Abstract
Obesity is often associated with insulin resistance, low-grade systemic inflammation, and reduced plasma adiponectin. Inflammation is also increased in adipose tissue, but it is not clear whether the reductions of adiponectin levels are related to dysregulation of insulin activity and/or increased proinflammatory mediators. In this study, we investigated the interactions of insulin, tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) in the regulation of adiponectin production using in vivo and in vitro approaches. Plasma adiponectin and parameters of insulin resistance and inflammation were assessed in a cohort of lean and obese insulin-resistant subjects. In addition, the effect of insulin was examined in vivo using the hyperinsulinemic-euglycemic clamp, and in adipose tissue (AT) cultures. Compared with lean subjects, the levels of total adiponectin, and especially the high-molecular-weight (HMW) isomer, were abnormally low in obese insulin-resistant subjects. The hyperinsulinemic clamp data confirmed the insulin-resistant state in the obese patients and showed that insulin infusion significantly increased the plasma adiponectin in lean but not obese subjects (P < 0.01). Similarly, insulin increased total adiponectin release from AT explants of lean and not obese subjects. Moreover, expression and secretion of TNF-α and IL-6 increased significantly in AT of obese subjects and were negatively associated with expression and secretion of adiponectin. In 3T3-L1 and human adipocyte cultures, insulin strongly enhanced adiponectin expression (2-fold) and secretion (3-fold). TNF-α, and not IL-6, strongly opposed the stimulatory effects of insulin. Intriguingly, the inhibitory effect of TNF-α was especially directed toward the HMW isomer of adiponectin. In conclusion, these studies show that insulin upregulates adiponectin expression and release, and that TNF-α opposes the stimulatory effects of insulin. A combination of insulin resistance and increased TNF-α production could explain the decline of adiponectin levels and alterations of isomer composition in plasma of obese insulin-resistant subjects.
Keywords: obesity, inflammation
obesity is frequently associated with insulin resistance and mild systemic inflammation (28). Invasion of adipose tissue by macrophages creates a microenvironment rich with proinflammatory cytokines (28) produced by both macrophages and adipocytes. With the excess of adiposity and infiltration of macrophages in adipose tissue, numerous proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), are concomitantly increased in adipose tissue, and this increase is significantly reflected in blood levels (20, 28). Although adipose tissue mass and adipocyte numbers are enhanced in obese subjects, blood adiponectin levels are paradoxically reduced in association with insulin resistance and low production of adiponectin (22). It is not clear whether insulin resistance at the level of the adipocyte is the underlying reason for the decline of adiponectin production, leading to a reduction of blood adiponectin. Furthermore, it is not clear whether proinflammatory cytokines have equally negative effects on adipocyte endocrine function. For instance, there is broad agreement that TNF-α induces insulin resistance (17), promotes lipolysis (34), and reduces adipogenesis (18). However, the role of IL-6 in obesity-induced insulin resistance and adipocyte metabolism is not fully understood. Indeed, the question of whether IL-6 has positive or negative effects on adipocyte function and carbohydrate metabolism is the subject of continuing controversy (7). For example, IL-6 production is enhanced in contracting muscle, concomitant with increased insulin sensitivity during exercise (31). Some studies reported that visceral adiposity is specifically linked to low serum levels of adiponectin and suggested that this association is in fact due to the production of more TNF-α and IL-6 and less adiponectin (14). In addition, an inverse correlation between circulating levels of TNF-α and adiponectin has been reported earlier in obese and diabetic patients (19), suggesting that TNF-α, and probably IL-6 among other cytokines, exerts suppressive roles on adipocyte production of adiponectin (12). However, ex vivo incubation of human adipose tissue with IL-6 did not alter adiponectin production unless IL-6-soluble receptor was added (5).
Adiponectin is an adipokine predominantly produced by adipocytes and exerts multiple beneficial actions, including insulin-sensitizing, anti-inflammatory, and anti-atherosclerotic properties (3, 8). Earlier studies have shown that adiponectin enhances insulin sensitivity by increasing glucose and fatty acid oxidation in muscle and other organs (46). Specific membrane receptors, adiponectin receptors 1 and 2 (adipoR1 and adipoR2), transmit adiponectin intracellular signals to activate AMP-activated protein kinase (AMPK) and other key metabolic regulators in muscle, liver, and heart (37, 45). In blood, adiponectin circulates in different multimer complexes (also named isomers), specifically classified as high-molecular-weight (HMW) multimers, medium-molecular-weight hexamers (MMW), and low-molecular-weight (LMW) trimers (2, 27). Recent clinical studies have revealed that the HMW complex is the most biologically potent form and plays a key role in the regulation of insulin sensitivity (23, 30). Furthermore, biochemical analysis of purified complexes and in vivo studies showed that different forms of adiponectin do not interconvert after secretion (2, 27). These findings suggest that factors that dysregulate adipocyte endocrine function, especially adiponectin production, could initiate a chain of events leading to insulin resistance and eventually diabetes (42).
Regulation of adiponectin production is not fully understood, and many factors may contribute individually or collectively to reduce adiponectin levels in blood (23). These studies were undertaken to 1) investigate the role of insulin in adiponectin production and 2) determine the effects of TNF-α and IL-6 in this regulatory relationship. In this regard, the roles of insulin, TNF-α, and IL-6 on adiponectin production were evaluated in vivo in lean and obese subjects and in adipocyte and adipose tissue explant culture.
MATERIALS AND METHODS
Human subjects.
A total of 68 subjects were recruited for this study prior to undergoing elective surgical procedures. Subject characteristics are shown in Table 1. Of the 68 subjects, 13 were lean (BMI 21.1–24.9 kg/m2), 10 were overweight (BMI 25–30 kg/m2), and 45 were obese (BMI 31–40 kg/m2) or morbidly obese (>40 kg/m2). Participants enrolled in these studies underwent repeated tests during multiple visits to the Vanderbilt University Medical Center or Clinical Research Center. These tests included physical examination, vital signs, blood analyses, and disclosure of medications. Inclusion criteria for lean subjects were based on the American Diabetes Association criteria for normal fasting blood glucose (21) and also included normal ranges for blood pressure, insulin and lipids (cholesterol, triglycerides), and hematology. In addition, standard tests of kidney function (blood creatine and glomerular filtration rate) were performed and used to exclude lean and obese patients when biochemical evidence of renal dysfunction was detected. Furthermore, blood inflammatory markers including monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), IL-6, and TNF-α were tested. All these tests indicated normal ranges of inflammatory markers in this group of lean subjects. Obese subjects underwent laparoscopic Roux-en-Y gastric bypass surgery (RYGB). Lean and obese subjects enrolled in these studies were not taking antidiabetic, antihyperlipidemic, or anti-inflammatory medications or supplements. Fat tissue biopsies were obtained from the subjects at the time of their operative procedures. The study protocol was approved by the Vanderbilt Institutional Review Board, and informed consent was obtained from each subject. Fasting serum glucose levels were determined using standard clinical assays as described elsewhere (16). In addition, fasting insulin, glycosylated hemoglobin (Hb A1C), and the homeostasis model assessment of insulin resistance index (HOMA-IR) were employed to assess the insulin resistance status of each subject (11). Obese patients were hyperinsulinemic but not diabetic according to the American Diabetes Association criteria (21).
Table 1.
Physical and metabolic characteristics of subjects according to BMI sub-divisions
| Lean | Overweight | Obese | |||
|---|---|---|---|---|---|
| BMI, kg/m2 | 20–25 | 25–30 | 31–40 | 41–50 | >51 |
| N | 13 | 10 | 12 | 18 | 15 |
| Age, yr | 43 ± 4 | 49 ± 4 | 45 ± 6 | 42 ± 3 | 41 ± 5 |
| Weight, kg | 64 ± 6 | 81 ± 8b | 101 ± 4a | 150 ± 6a | 155 ± 6a |
| BMI, kg/m2 | 24 ± 1 | 28 ± 2b | 38 ± 2a | 45 ± 1a | 57 ± 5a |
| Hb A1C, % | 5.5 ± 0.1 | 5.6 ± 0.3 | 5.9 ± 0.5 | 5.9 ± 0.3 | 5.8 ± 0.3 |
| HOMA-IR | 0.9 ± 0.2 | ND | 1.9 ± 0.2a | 2.8 ± 0.2a | 3.9 ± 0.2a |
| Glucose, mg/dl | 96 ± 2 | 89 ± 4 | 102 ± 9 | 109 ± 5 | 108 ± 6 |
| Insulin, μU/ml | 4.9 ± 0.5 | 14.6 ± 5a | 16 ± 2a | 21.5 ± 1.1a | 27.9 ± 3a |
| Adiponectin, μg/ml | 28.4 ± 1.3 | ND | 11.3 ± 0.8a | 6.1 ± 0.4a | 4.8 ± 0.7a |
| IL-6, ng/ml | 11 ± 6 | ND | 126 ± 28a | 202 ± 29a | 214 ± 35a |
| TNF-α, pg/ml | 39 ± 14 | ND | 294 ± 41a | 478 ± 55a | 713 ± 62a |
Values are means ± SE. ND, not determined; HOMA-IR, homeostasis model assessment of insulin resistance. Significantly different from values of lean for:
P < 0.001,
P < 0.05.
Hyperinsulinemic-euglycemic clamp.
Insulin sensitivity was assessed using the hyperinsulinemic-euglycemic clamp in lean (n = 7, BMI 23.2 ± 2.1 kg/m2) and obese subjects (n = 21, BMI 38 ± 4 kg/m2) according to the protocol described elsewhere (11). Subjects were admitted to the Vanderbilt Clinical Research Center (CRC) in the evening before the study and given a standard meal. The subjects were fasted overnight (10–12 h). Catheters were inserted into a forearm vein and used for insulin and glucose infusion and into a contralateral superficial hand vein, which was heated to 55°C. A two-step insulin clamp was then performed. During the first step, insulin was infused at 0.6–1.0 mU·kg−1·min−1 for a total of 2 h followed by a second step infusion of 2.0–3.0 mU·kg−1·min−1 for an additional 2 h. Euglycemia (∼100 mg/dl) was maintained by a variable infusion of 20% dextrose. Blood samples for rapid glucose determination were obtained every 5 min throughout the clamp, and additional samples for insulin determinations were obtained during the last 30 min of the basal and insulin infusion periods. Plasma glucose levels were measured by the automated glucose oxidase method (16).
Adipose tissue biopsies.
Paired samples of subcutaneous and omental adipose tissues were obtained from lean and obese subjects at the time of their operations. Subcutaneous abdominal adipose tissue was obtained from the lower abdominal region, and omental adipose tissue was obtained from the greater omentum. The tissue samples were immediately placed in sterile saline (0.9%) and were used for explant cultures or frozen in liquid nitrogen and stored at −80°C until analysis.
Adipose explant culture.
All procedures for cultures of adipose tissue explants were carried out using sterile technique as described earlier (15). Following biopsy, adipose tissue was placed into sterile filtered HEPES-salts buffer containing (in mmol/l) 150 NaCl, 5 KC1, 1.2 MgSO4, 1.2 CaC12, 2.5 NaH2PO4, 10 HEPES, and 2 pyruvate, pH 7.4, and supplemented with 4% BSA (Roche Diagnostic, Indianapolis, IN) and immediately transported under aseptic conditions to the laboratory. Adipose tissue was washed free of lipid and blood clots with saline and then processed further by cutting into 5- to 10-mg pieces, washed, and placed in DMEM-F12 medium, as described earlier (15). Explants were cultured (∼0.5 g/2 ml) under an atmosphere of 5% CO2 at 37°C in a defined medium (1:1 DME low-Ham's F-10 containing 5 mM glucose supplemented with 25 mM HEPES, 15 mM NaHCO3, 2 mM glutamine, and 100 U/ml penicillin-0.1 mg/ml streptomycin). Culture medium was replenished each day, and after a 2-day recovery period experiments were started. Each experiment was carried in triplicate for paired subcutaneous and omental adipose tissue from the same patient. To test the effects of insulin on adiponectin production, explant cultures were pretreated with either insulin (100 nM), or vehicle (CNT) for 24 h. The medium was then changed with appropriate ingredients, and samples of 200 μl were taken at 4, 8, 12, and 24 h of incubation. At the end of the experiment, medium and adipose tissues were immediately frozen for later analysis.
3T3-L1 adipocyte culture and treatments.
3T3-L1 cells were grown in DMEM (GIBCO, Grand Island, NY) with 10% delipidated fetal calf serum (FCS). Differentiation of 3T3-L1 preadipocytes to mature adipocytes was performed as described previously (41). Briefly, after 2 days of confluence, cells were induced by incubation in DMEM with 10% FCS and differentiation cocktail containing 0.5 mM isobutyl-1-methylxanthine (IBMX), 1 μg/ml dexamethasone, and 10 μg/ml insulin for 48 h. Then, cells were incubated in DMEM with 10% delipidated FCS supplemented with 10 μg/ml insulin for another 6 days. The medium was replaced every 2 days. After mature adipocytes were achieved, cells were washed three times with phosphate-buffered solution (PBS), and the 10% FBS was replaced by 0.2% fatty acid-free BSA. The cells were preincubated for 24 h with insulin (100 nM) or vehicle (CNT). Thereafter, the medium was replaced, and dishes were divided in subgroups designated as 1) basal control cells (CNT), 2) insulin treated (100 nM), 3) IL-6 treated with 20, 40, or 80 ng/ml), 4) TNF-α treated with either 10, 20, or 40 ng/ml), 5) insulin + IL-6 treated (100 nM insulin + 20, 40, or 80 ng/ml IL-6), and 6) insulin + TNF-α (100 nM insulin + 10, 20, or 40 ng/ml TNF-α). All tests were performed in triplicate, and media were harvested at indicated time points (0, 2, 4, and 8 h). At the end of the experiment, cells were collected for protein and mRNA extraction and analysis as described elsewhere (41).
Human preadipocyte isolation, differentiation, and treatments.
Human subcutaneous adipose tissue was cut into small pieces after cleaning and vessel removal and digested with 1.0 mg/ml collagenase type I (Sigma-Aldrich) in Hank's buffered salt solution (HBSS) containing 2% BSA with shaking for 1 h (41). The digested material was filtered and centrifuged at 500 g for 10 min. The resulting pellets were resuspended in erythrocyte lysis buffer (Lonza, Wakersville, MD). After washing with PBS, the cells were suspended in DMEM-F12 with 10% FCS and used for cell culture at passage 3 to eliminate non-preadipocyte cell contamination. At this stage, control tests using specific antibodies against markers for macrophages and preadipocytes confirmed that the growing cells were preadipocytes. For preadipocyte differentiation, cells were cultured over a period of 21 days in differentiation medium [FCS-free adipocyte medium supplemented with 10 μg/ml insulin, 1 nM triiodothyronine (T3), 0.5 mM IBMX]. Culture medium was changed every 3 days. Fully mature adipocytes were pretreated for 24 h with either 100 nM insulin (INS) or vehicle (CNT). Then, the medium was replaced, and cells were treated with insulin (100 nM) alone, or in combination with either IL-6 (80 ng/ml) or TNF-α (40 ng/ml), as described for 3T3-L1 cells. Aliquots of culture medium were harvested at the indicated time. At the end of the experiment, cells were collected for mRNA extraction and qPCR analysis as previously described (41).
Measurement of circulating insulin, adiponectin, and cytokines.
Insulin and total adiponectin content in plasma were measured using an ELISA (Ray Biotech, San Diego, CA) according to the manufacturer's instructions. Contents of IL-6 and TNF-α in plasma and culture medium were performed by ELISA (R&D Systems, Minneapolis, MN). Preliminary assays showed that TNF-α concentrations were lower in serum of lean subjects than in that of obese subjects. Therefore, optimization of the assay was performed to ensure that optical density readings for samples of lean and obese subjects were within the linear range of the standard curve. To achieve this, plasma samples from lean subjects were not diluted, whereas those from obese subjects were diluted, and assays were performed in equal final volumes in duplicates of 200 μl for both groups. This procedure allows appropriate detection of low contents of IL-6 and TNF-α within the standard curve range. The interassay coefficients of variations (CVs) were less than 10%, and the detection limits of the assays were less than 3 pg/ml.
Distribution of adiponectin isoforms in serum and culture media.
To investigate the distribution of adiponectin polymeric isoforms, plasma of lean and obese subjects was tested using an adiponectin multimeric ELISA assay (Alpco Immunoassays no. 47-ADPHU-E01, Salem, NH). Based on protease digestion, this assay allows the determination of total adiponectin as well as HMW, MMW, and LMW isomers. The interassay CVs were less than 10%, and the sensitivity of assays was 0.2 ng/ml. Values of plasma total adiponectin were also measured with an Alpco ELISA kit and were comparable to those tested by a Raybiotech ELISA kit for the same plasma (<5% difference). The correlation between both tests was very high (r = 0.98). The interassay CVs for total and HMW adiponectin measured by ELISA were 9.8 and 12.9%, respectively; the intra-assay CVs for total and HMW adiponectin were less than 6.9 and 10%, respectively.
In addition, adiponectin isomers in culture medium and cell lysates were analyzed by Western blot after protein separation in nondenaturing (native) polyacrylamide gel electrophoresis conditions as described by others (27, 30). Briefly, proteins were separated using Tris-acetate 3–8% polyacrylamide gradient gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membrane, followed by the application of primary anti-adiponectin antibody (Epitomics, Burlingame, CA) and an appropriate second antibody (Santa Cruz Biotechnology, Santa Cruz, CA). A mixture of protein standards and known amounts of recombinant adiponectin protein (HMW) applied in the same gel was used to identify adiponectin isomers according to their molecular weights (41). Densitometric units were determined for each band by use of ChemiDoc apparatus and Quantity One software (Bio-Rad), and relative density for each protein band was expressed as percent total adiponectin.
Gene expression and qPCR.
Total RNA was extracted using a total RNA fatty and fibrous tissue pack (Bio-Rad, Oakland, CA) according to the manufacturer's protocol. Complementary DNA was synthesized from 1 μg of total RNA with iScript reverse transcriptase (Bio-Rad). qPCR was performed using SYBR Green Supermix with iTaqDNA polymerase on Bio-Rad's IQ5 thermocycler, as described earlier (41). Oligonucleotides (Table 2) were designed, optimized, and efficiently tested before using. For each primer set, the annealing temperature was optimized, and the solitary product formed was confirmed through the melt curve analysis in each PCR run. The PCR program included 36 cycles of heat denaturing at 95°C for 30 s, annealing at 52–62°C for 30 s, followed by a melt curve cycle. qPCR data were obtained as CT values, where CT was defined as the threshold cycle of PCR at which products amplify exponentially. As an internal control, β-actin expression was measured in parallel. The difference in the CT values (ΔCT) was derived from the specific gene tested and CT of the control gene (β-actin) according to the equation 2[CT actin − CT target gene] (25). Final results are presented as relative expression (ΔCT).
Table 2.
Primers used for real-time RT-PCR
| Forward | Reverse | GenBank No. | |
|---|---|---|---|
| Human adiponectin | 5′-AGGCACAGGGAACAAGCA-3′ | 5′-AGGTCCACAGCTCTGGGTTTGA-3′ | NM_004797 |
| AdipoR1 | 5′-CCTTCTACTGCTCCCCACAG-3′ | 5′-GACAAAGCCCTCAGCGATAG-3′ | NM_015999 |
| AdipoR2 | 5′-GGCATGTCCCCTCTCTTACA-3′ | 5′-TGTGTCCAAATGTTGCCTGT-3′ | NM_024551 |
| Human IL-6 | 5′-AAATTCGGTACATCCTCGACGGCA-3′ | 5′-AGTGCCTCTTTGCTGCTTTCACAC-3′ | NM_000600 |
| Human TNF-α | 5′-GAGCTGAACAATAGGCTGTTCCCA-3′ | 5′-AGAGGCTCAGCAATGAGTGACAGT-3′ | NM_000594 |
| Mouse adiponectin | 5′-ACTGCAACATTCCGGGACTCTACT-3′ | 5′-AGAGAACGGCCTTGTCCTTCTTGA-3′ | NM_009605 |
AdipoR, human adiponectin receptor.
Statistical analysis.
Results are presented as means ± SE for indicated numbers of subjects. Due to limitations in sample availability, not all analyses were performed in every subject. The number of subjects studied is given in the legend of each figure. Clinical characteristics of the subsets of subjects studied in the different experiments did not differ from the average of the total group. Statistical analysis was performed using the GraphPad Prism program v.4 (Intuitive Software, San Diego, CA). For multiple groups comparison (Table 1), a two-way ANOVA test was used, and when ANOVA indicated a significant effect (P < 0.05) between groups, differences were determined by the Tukey-Kramer post hoc test. Statistical significance was evaluated with Student's paired t-test for comparison between repeated measurements of adiponectin in the same group during the hyperinsulinemic-euglycemic clamp (see Fig. 2). For comparison between two groups, statistical significance was evaluated with Student's unpaired t-test. Pearson's correlation coefficients were computed to determine significant associations among variables. Significance was accepted at the P < 0.05 level.
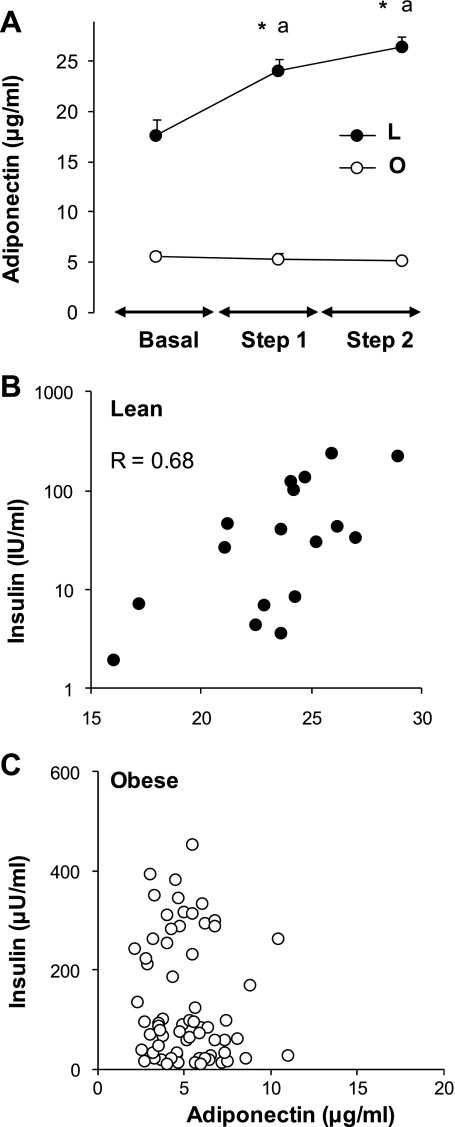
Fig. 2.
Relationship of plasma insulin levels in lean and obese subjects. A: effect of varying insulin concentrations on plasma adiponectin levels in lean (L, n = 7) and obese (O, n = 21) subjects undergoing a 2-step (each for 2 h) hyperinsulinemic-euglycemic clamp; basal levels were obtained after a 12-h fast prior to insulin infusion. Steady-state low (3- to 4-fold; step 1) and high (8- to 10-fold basal; step 2) insulin levels were achieved following insulin infusions of 0.6–1.0 and 2–3.0 mU·kg−1·min−1, each for 2 h while maintaining euglycemia using a variable infusion of 20% dextrose. Values are means ± SE. Statistical differences between obese and lean for each time point are presented with an asterisk: *P < 0.001. Differences between values before (basal) and after insulin infusion for each group are indicated by letter (aP < 0.01). Correlations are shown between plasma insulin and adiponectin levels in lean (B) and obese (C) subjects during the euglycemic-hyperinsulinemic clamps. Pearson correlation coefficient is shown for lean subjects; there was no significant correlation for obese subjects.
RESULTS
Serum adiponectin levels in lean and obese insulin-resistant subjects.
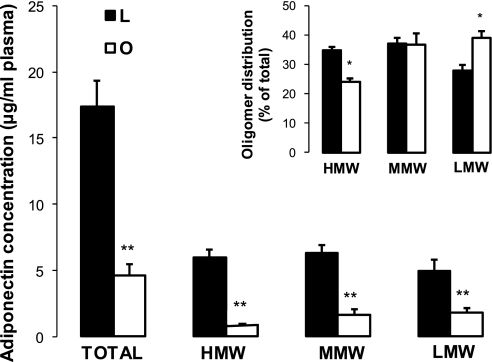
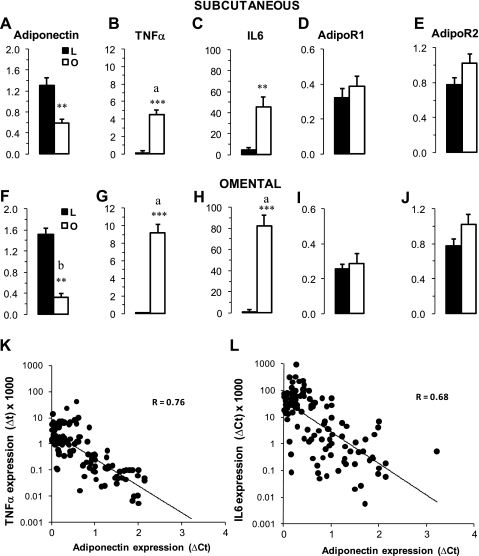
To compare the effects of obesity and insulin resistance on blood adiponectin, we recruited subjects with different degrees of obesity (BMI 21–70 kg/m2). In addition to the preliminary clinical analysis used for exclusion/inclusion criteria, we performed additional analyses of circulating cytokines and markers of inflammation in adipose tissue samples. The levels of proinflammatory cytokines TNF-α and IL-6 were low in lean subjects, however, within the range of published values for lean subjects (26). In addition, blood levels of IL-6 and TNF-α were higher in obese subjects (BMI >31 kg/m2), consistent with the mild systemic inflammation associated with obesity (19). Fasting plasma glucose levels were elevated in the obese, but these elevations did not achieve statistical significance. Plasma insulin levels were low in normal lean subjects and increased gradually with the increase of BMI in obese subjects (Table 1). HOMA-IR was progressively increased in obese subjects, suggesting a state of insulin resistance, especially in the severely obese subjects (Table 1). In contrast to insulin, adiponectin concentrations gradually decreased in obese subjects (Table 1). Concentrations of plasma adiponectin were negatively correlated with BMI (P < 0.001), plasma insulin (P < 0.001), TNF-α (P < 0.05), and IL-6 (P < 0.05). We also questioned whether insulin resistance is associated with a modification of circulating adiponectin isomers (Fig. 1). The absolute levels of adiponectin isomers were significantly lower in obese subjects, but the decline of the HMW isomer (5-fold decrease) was stronger than those of MMW (3.5-fold) and LMW (2-fold) isomers. Consequently, the composition of adiponectin isomers was significantly altered in obese subjects to the benefit of LMW and disadvantage of the HMW isomers (Fig. 1, inset).
Fig. 1.
Total and adiponectin isomers in plasma of lean and morbidly obese subjects. Analysis of plasma adiponectin isomers was performed with ELISA, using an adiponectin multimeric assay kit from Alpco Immunoassays, as described in materials and methods. Results are presented as means ± SE. Statistical differences between lean and obese subjects were performed by unpaired Student's t-test. Significant differences are indicated by asterisks: **P < 0.01, *P < 0.05.
Effects of insulin on blood adiponectin levels in vivo.
In lean subjects, the two-step hyperinsulinemic clamp resulted in a significant increase of circulating adiponectin levels for the low (+25%) and high (+36%) insulin infusion rates (Fig. 2A). However, the effects of insulin infusion on plasma adiponectin levels were virtually abolished in obese subjects (Fig. 2A). Interestingly, plasma adiponectin levels correlated positively (P < 0.05) with plasma insulin levels in the lean (Fig. 2B) but not in the obese (Fig. 2C) subjects.
Impact of fat depot and insulin on adiponectin secretion in vitro.
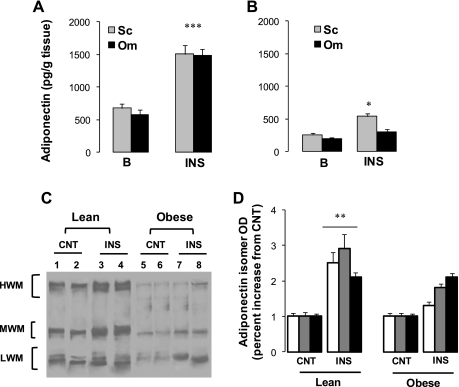
To determine the effects of insulin on adiponectin secretion in vitro, human subcutaneous and omental adipose tissue explants were cultured in medium with or without insulin. The degree of inflammation varies according to the location of adipose tissue (33); therefore, cultures of subcutaneous and omental fats were performed separately. Insulin treatment of subcutaneous adipose tissue explants resulted in enhanced total adiponectin secretion in a time-dependant manner (results not shown). The effects of insulin on total adiponectin secretion in omental and subcutaneous adipose tissues of lean subjects were comparable, with an approximately twofold increase in both fat depots (Fig. 3A). In the culture of adipose tissue of obese subjects (Fig. 3B), basal control rates (CNT) of total adiponectin secretion were lower than those of adipose tissues from lean subjects (P < 0.001). In addition, total adiponectin production was lower (P < 0.05) in omental than in subcutaneous fat depots (Fig. 3B). Insulin stimulation of adiponectin secretion in subcutaneous (+31%) and omental (+11%) adipose tissues of obese subjects was significantly (P < 0.001) lower than in lean fat depots. These data show that insulin was effective in the stimulation of adiponectin secretion in fat depots of lean subjects. However, the insulin-stimulated adiponectin production was significantly blunted in fat depots of obese subjects.
Fig. 3.
Adiponectin secretion from subcutaneous (Sc) and omental (Om) adipose tissue cultures. Sc adipose tissues of lean (A) and obese (B) subjects were pretreated (24 h) with insulin (INS) or vehicle (CNT), and adiponectin secretion was tested and compared with control nontreated explants (CNT). Adiponectin secretions (24 h) in Sc and Om fats are shown for lean (A) and obese subjects (B). Analysis of adiponectin isomers in culture medium (D) was performed by Western blotting followed by separation in a nondenaturing polyacrylamide gel electrophoresis condition as described in materials and methods. Representative blot and mean of optic density are shown in C and D, respectively. Data are presented as means ± SE; n = 6 for lean subjects (BMI 24 ± 1 kg/m2) and n = 12 for obese subjects (BMI 45 ± 5 kg/m2). Statistical differences between insulin-treated (INS) and control (CNT) for the same fat depot and the same subject group are indicated by asterisks: ***P < 0.001, **P < 0.01, and *P < 0.05.
To determine the effects of insulin on the production of adiponection isomers, proteins in culture medium were analyzed with native electrophoresis and Western blot (Fig. 3, C and D). Insulin treatment of adipose tissue from lean subjects increased the secretion of adiponection isomers without discrimination (∼2- to 2.7-fold vs. controls). In agreement with the ELISA analysis for total adiponectin (Fig. 3D), secretion of adiponectin isomers was significantly blunted in obese subjects. In addition, insulin induction of the HMW isomer (+10%) was significantly lower than MMW and LMW isomers (+24%). Analysis of adiponectin isomers in cell lysate showed similar results to those in culture medium, indicating that intracellular isomer composition and secretion rates were altered in the fat of obese subjects.
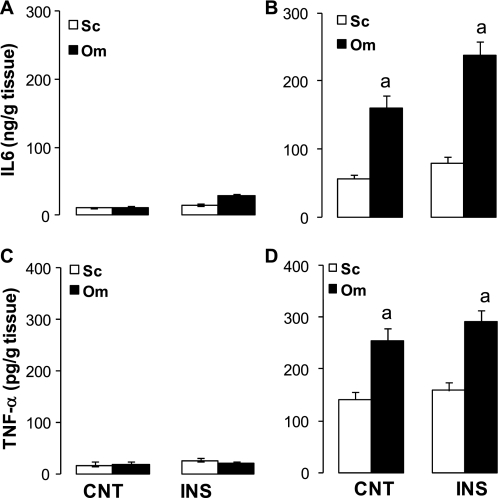
Effects of insulin on IL-6 and TNF-α secretion in fat depots.
The basal secretion rates of IL-6 (Fig. 4A) and TNF-α (Fig. 4C) in adipose tissue of lean subjects were low and were not altered by insulin. In contrast, the basal secretion rates of IL-6 (Fig. 4B) and TNF-α (Fig. 4D) were significantly higher in fat depots of obese subjects. These rates were higher in omental than in subcutaneous explants and were not affected by insulin treatment. There were significant inverse relationships (P < 0.001) between the secretion rates of adiponectin and those of TNF-α (r = 0.62) and IL-6 (r = 0.69) in subcutaneous and omental adipose tissue cultures.
Fig. 4.
Secretion rates of IL-6 (A and B) and TNF-α (C and D) from Sc and Om adipose tissue cultures. Adipose tissue explants of lean (A and C) and obese (B and D) subjects were pretreated (24 h) with insulin or vehicle, and adiponectin secretion was tested for the subsequent 24 h as described in materials and methods. Data are presented as means ± SE; n = 6 for lean subjects and n = 12 for obese subjects. Statistical differences between fat depots of lean and obese subjects for the same treatment (CNT vs. CNT and INS vs. INS) are indicated by letter: aP < 0.001.
Expression of adiponectin, IL-6, and TNF-α genes in adipose tissue depots.
The expression of adiponectin, TNF-α, and IL-6 was also investigated in subcutaneous and omental adipose tissues (Fig. 5). In lean subjects, adiponectin mRNA abundances in subcutaneous (Fig. 5A) and omental (Fig. 5F) tissues were comparable and were significantly higher than those in adipose tissues of obese subjects (P < 0.001). In obese insulin-resistant subjects, adiponectin expression was ∼40% lower in the omental than in subcutaneous tissues (Fig. 5, A and F). In contrast, mRNA abundances of TNF-α were 10- and 14-fold higher in subcutaneous (Fig. 5B) and omental (Fig. 5G) adipose tissue of obese compared with lean subjects. Similarly, IL-6 expression was dramatically increased in subcutaneous and omental adipose tissue of obese subjects (Fig. 5, C and H). There were strong inverse relationships (P < 0.001) between the abundance of mRNA of adiponectin and TNF-α (Fig. 5H) and adiponectin and IL-6 in subcutaneous tissues (Fig. 5L). Similar negative relationships were also observed for omental adipose tissue.
Fig. 5.
Expression of adiponectin (A and F), TNF-α (B and G), IL-6 (C and H), AdipoR1 (D and I) and AdipoR2 (E and J) in Sc and Om adipose tissue of lean (L) and obese (O) subjects: Data for qPCR are calculated as relative expression of genes and represent ΔCT (cycle threshold) normalized to β-actin as described in materials and methods. Data are presented as means ± SE; n = 13 for lean subjects (BMI: 23.8 ± 1.2 kg/m2) and n = 36 for obese subjects (BMI: 45 ± 9 kg/m2). Statistical analysis was performed by Student's t-test. Differences between lean and obese subjects for the same fat depots are indicated by asterisks: ***P < 0.001 and **P < 0.01. Differences between Sc and Om of obese subjects are indicated by letters: aP < 0.01 and bP < 0.05. Relations between expression of adiponectin and TNF-α (log) and IL-6 (log) are presented in K and L, respectively. Pearson correlation coefficients are shown.
Expression of adiponectin receptors in fat depots.
Because adiponectin receptors are crucial in the transmission of intracellular adiponectin signals, we analyzed the expression of adipoR1 and adipoR2 in subcutaneous (Fig. 5, D and E) and omental (Fig. 5, I and J) adipose tissues. The mRNA abundances of both receptors in adipose tissue of obese patients were comparable to those of lean subjects, although there was a modest (not significant) increase (+15–20%) of adipoR2 expression in adipose tissues of obese subjects.
Effects of insulin, IL-6, and TNF-α on adiponectin secretion and expression in human adipocytes.
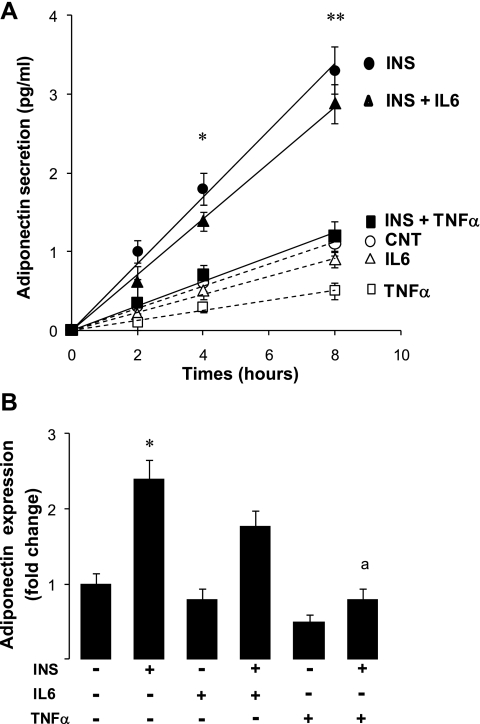
In adipose tissue explants, TNF-α and IL-6 were secreted together, and their independent effects on adiponectin production could not be distinguished. Therefore, we explored these specific effects in human adipocytes differentiated in vitro. The standard procedure used to isolate mature adipocytes induces inflammation (39), and this could interfere with the outcome of the experiment. Therefore, we chose to use the model of human preadipocytes differentiated in vitro in which we controlled the amounts of IL-6 and TNF-α added. Preadipocytes were differentiated in vitro, and the effects of insulin, TNF-α, and IL-6 on adiponectin secretion and expression were tested (Fig. 6). Because of the limitations in these primary cell cultures, the effects of single doses of IL-6 and TNF-α were tested. The choice of these doses was based on our own results with 3T3-L1 cells (see next section below) and previous studies showing the effectiveness of these doses (22). Treatment with insulin increased adiponectin secretion in a time-dependent manner (Fig. 6A). In addition, TNF-α, and not IL-6, inhibited the stimulatory effect of insulin on adiponectin secretion. Adiponectin mRNA abundance, examined at the end of the experiment, was induced by insulin (+150% vs. nontreated cells; Fig. 6B). TNF-α countered the induction by insulin (−170%) compared with insulin-treated cells, whereas the effect of IL-6 was not significant (Fig. 6B). Moreover, treatment with TNF-α alone reduced adiponectin expression by ∼45% (P < 0.05).
Fig. 6.
Adiponectin secretion and expression in human adipocytes. Conditions for isolation and differentiation of human adipocytes are detailed in materials and methods. Adipocytes were pretreated for 24 h with 100 nM insulin or vehicle (A). Aliquots of medium were harvested at indicated times for the measurement of adiponectin secretion. Adiponectin expression was examined at the end of the experiment by qPCR (B). Statistical analysis was performed with Student's t-test. Differences between insulin-treated (INS) and nontreated cells (CNT) are indicated with asterisks: **P < 0.001 and *P < 0.05. Differences between INS + TNF-α treatment and INS treatment are indicated by letter: aP < 0.01.
Effects of insulin, IL-6, and TNF-α on adiponectin expression and secretion in 3T3-L1 cells.
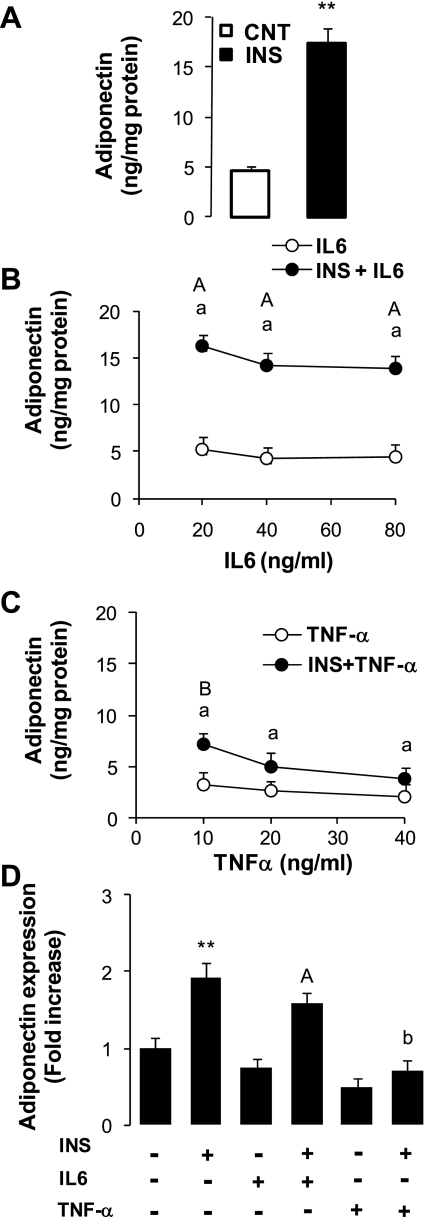
We also questioned the effects of insulin, IL-6, and TNF-α on adiponectin secretion and expression in 3T3-L1 adipocytes. Using this cell line, we were able to have more flexibility and include additional experiments to test different doses of IL-6 and TNF-α. The results (Fig. 7) show that insulin induced total adiponectin secretion ∼260% above basal control rates (Fig. 7A). With or without the presence of insulin, total adiponectin secretion was not significantly changed by varying IL-6 concentrations in the medium (Fig. 7B). However, treatment with TNF-α opposed the stimulating effects of insulin and induced a dose-dependent inhibition of total adiponectin secretion (Fig. 7C) with decreases of 55, 64, and 76% for the three doses of TNF-α (Fig. 7C) compared with the insulin-induced rate (Fig. 7A).
Fig. 7.
Total adiponectin secretion and expression in 3T3-L1 adipocytes. Culture and treatments of adipocytes were performed according to the conditions described in materials and methods. Results are presented as secretion rates per 8 h. A: adiponectin secretion after incubation with insulin or vehicle. B: effects of IL-6 alone or with insulin (INS + IL-6). C: effects of TNF-α alone or with insulin (INS + TNF-α). D: effects of these treatments on adiponectin expression measured by qPCR as indicated in materials and methods. Results are presented as means ± SE. Statistical differences between CNT and INS (A and D) are indicated by asterisk: **P < 0.001. Statistical differences between combined treatments (INS + IL-6 or TNF-α) and insulin treatment (INS) (B and C vs. A) are indicated with lower-case letters: aP < 0.001 and bP < 0.01. Statistical difference between combined treatment (INS + IL-6 or TNF-α) and single treatment with either IL-6 or TNF-α are indicated with upper-case letters: AP < 0.01 and BP < 0.05.
We also tested the effects of insulin, IL-6, and TNF-α on adiponectin expression in 3T3-L1 cells (Fig. 7D). Treatment of cells with insulin increased the abundance of adiponectin mRNA by 92% compared with nontreated cells (Fig. 7D). Furthermore, adiponectin expression was reduced (P < 0.05) by TNF-α alone (−38% vs. nontreated) or in combination with insulin (−82% vs. insulin-treated cells). By contrast, the effect of IL-6 was not significant (Fig. 7D).
Effects of insulin, IL-6, and TNF-α on adiponectin isomers in 3T3-L1 cells.
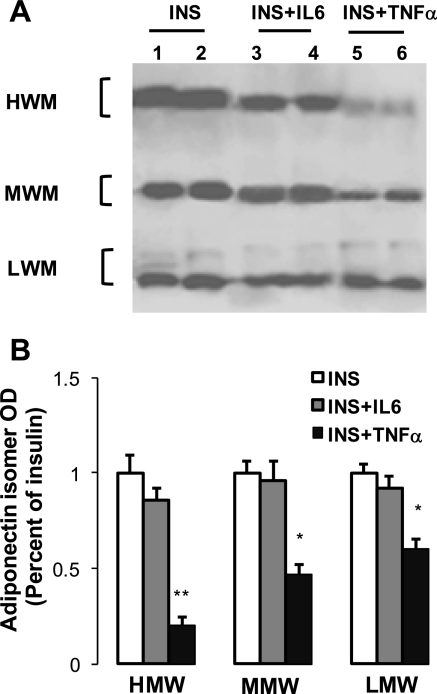
Analysis of adiponectin isomers in culture medium (Fig. 8) showed that the inhibitory action of TNF-α is stronger for the HMW (−75%) than for the MMW (−50%) and LMW (−42%) isomers. A similar profile of isomer distribution was also detected in adipocyte lysate (not shown), suggesting that intracellular HMW isomer formation is greatly altered by TNF-α.
Fig. 8.
Effect of insulin and cytokines on adiponectin isomer composition in 3T3-L1 adipocytes. Conditions of culture and treatments were performed as described in materials and methods. Proteins of culture medium were separated by nondenaturing electrophoresis, and Western blotting was performed using specific anti-adiponectin antibody as described in materials and methods. Representative blot and means of optic density are shown in A and B, respectively. Data are presented as means ± SE; n = 3 experiments, each performed in triplicate. Statistical difference between combined treatment (INS + IL-6 or INS + TNF-α) and insulin treatment (INS) are indicated by asterisk: **P < 0.01 and *P < 0.05.
DISCUSSION
Obesity is considered to be a chronic mild inflammatory condition with adipose tissue playing a major role in this inflammatory process (28). It is now recognized that proinflammatory cytokines exacerbate whole body insulin resistance and alter the normal metabolic and endocrine functions of adipocytes (38). In this study, we investigated the roles of insulin, TNF-α, and IL-6 in regulating the production of the insulin-sensitizing protein adiponectin, utilizing both in vivo (human) and in vitro (explant and adipocyte culture) approaches. Our studies demonstrate some important findings. First, the decline of circulating total adiponectin levels in obese insulin-resistant subjects is associated with disturbances in the relative distribution of adiponectin isomers. Second, insulin upregulates adiponectin production only in lean and not obese subjects. Third, the effects of cytokines on insulin-stimulated adiponectin production are selective, with TNF-α, and not IL-6, opposing insulin's effects and altering adiponectin isomer composition.
In this study, we analyzed the blood levels of adiponectin, TNF-α, and IL-6 in parallel with their secretion and expression in adipose tissue depots from a cohort of lean and obese subjects. We correlated the findings to the degree of insulin resistance as determined by basal insulin and glucose levels and as measured by the hyperinsulinemic-euglycemic clamp. Consistent with previous findings (19), our results demonstrate strong inverse relationships (P < 0.01) between adiponectin levels and BMI as well as with fasting insulin levels in blood and HOMA-IR (not shown). To examine the role of insulin in adiponectin production, we investigated the changes induced by insulin treatment in vivo (clamp) and in vitro (explant cultures) in lean and obese subjects. The results from the hyperinsulinemic-euglycemic clamp show a significant increase of plasma adiponectin noticeable within a relatively short time of insulin infusion. The in vivo findings are supported by in vitro experiments showing that insulin stimulation of adiponectin production was detected only in adipose tissue explants derived from lean subjects. Furthermore, the effects of insulin stimulation of the 3T3-L1 cells and human adipocytes confirm the role of insulin in the induction of adiponectin secretion (Figs. 6 and 7). Taken together, these results show that insulin upregulates adiponectin expression and secretion and suggest that insulin sensitivity is a requirement for adiponectin secretion.
In addition to the decline of the absolute amount of total adiponectin in the plasma of obese subjects, the composition of adiponectin isomers was altered, showing a shift from the HMW to the LMW (Fig. 1). In line with these data, the results of adipose tissue cultures show that the insulin effect in adipose tissue of obese subjects was blunted and selective, with relatively less HMW than LMW isomer secretion. In obese subjects, the changes of adiponectin isomer composition at the level of adipose tissue were reflective of the changes observed in the plasma, suggesting that adipocytes are key regulators of the relative distribution of adiponectin isomers present in plasma. In agreement with our finding, previous investigations had suggested that oligomerization of adiponectin occurs within the adipocyte (23, 30). The subcellular control of adiponectin oligomerization and release from adipocytes is not clearly understood (23). Insulin and glucose have been reported as inducers of total adiponectin secretion in 3T3-L1 cells (32, 36). Moreover, elective inhibition of phosphatidylinositol 3-kinase in adipocytes prevented the stimulatory effect of insulin (32), indicating that insulin signaling is needed for insulin-stimulated adiponectin release, and this could explain the blunted effect of insulin in obese insulin-resistant subjects. The fact that HMW adiponectin production in adipose tissue of obese subjects (Fig. 3, C and D) is the most affected suggests that intracellular oligomerization of adiponectin is also sensitive to insulin action. Recent studies have shown that some intracellular chaperone proteins are critical for the maturation of adiponectin complexes and formation of the HMW isomer. It is possible that disruption of insulin signaling in the adipose tissue of obese subjects alters adiponectin interaction with endoplasmic reticulum proteins and reduces adiponectin oligomerization and release. In agreement with this hypothesis, Frizzell et al. (13) showed that HMW isomer production is reduced in adipose tissue of obese insulin-resistant db/db mice due to the inhibition of the oligomerization mechanism.
The second focus of our studies was to compare the regulatory effects of the inflammatory cytokines TNF-α and IL-6 on adiponectin production. Adiponectin concentrations in plasma and expression levels in adipose tissue (Fig. 5) were inversely correlated with IL-6 and TNF-α. These relationships did not show a clear distinction between the specific regulatory role of IL-6 and TNF-α on adiponectin production. Earlier studies had examined the relationship between adiponectin and TNF-α, and adiponectin and IL-6, and have reported similar negative relationships (20). In addition, we noticed that TNF-α secretion from adipose tissue explants was positively associated with IL-6 secretion (results not shown). These results are in agreement with the known regulatory relationship between TNF-α and IL-6. In fact, TNF-α induces IL-6 production via nuclear factor-κ,B (NF-κB) activation (29) and induction of IL-6 expression is expected when TNF-α is increased. Therefore, in this experimental framework it is not possible to distinguish the specific effects of either TNF-α or IL-6 on adiponectin production; hence, the data generated in vivo or in adipose tissue explants could be the result of a combined effect of TNF-α and IL-6 and possibly other factors. It is also possible that TNF-α released from macrophages residing within adipose tissue could act in a paracrine fashion, stimulating the secretion IL-6 from adipocytes and simultaneously inhibiting the release of adiponectin.
To dissociate between the effects IL-6 and TNF-α, we investigated the production of adiponectin in 3T3-L1 and human adipocytes. Our results show that TNF-α, and not IL-6, significantly blunted insulin's induction of adiponectin secretion and expression in both 3T3-L1 (Fig. 7) and human adipocytes (Fig. 6). Interestingly, TNF-α strongly reduced HMW secretion compared with MMW and LMW isomers (Fig. 8). The mechanisms behind the selective effects of TNF-α are not clear, but previous studies suggested that TNF-α inhibits intracellular oligomerization of HMW isomers through the downregulation of disulfide-bond-A oxidoreductase-like protein (DsbA-L), a protein needed for disulfide bond formation and adiponectin oligomerization (23, 43). It is possible that similar mechanisms occur in adipose tissue of obese patients, since the production of TNF-α is significantly enhanced. TNF-α is an important activator of inflammatory proteins through the NF-κB pathway (1), leading to downregulation of many adipocyte-abundant genes that are critical for insulin responsiveness, particularly those involved in inflammation and the endoplasmic reticulum stress response (17, 38). These proteins are known to interfere with intracellular protein maturation, transport, and secretion (10). In addition, TNF-α and IL-6 differ in their interaction with PPARγ (35). In this regard, TNF-α reduces PPARγ activity while IL-6 increases PPARγ expression (35). Moreover, earlier studies had reported the anti-inflammatory effects of PPARγ (9, 23, 28, 44), which also suppresses NF-κB activation (4) and induces adiponectin expression and secretion (23, 24). It is conceivable that increased levels of TNF-α in adipose tissue of obese subjects activates NF-κB and at the same time downregulates PPARγ-induced adiponectin production.
The differential regulation of adiponectin isomers may be of relevance, given that specific biological functions of the adiponectin oligomers have been reported. Our investigations show that, among adiponectin isomers, the HMW isomer is the most affected by insulin resistance and TNF-α, while the expression of adiponectin receptors is not significantly altered in adipose tissue of obese subjects. Taken together, these findings suggest that a shortage of total adiponectin availability, especially the most active HMW isomer, rather than the expression of the receptors, is responsible for the eventual reduction of the biological activity of adiponectin within adipose tissue. However, we cannot exclude the possibility that the expression of adiponectin receptors in other tissues of obese subjects is affected, since previous reports showed conflicting results with up- or downregulation in liver or muscle tissues (6, 40).
In conclusion, the present work shows that insulin stimulates adiponectin production in insulin-sensitive adipose tissue of lean subjects. However, in adipose tissue of obese subjects, insulin stimulation of adiponectin secretion and isomer composition is significantly altered. Of the inflammatory cytokines, TNF-α strongly blunted the stimulatory effects of insulin while IL-6 did not alter insulin action. Reduced insulin stimulation and increased TNF-α production are among the factors that contribute to the decline of adiponectin production and alteration of isomer composition in obese insulin-resistant subjects.
GRANTS
This work was supported by Development Award AHA0730356N from the American Heart Association (T. Hajri), Development Grant 1047089103 form the Department of Surgery, Vanderbilt University (T. Hajri), and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-070860-01S1 (N. Abumrad).
DISCLOSURES
No conflicts of interest are reported by the authors.
REFERENCES
- 1. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes 56: 2174–2177, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bogan JS, Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol 146: 609–620, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 285: E527–E533, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290: E961–E967, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Carey AL, Bruce CR, Sacchetti M, Anderson MJ, Olsen DB, Saltin B, Hawley JA, Febbraio MA. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness. Diabetologia 47: 1029–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care 26: 2442–2450, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 143: 998–1007, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Das SK, Chu WS, Mondal AK, Sharma NK, Kern PA, Rasouli N, Elbein SC. Effect of pioglitazone treatment on endoplasmic reticulum stress response in human adipose and in palmitate-induced stress in human liver and adipose cell lines. Am J Physiol Endocrinol Metab 295: E393–E400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139: 448–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Chambaut-Guerin AM, Klein J, Paschke R. Interleukin-6 is a positive regulator of tumor necrosis factor alpha-induced adipose-related protein in 3T3-L1 adipocytes. FEBS Lett 560: 153–157, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Frizzell N, Rajesh M, Jepson MJ, Nagai R, Carson JA, Thorpe SR, Baynes JW. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J Biol Chem 284: 25772–25781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88: 4823–4831, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109: 1381–1389, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 58: 1550–1557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes 52: 1779–1785, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745–E751, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lee ET, Howard BV, Go O, Savage PJ, Fabsitz RR, Robbins DC, Welty TK. Prevalence of undiagnosed diabetes in three American Indian populations. A comparison of the 1997 American Diabetes Association diagnostic criteria and the 1985 World Health Organization diagnostic criteria: the Strong Heart Study. Diabetes Care 23: 181–186, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Lihn AS, Richelsen B, Pedersen SB, Haugaard SB, Rathje GS, Madsbad S, Andersen O. Increased expression of TNF-α, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab 285: E1072–E1080, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 425: 41–52, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, Xu A, Marette A, Sweeney G. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab 297: E657–E664, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr 79: 606–612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murdolo G, Hammarstedt A, Schmelz M, Jansson PA, Smith U. Acute hyperinsulinemia differentially regulates interstitial and circulating adiponectin oligomeric pattern in lean and insulin-resistant, obese individuals. J Clin Endocrinol Metab 94: 4508–4516, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246 [DOI] [PubMed] [Google Scholar]
- 29. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853–6866, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Pereira RI, Draznin B. Inhibition of the phosphatidylinositol 3′-kinase signaling pathway leads to decreased insulin-stimulated adiponectin secretion from 3T3-L1 adipocytes. Metabolism 54: 1636–1643, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Phillips SA, Ciaraldi TP, Oh DK, Savu MK, Henry RR. Adiponectin secretion and response to pioglitazone is depot dependent in cultured human adipose tissue. Am J Physiol Endocrinol Metab 295: E842–E850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab 89: 447–452, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Pitulis N, Papageorgiou E, Tenta R, Lembessis P, Koutsilieris M. IL-6 and PPARgamma signalling in human PC-3 prostate cancer cells. Anticancer Res 29: 2331–2337, 2009 [PubMed] [Google Scholar]
- 36. Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27: 4698–4707, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 29: 449–457, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 51: 1319–1336, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J Biol Chem 278: 47585–47593, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Saito Y, Fujioka D, Kawabata K, Kobayashi T, Yano T, Nakamura T, Kodama Y, Takano H, Kitta Y, Obata JE, Kugiyama K. Statin reverses reduction of adiponectin receptor expression in infarcted heart and in TNF-α-treated cardiomyocytes in association with improved glucose uptake. Am J Physiol Heart Circ Physiol 293: H3490–H3497, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Tao H, Aakula S, Abumrad NN, Hajri T. Peroxisome proliferator-activated receptor-γ regulates the expression and function of very-low-density lipoprotein receptor. Am J Physiol Endocrinol Metab 298: E68–E79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin J Biol Chem 278: 40352–40363, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Wang ZV, Scherer PE. DsbA-L is a versatile player in adiponectin secretion. Proc Natl Acad Sci USA 105: 18077–18078, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White PJ, Marette A. Is omega-3 key to unlocking inflammation in obesity? Diabetologia 49: 1999–2001, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52: 1355–1363, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55: 2562–2570, 2006 [DOI] [PubMed] [Google Scholar]