Fig. 1, A and B]. A fivefold increase in luminal flow rate in the bumetanide-treated CCDs to 4.9 ± 0.2 nl·min−1·mm−1 led to a typical increase in JNa from 13.9 ± 1.2 to 55.7 ± 10.7 pmol·min−1·mm−1 (P < 0.05), but not JK. JK in bumetanide-treated CCDs perfused at a fast flow rate (−10.1 ± 2.6 pmol·min−1·mm−1) was not significantly different than that measured in the same tubules perfused at a slow flow rate (−9.0 ± 0.4 pmol·min−1·mm−1; P = NS; Fig. 1B).
As a 100-μM concentration of bumetanide may have nonspecific effects on cation transport, we performed another set of experiments using 10 μM bumetanide. In four CCDs perfused at a fast flow rate of 5.1 ± 0.2 nl·min−1·mm−1, addition of 10 μM bumetanide to the bathing solution had no significant effect on JNa (74.4 ± 3.3 to 70.3 ± 6.0 pmol·min−1·mm−1; P = NS) but led to a significant reduction in JK from −15.7 ± 1.5 to −8.1 ± 2.7 pmol·min−1·mm−1 (P < 0.05; Fig. 2, A and B). These data suggest that flow-stimulated but not basal JK is dependent on a basolateral bumetanide-sensitive transport pathway.
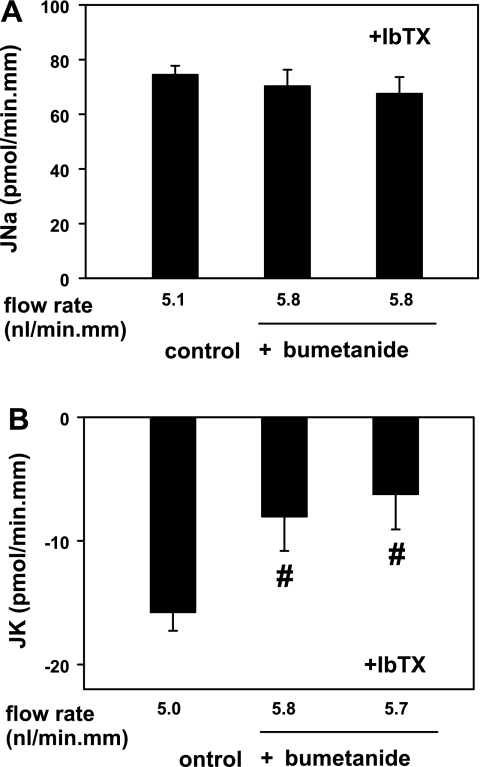
Fig. 2.
Effect of basolateral bumetanide (10 μM) on flow-induced net cation transport (in pmol·min−1·mm−1) in isolated, perfused rabbit CCDs. A: flow-stimulated JNa was not affected by 10 μM bumetanide added to the bath in the absence or presence of luminal iberiotoxin (IbTX), a specific inhibitor of the BK channel, in CCDs perfused at fast flow rates of ∼5 nl·min−1·mm−1. B: 10 μM bumetanide added to the bath inhibited FIKS in CCDs perfused at a fast flow rate. Addition of IbTX to the luminal perfusate of these same tubules did not further inhibit JK, suggesting that bumetanide and IbTX inhibit the same transport pathway. Means ± SE; n = 4 CCDs. #P < 0.05 compared with Jx in control tubules.
To determine whether the bumetanide-induced inhibition of FIKS reflected a reduction of BK channel-dependent K+ secretion, the effect of luminal addition of the specific BK channel inhibitor IbTX (50 nM) on JK in the four bumetanide-treated (10 μM) CCDs was examined. As shown in Fig. 2B, JK in IbTX-treated CCDs pretreated with basolateral bumetanide and perfused at a fast flow rate of 5.8 ± 0.6 nl·min−1·mm−1 (−6.3 ± 2.0 pmol·min−1·mm−1) was similar to that measured in these same CCDs before luminal addition of IbTx and thus treated with bumetanide alone (−8.1 ± 2.7 pmol·min−1·mm−1; P = NS). JNa was similar in the bumetanide-treated CCDs before (70.3 ± 6.0 pmol·min−1·mm−1) and after (67.6 ± 6.0 pmol·min−1·mm−1) luminal addition of IbTX (P = NS; Fig. 2A). The lack of an additive effect of luminal IbTX and basolateral bumetanide on FIKS suggests that the targets of these two inhibitors participate in same transport pathway.
Effects of basolateral bumetanide on [Ca2+]i.
FIKS is dependent on a flow-induced increase in [Ca2+]i (33). To test whether the inhibition of FIKS by basolateral bumetanide was due to an inhibitor-induced blockade of the flow-induced increase in [Ca2+]i, the effect of basolateral bumetanide on [Ca2+]i in principal and intercalated cells was examined in CCDs perfused at a low flow rate and then in response to an acute increase in luminal flow rate.
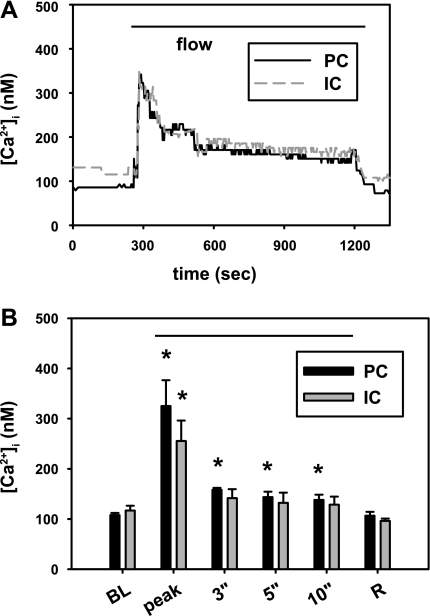
Basolateral bumetanide (100 μM) had no apparent effect on resting [Ca2+]i which averaged 108.1 ± 3.9 and 117.0 ± 9.5 nM in principal and intercalated cells, respectively (n = 4; Fig. 3), values similar to those previously reported by us in 10 untreated CCDs (109.5 ± 11.9 and 133.2 ± 15.7 nM; P = NS vs. bumetanide-treated specific cell types) (33). An acute increase in luminal flow rate in these bumetanide-pretreated CCDs led to a rapid, albeit brief rise in [Ca2+]i in principal and intercalated cells to 325.2 ± 51.3 and 256.0 ± 40.2 nM, respectively (Fig. 3), increases similar to those previously reported in 10 untreated CCDs (322.2 ± 38.0 and 336.1 ± 36.3 nM; P = NS vs. bumetanide-treated specific cell types) and attributed to release of Ca2+ from internal stores and/or mechanosensitive Ca2+ influx (33). These data suggest that the inhibitory effect of bumetanide on FIKS is not due to inhibition of the flow-induced increase in [Ca2+]i required for BK channel activation.
Fig. 3.
Effect of basolateral bumetanide (100 μM) on the flow-induced increase in [Ca2+]i in microperfused CCDs. A: representative tracing of the effect of an acute increase in flow rate on [Ca2+]i in an intercalated (IC) and principal (PC) cell in a CCD treated with bumetanide. An acute increase in luminal flow rate led to a rapid, transient rise in [Ca2+]i in both cell types, similar to the response we previously described in detail and attributed to release of Ca2+ from internal stores and/or mechanosensitive Ca2+ influx. B: summary of the effect of flow on [Ca2+]i in PC (black) and IC (gray) cells in bumetanide-treated CCDs (n = 4). Baseline resting (BL; at slow flow rates) and flow-induced peak [Ca2+]i values are given, as are the values detected at specific times (min) after initiation of high flow and after the flow rate was reduced (R; recovery). Means ± SE. *P < 0.05 vs. baseline value for that cell type.
Effects of basolateral Cl− removal on basal and flow-stimulated net cation transport.
To strengthen the evidence that basolateral NKCC is required for FIKS, we sought to examine the effect of basolateral Cl− removal (replacement with gluconate) on basal and flow-stimulated net cation transport in the CCD. Removal of Cl− from the bath was expected to reverse the transcellular Cl− gradient and therefore reduce NKCC activity.
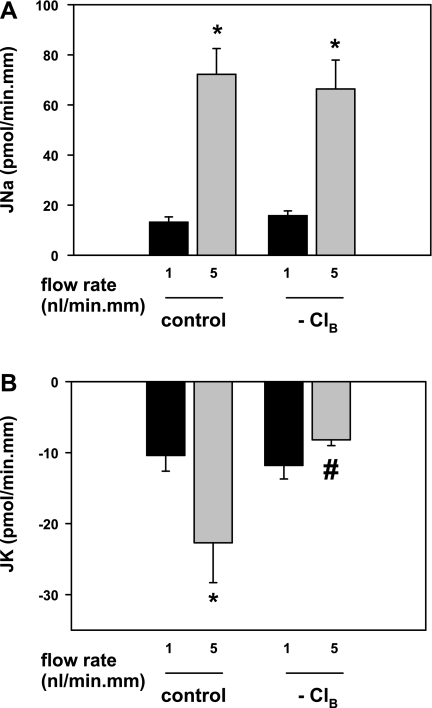
In five CCDs bathed in a Cl−-free Burg's perfusate, an increase in luminal flow rate from 1.1 ± 0.1 to 4.6 ± 0.2 nl·min−1·mm−1 led to a typical increase in JNa from 15.8 ± 1.9 to 66.4 ± 11.5 pmol·min−1·mm−1 (P < 0.05; Fig. 4). In these same tubules, the increase in luminal flow rate failed to stimulate JK (−11.8 ± 1.9 to −8.2 ± 0.8 pmol·min−1·mm−1; P = NS; Fig. 4). In two CCDs perfused at the fast flow rate, restoration of Cl− to the bath at the conclusion of the experiment uncovered FIKS (−7.8 to −42.0 and −11.2 to −26.5 pmol·min−1·mm−1). These data are consistent with the requirement of a basolateral Cl−-dependent transport pathway for FIKS in the CCD.
Fig. 4.
Effect of basolateral Cl− removal (replacement with gluconate) on basal and flow-induced net cation transport in the CCD. A: JNa increased in tubules bathed in Cl−-free perfusate as the luminal flow rate was increased from ∼1 to 5 nl·min−1·mm−1. B: removal of basolateral Cl− eliminated FIKS but not basal JK. These data are consistent with the requirement for FIKS of a basolateral Cl−-dependent transport pathway in the CCD. Means ± SE. *P < 0.05 compared with Jx at 1 nl·min−1·mm−1 in same tubules. #P < 0.05 compared with Jx in control tubules studied at same flow rate.
Identification of cell population with basolateral bumetanide-sensitive NH4+ uptake.
To determine whether the basolateral bumetanide-sensitive transport pathway is localized to intercalated and/or principal cells in the CCD, we exploited the finding that NKCC accepts NH4+ at its K+-binding sites (28) and that NH4+ uptake into cells can be assessed in CCDs loaded with the pH indicator BCECF using the NH4Cl pulse technique. A typical experiment is shown in Fig. 5. Each tracing shows three phases of the cellular response to NH4Cl addition to the bath: 1) a rapid initial alkalinization (increase in FIR) due to NH3 entry, 2) a slower acidification (reduction in FIR) from the peak FIR reflecting NH4+ entry into the cell, and 3) a rapid fall in pHi associated with washout of NH4+ from the bath, secondary to rapid exit of NH3. As we previously reported that BK channel-mediated (IbTX-sensitive) K+ secretion in the CCD occurs only at fast flow rates (∼5 nl·min−1·mm−1), this series of studies was performed exclusively at fast flow rates.
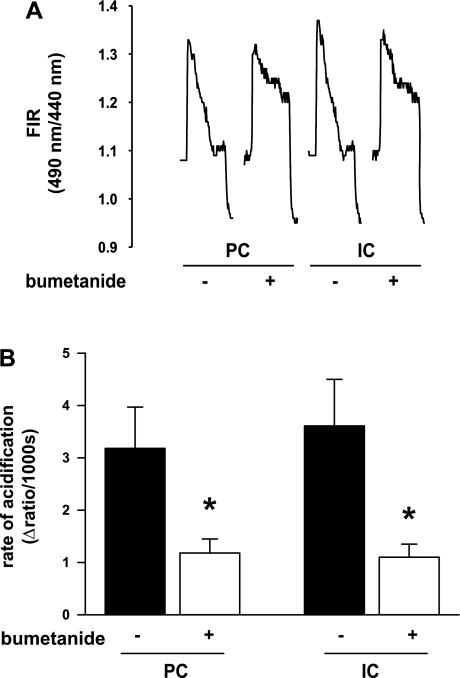
Fig. 5.
Effect of 10 μM bumetanide on the response of PC and IC cells to an NH4Cl pulse. A: fluorescence intensity ratios (FIRs; 490/440 nm) of individually identified BCECF-loaded cells were monitored before and then after 20 mM NH4+ was added to the CO2/HCO3−-free bathing solution. Addition of NH4+ to the bath led to a rapid increase in FIR in both cell types due to the initial alkalinization caused by NH3 entry. This was followed by a slower acidification (second phase; monitored for 3 min), reflecting in part NKCC-mediated NH4+ entry into the cell. On washout of the basolateral NH4Cl, the FIR dropped rapidly as pHi acidified. B: initial rate of change in FIR (2nd phase) was calculated using an exponential fit and was expressed as the change in FIR units per time. This rate was reduced in both cell types by basolateral bumetanide; the difference between the rates in the absence and presence of bumetanide presumably represents NKCC-mediated NH4+ uptake. Means ± SE. *P < 0.05 compared with control; n = 5 CCDs in each group (±bumetanide).
In CCDs (n = 5) perfused at a fast flow rate of 5.2 ± 0.2 nl·min−1·mm−1, basolateral bumetanide (10 μM) partially inhibited the acidification phase following the NH4Cl prepulse (presumably reflecting NH4+ uptake) in both principal and intercalated cells compared with the rates measured in five CCDs perfused at 5.3 ± 0.2 nl·min−1·mm−1 in the absence of inhibitor. Bumetanide reduced the acidification slope both in principal (3.18 ± 0.79 to 1.18 ± 0.27 ΔFIR/1,000 s; P < 0.05) and intercalated (3.61 ± 0.89 to 1.10 ± 0.25 ΔFIR/1,000 s; P < 0.03) cells. The observation that the inhibition of the acidification phase by bumetanide (and Ba2+) is incomplete is consistent with the presence of alternate NH4+ uptake pathways in these cells, including the basolateral Na+-K+-ATPase and K+ channels as well as other acid-base transporting pathways that modulate cytosolic pH (e.g., Cl−/HCO3− and Na+/H+ exchangers) (22). As Ba2+ was present in all CO2 and HCO3−-free bath solutions used for this series of experiments, we consider that most of the NH4+ entry was mediated by a basolateral NKCC.
Expression of NKCC1 subunit mRNA in the CCD.
Quantitative PCR was performed on single CCD samples (∼15-mm total length/sample; n = 5) using NKCC1- and 18S-specific primers and probes and the TaqMan assay to confirm the presence of the transcript in this nephron segment. Gene-specific transcripts were identified in all samples of single CCDs.
Immunolocalization of NKCC1.
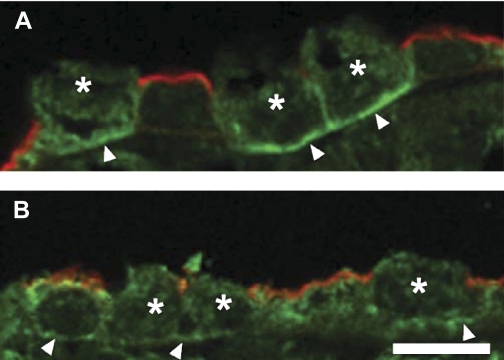
Since a previous immunolocalization study was able to detect NKCC1 in intercalated cells in the OMCD but not in principal cells (17), immunolocalization studies were undertaken to determine whether NKCC1 could be detected in both cell types in the CCD. While efforts to obtain a specific NKCC1 localization in rabbit kidney sections were not successful, we were able to carry out localizations on rat kidney using a previously well-characterized antibody (17) and antigen retrieval methods. These immunolocalizations confirmed the previous finding that NKCC1 is strongly detected in the basolateral membrane of intercalated cells but not in principal cells in the OMCD (Fig. 6A). In CCDs in the same section, distinct basolateral labeling in both principal and intercalated cells was apparent (Fig. 6B). The NKCC1 labeling of intercalated cells appeared weaker in the cortex than in the outer medulla. Diffuse cytoplasmic NKCC1 labeling was greater in CCD principal than intercalated cells. As an increase in luminal flow rate appears to stimulate trafficking of BK and/or Ca2+ channels from the cytoplasm to the membrane in the CCD (33), it may be that the cytoplasmic component of the NKCC1 signal in the CCD may traffic to the membrane with increased flow, a possibility that deserves future study.
Fig. 6.
Immunolocalization of NKCC1 along the rat collecting duct. A: immunolocalization of NKCC1 (green) in collecting ducts in the outer medulla showed strong labeling in the basolateral membrane (arrowheads) of IC cells (*) but anti-NKCC1 labeling could not be detected in adjacent PC cells identified by the presence of aquaporin-2 (AQP2; red) in their apical membrane. B: in the CCD, however, antibody to NKCC1 (green) labeled the basolateral surface (arrowheads) of both IC (*) and PC identified by their apical AQP2 labeling (red). Bar = 10 μm.
DISCUSSION
The results of the present study demonstrate that 1) FIKS but not JNa nor basal JK is inhibited by basolateral bumetanide, a relatively specific inhibitor of NKCC at the 10-μM concentration used; 2) FIKS is also inhibited by basolateral Cl− removal; 3) the effects of basolateral bumetanide and IbTX on FIKS are not additive; and 4) both intercalated and principal cells in rabbit appear to have a basolateral bumetanide-sensitive NH4+ entry step and, in rat, immunodetectable NKCC1. Importantly, bumetanide (100 μM) did not inhibit the flow-induced [Ca2+]i transient, suggesting that the inhibitor-induced reduction in FIKS was not directly due to loss of this critical trigger necessary for BK channel activation. These results lead us to conclude that a bumetanide-sensitive, basolateral Cl−-dependent transport pathway, specifically the secretory isoform of NKCC (NKCC1), contributes to BK channel-mediated FIKS and that basolateral NKCC1 may be present in both cell types in the CCD. In support of this conclusion is the finding that mice with genetic disruption of NKCC1 exhibit higher serum K+ concentrations with inappropriately low urinary K+ excretion compared with wild-type mice (42, 74).
NKCC1 is a widely distributed cotransporter that is expressed on the basolateral membrane of polarized epithelia, including the colon, salivary glands, and α-intercalated cells of rat OMCD (17, 21, 37). Studies in a variety of cell types demonstrate that this cotransporter mediates vectorial transport of Na+, K+, H+, and Cl− (21, 56). In OMCDs isolated from deoxycorticosterone-treated rats and microperfused in HCO3−/CO2-buffered solutions, basolateral bumetanide (100 μM) uncovered net Na+ absorption and inhibited net Cl− secretion but it was without significant effect on the net K+ flux (72, 73). However, the latter studies were performed in tubules perfused at ∼1.5 nl·min−1·mm−1, a flow rate below that necessary to activate FIKS. Indeed, in CCDs perfused at a similar slow rate in the present study, bumetanide had no effect on JK (Fig. 1B). In CCDs isolated from dietary K+-loaded rabbits and perfused at flow rates of ∼4.5 nl/min (tubule length not indicated), basolateral bumetanide (1 μM) failed to alter the net K+ flux, although basolateral Cl− removal significantly inhibited K+ secretion in these tubules; based on additional findings that reducing the luminal Cl− concentration stimulated K+ secretion and K+ secretion could be reversed to K+ absorption by reversing the Cl− gradient, these investigators concluded that there exists an apical KCl cotransport pathway in the CCD (76). However, as bumetanide inhibits JCl in a dose-dependent manner (50% by 10 μM and 78% by 100 μM) (73), an effect of this inhibitor applied in a concentration of 1 μM may not have been detected.
An unresolved question is how Na+, taken up at the basolateral membrane by NKCC, is extruded back out of intercalated cells. Certainly, a small fraction of this Na+ is pumped out by the basolateral Na+-K+-ATPase, as would be expected for the principal cell. Indeed, Sabolic et al. (57) proposed that the limited abundance of Na+-K+-ATPase in intercalated cells detected by their antigen retrieval technique might play a “housekeeping” role to maintain normal intracellular concentrations of Na+ and K+. But under conditions of high urinary flow, it is unlikely that the presumed low abundance of Na+-K+ pump in intercalated cells could handle the high-Na+ entry rates that would be necessary for basolateral NKCC to sustain FIKS. However, a second basolateral Na+ pump, a ouabain-insensitive furosemide-sensitive Na+-ATPase, has been identified biochemically in the kidney (40, 50). This Na+-stimulated ATPase activity is inhibited by millimolar concentrations of furosemide (8), and in glomerular, distal, and proximal suspensions of rat tubules, it is activated by increases in cell volume (40, 50). Na+-ATPase Vmax is increased by addition of micromolar concentrations of Ca2+ to the assay medium (51) while the Na+-K+-ATPase is inhibited by this concentration of Ca2+ (9). Madin-Darby canine kidney (MDCK) C11 cells, the majority of which possess β-intercalated cell characteristics (including peanut lectin agglutin binding), exhibit significantly greater Na+-ATPase activity and far lower Na+-K+-ATPase activity than that measured in C7 principal-like MDCK cells (58).
In assigning a role for principal and intercalated cell NKCC1 in FIKS, it is of interest that the activation profile of the cotransporter complements that of the BK channel. It is well-established that NKCC1 plays a major role in cell volume regulation; in general, activation of NKCC1-mediated transmembrane fluxes restores cell volume in response to osmotic cell shrinkage induced by extracellular hypertonicity (regulatory volume increase) (21, 56). As BK channels are also activated by cell swelling (23, 69), an NKCC1-induced increase in cell volume could lead to activation of these channels. Conversely, a reduction in NKCC1 activity leads to cell shrinkage and would be expected to lead to BK silencing. Indeed, the BK channel (α-β4) has been reported to mediate shear-induced K+ loss from intercalated-like MDCK cells (24), implicating a role for this channel in regulation of cell volume under conditions of high flow. NKCC1 activity is also influenced by the extracellular K+ concentration (56, 79), with a Km in the low millimolar range (25). As such, one may speculate that NKCC1 acts as a K+-controlled tunable switch that modulates cell volume and BK channel activity (Fig. 7), and as such, allows for FIKS to be regulated to meet body K+ homeostatic needs. In the face of a low extracellular K+ concentration, as follows chronic dietary K+ restriction (45), NKCC1 activity would be low, as has also been reported for BK channel-mediated K+ secretion, even at high urinary flow rates (45). Finally, both NKCC1 and the BK channel are regulated by a number of kinases. Among these, the observation that NKCC1 is regulated by WNKs (26) raises the possibility that FIKS may be regulated indirectly by regulation of NKCC1.
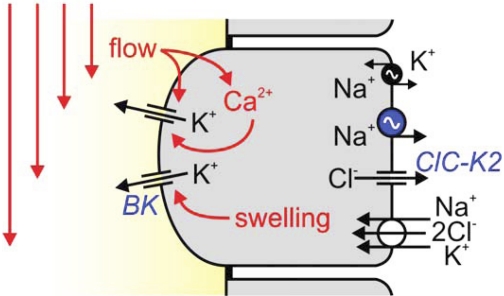
Fig. 7.
Hypothetical model for IC-mediated FIKS. Apical BK channels are activated by flow-induced increases in [Ca2+]i (33, 46, 47) and cell swelling (23, 69). Results of the present study suggest that basolateral NKCC1 is critical for BK channel-mediated FIKS. Basolateral NKCC1 may support apical K+ secretion by IC by 1) serving as a major uptake pathway for K+, 2) leading to cell swelling that activates apical BK channels, and 3) leading to uptake of Cl−, which in turn increases Cl− efflux through channels (e.g., ClC-K2), necessary to preserve intracellular electroneutrality when K+ is secreted across the apical membrane. Whereas Na+ taken up by basolateral NKCC1 can be extruded at the same membrane by the Na+-K+-ATPase, pump activity is proposed to be low in these cells (vs. PC), raising the possibility that a ouabain-insensitive Na+-ATPase similar to that identified in IC-like Madin-Darby canine kidney cells is present in native IC. It should be noted that urinary K+ secretion requires that the apical membrane be more depolarized than the equilibrium potential of K+, which may be achieved in IC cells by “intraepithelial current flow” between IC and PC (31). The electrophysiologic properties of the CCD can also be regulated by the paracellular Cl− permeability (59).
Clinically, the 24-h kaliuresis that follows once-daily administration of furosemide is lower than following administration of an equinatriuretic dose of a thiazide diuretic (52, 53). This finding has been attributed to the fact that Na+ that escapes reabsorption in the “inhibited” thick ascending limb of Henle can be reabsorbed in the early distal tubule, thereby reducing Na+ and fluid delivery to more distal K+-secretory sites (52, 53). Our data offer another explanation for this clinical finding. Specifically, loop diuretic-induced K+ losses may be minimized if inhibition of basolateral NKCC1 by furosemide in the K+-secretory segments of the nephron also prevents FIKS.
GRANTS
This work was supported by National Institutes of Health Grants DK038470 (to L. M. Satlin), DK051391 (to T. R. Kleyman), P30 DK079307 (The Pittsburgh Center for Kidney Research), American Heart Association Fellowship Grant (C. Schreck), DK32839 (to J. Wade), and Deutsche Forschungsgemeinschaft Grant SFB699 (to R. Warth).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Binder HJ, Sandle GI. Electrolyte transport in the mammalian colon. In: Physiology of the GI Tract, 3rd ed., edited by Johnson LR. New York: Raven, 1994, p. 2133–2171 [Google Scholar]
- 5. Boyarsky G, Hanssen C, Clyne LA. Superiority of in vitro over in vivo calibrations of BCECF in vascular smooth muscle cells. FASEB J 10: 1205–1212, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Del Castillo JR, Marin R, Proverbio T, Proverbio F. Partial characterization of the ouabain-insensitive, Na+-stimulated ATPase activity of kidney basal-lateral plasma membranes. Biochim Biophys Acta 692: 61–68, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Del Castillo JR, Robinson JW. Na+-stimulated ATPase activities in basolateral plasma membranes from guinea-pig small intestinal epithelial cells. Biochim Biophys Acta 812: 413–422, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci USA 83: 8415–8419, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emmons C, Kurtz I. Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Grantham JJ, Burg MB, Orloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest 49: 1815–1826, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haas M, Forbush B., 3rd The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Heitzmann D, Warth R, Bleich M, Henger A, Nitschke R, Greger R. Regulation of the Na+-2Cl-K+ cotransporter in isolated rat colon crypts. Pflügers Arch 439: 378–384, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Hirsch J, Frobe U, Schlatter E. Regulation and possible physiological role of the Ca2+-dependent K+ channel of cortical collecting ducts of the rat. Pflügers Arch 422: 492–498, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Holtzclaw JD, Liu L, Grimm PR, Sansom SC. Shear stress-induced volume decrease in C11-MDCK cells by BK-α/β4. Am J Physiol Renal Physiol 299: F507–F516, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isenring P, Jacoby SC, Payne JA, Forbush B., 3rd Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-L-Cl cotransporter. J Biol Chem 273: 11295–11301, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta 1802: 1150–1158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975 [DOI] [PubMed] [Google Scholar]
- 28. Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature 339: 478–480, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem 269: 17274–17278, 1994 [PubMed] [Google Scholar]
- 31. Koeppen BM. Electrophysiological identification of principal and intercalated cells in the rabbit outer medullary collecting duct. Pflügers Arch 409: 138–141, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct (CCD): role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lytle C, Xu JC, Biemesderfer D, Forbush B., 3rd Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Malnic G, Klose RM, Giebisch G. Microperfusion study of distal tubular potassium and sodium transfer in rat kidney. Am J Physiol 211: 548–559, 1966 [DOI] [PubMed] [Google Scholar]
- 40. Marin R, Gomez DC, Rodriguez GA, Proverbio T, Proverbio F. Ouabain-insensitive, Na-ATPase activity in pure suspensions of rat kidney proximal tubules. FEBS Lett 269: 77–78, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593–F601, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol 283: H1846–H1855, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang W, Seldin D, Giebisch G. Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int 75: 25–30, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-b1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Proverbio F, Del Castillo JR. Na+-stimulated ATPase activities in kidney basal-lateral plasma membranes. Biochim Biophys Acta 646: 99–108, 1981 [DOI] [PubMed] [Google Scholar]
- 51. Proverbio F, Proverbio T, Marin R. Ouabain-insensitive Na+-stimulated ATPase activity of basolateral plasma membranes from guinea-pig kidney cortex cells. II. Effect of Ca2+. Biochim Biophys Acta 688: 757–763, 1982 [DOI] [PubMed] [Google Scholar]
- 52. Reyes AJ. Effects of diuretics on outputs and flows of urine and urinary solutes in healthy subjects. Drugs 41, Suppl 3: 35–59, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Reyes AJ, Taylor SH. Diuretics in cardiovascular therapy: the new clinicopharmacological bases that matter. Cardiovasc Drugs Ther 13: 371–398, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Ridderstrale Y, Kashgarian M, Koeppen B, Giebisch G, Stetson D, Ardito T, Stanton B. Morphological heterogeneity of the rabbit collecting duct. Kidney Int 34: 655–670, 1988 [DOI] [PubMed] [Google Scholar]
- 55. Roos A, Boron WF. Intracellular pH. Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 56. Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Sampaio MS, Bezerra IP, Pecanha FL, Fonseca PH, Capella MA, Lopes AG. Lack of Na+, K+-ATPase expression in intercalated cells may be compensated by Na+-ATPase: a study on MDCK-C11 cells. Cell Mol Life Sci 65: 3093–3099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sansom SC, Weinman EJ, O'Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 247: F291–F302, 1984 [DOI] [PubMed] [Google Scholar]
- 60. Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Satlin LM, Evan AP, Gattone VH, 3rd, Schwartz GJ. Postnatal maturation of the rabbit cortical collecting duct. Pediatr Nephrol 2: 135–145, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Satlin LM, Matsumoto T, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. III. Peanut lectin-binding intercalated cells. Am J Physiol Renal Fluid Electrolyte Physiol 262: F199–F208, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 270: F391–F397, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Sauer M, Flemmer A, Thurau K, Beck FX. Sodium entry routes in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflügers Arch 416: 88–93, 1990 [DOI] [PubMed] [Google Scholar]
- 66. Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 67. Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974 [DOI] [PubMed] [Google Scholar]
- 69. Stoner LC, Morley GE. Effect of basolateral or apical hyposmolarity on apical maxi K channels of everted rat collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 268: F569–F580, 1995 [DOI] [PubMed] [Google Scholar]
- 70. Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280: C192–C198, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Wall SM, Fischer MP. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to transepithelial transport of H+, NH4+, K+, and Na+ in rat outer medullary collecting duct. J Am Soc Nephrol 13: 827–835, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter NKCC1 to Cl− secretion in rat OMCD. Am J Physiol Renal Physiol 280: F913–F921, 2001 [DOI] [PubMed] [Google Scholar]
- 74. Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 76. Wingo CS. Reversible chloride-dependent potassium flux across the rabbit cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F697–F704, 1989 [DOI] [PubMed] [Google Scholar]
- 77. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush B., 3rd Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci USA 91: 2201–2205, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]