Abstract
Amyloid beta (Aβ) plays a critical role in the pathophysiology of Alzheimer’s disease. Increasing evidence indicates mitochondria as an important target of Aβ toxicity; however, the effects of Aβ toxicity on mitochondria have not yet been fully elucidated. Recent biochemical studies in vivo and in vitro implicate mitochondrial permeability transition pore (mPTP) formation involvement in Aβ mediated mitochondrial dysfunction. mPTP formation results in severe mitochondrial dysfunction such as reactive oxygen species (ROS) generation, mitochondrial membrane potential dissipation, intracellular calcium perturbation, decrease in mitochondrial respiration, release of pro-apoptotic factors and eventually cell death. Cyclophilin D (CypD) is one of the more well-known mPTP components and recent findings reveal that Aβ has significant impact on CypD-mediated mPTP formation. In this review, the role of Aβ in the formation of mPTP and the potential of mPTP inhibition as a therapeutic strategy in AD treatment are examined.
Keywords: Amyloid beta, Alzheimer’s disease, mitochondria, mitochondrial permeability transition pore, cyclophilin D, cyclosporine A
Introduction
Alzheimer disease (AD), a commonly occurring progressive neurodegenerative disorder in aged people results in severe cognitive deficits [1, 2] with typical pathological changes such as amyloid beta (Aβ) deposition in brain parenchyma and vessels[3–6], neurofibrillary tangles formation[7–10], and neuronal loss. Even though a small percentage of the patients carry AD associated genetic mutants, approximately 98% of AD onset occurs in a sporadic manner and the mechanism of AD pathogenesis remains largely unknown. Among the reported possible pathogenic factors for AD, extracellular as well as intracellular Aβ [11, 12] is considered to play a central role. It remains unclear however, which type of Aβ species plays a major role and whether Aβ is the initiating factor in AD. Aβ is reported to impact a diverse array of cellular properties, including disruption of cell membrane integrity, and disturbance of organelle function and intracellular homeostasis. Despite its various impact on neurons, increasing evidence is focused on Aβ-mediated mitochondrial dysfunction in AD. Known Aβ related mitochondrial dysfunctions including mitochondrial DNA mutation [13–15], decreased glucose metabolism [16, 17], decreased mitochondrial respiratory chain activity [18, 19], deactivation of certain key enzymes [16, 18–22], increased reactive oxygen species (ROS) generation [18, 19, 23–30] and perturbed calcium homeostasis [18, 22, 31–33] have been widely observed in AD patients, AD animal models and in Aβ-treated cell cultures. Recent studies have demonstrated that Aβ induced mitochondrial dynamic changes, including decrease in mitochondrial movement and mitochondrial fission/fusion perturbations [34, 35]. These observations significantly increase our understanding of mitochondrial perturbation relevant to the pathogenesis of AD. Furthermore, progressive accumulation of mitochondrial Aβ in the AD brain and in the AD mouse model implicates the role of mitochondrial Aβ in mitochondrial malfunction [16, 18, 27, 36]. Although the precise role of Aβ in mitochondria is not yet defined, reports clearly show that interaction of mitochondrial Aβ with mitochondrial proteins, amyloid-beta (Aβ) binding alcoholdehydrogenase (ABAD), and cyclophilin D exacerbates mitochondrial and neuronal stress in transgenic AD mouse models [16, 18, 22, 27].
Notably, the involvement of mitochondrial permeability transition pore (mPTP) is implicated in Aβ-induced mitochondrial dysfunction, such as perturbation of intracellular calcium regulation, ROS generation, release of pro-apoptotic factors and changes in mitochondrial morphology. This assumption is deduced from the facts that calcium and ROS are strong inducers of the mPTP formation and that the consequence of mPTP formation are increased ROS generation, decreased ATP production and apoptogenic substance release accompanied by mitochondrial swelling [37–40]. For instance, the absence of cyclophilin D, a key component of mPTP, protects against Aβ-mediated mitochondrial, neuronal and synaptic dysfunction [18, 22]. These studies added significant dimension to our knowledge about the mechanism of Aβ toxicity as well as AD pathogenesis. This review contains a discussion of the role of Aβ-induced mPTP formation in the induction of mitochondrial dysfunction and its relevance to the pathogenesis of AD.
1. Mitochondria and mitochondrial permeability transition pore (mPTP)
Mitochondria are pivotal organelles in all eukaryotic cells and play a vital role in cell survival by providing energy, maintaining intracellular calcium homeostasis and regulating cellular redox status. Another important role of mitochondria in cell survival is as an initiating organelle for apoptosis due to its ability to release apoptogenic factors in response to mitochondrial stress. The basic structure of a mitochondrion consists of the outer mitochondrial membrane (OMM), the inner mitochondrial membrane (IMM) [41] and the matrix. OMM is quite permeable to ions, solutes and even some small proteins, while IMM is impermeable to most ions and solutes. The impermeable property of IMM is important in maintaining homeostasis of the inner mitochondrial environment as well as its morphology, while blocking the free exchange of substances between matrix and cytosol. The exchange (especially the import) of mitochondria with their outer environment is strictly controlled by the channels and transporters in the IMM. Any disturbance in this control can lead to mitochondrial damage. For example, cytosolic calcium cannot freely move into mitochondria in an ion gradient driven manner but does so via the energy driven uniporter [42, 43]. On the other hand, the extrusion of calcium from mitochondria under normal conditions is mainly achieved via antiporter driven by ion gradient [44]. In the circumstances of mitochondrial calcium or phosphoate overloading, and intracellular oxidative stress, mitochondria lose their calcium handling ability and intramitochondrial calcium quickly effluxes via a nonselective pathway, named mitochondrial permeability transition pore (mPTP) [45–48].
The molecular basis of mPTP
Although the presence of mPTP formation has long been proposed and widely proven, until recent years the molecular basis of mPTP was not clearly delineated. The structure of the pore is believed to composed of three compartments: voltage-dependent anion channel (VDAC) in the OMM [49], adenine nucleotide translocator (ANT) in the IMM [50] and cyclophilin D (Cyp D) in the mitochondrial matrix[40]. According to the prevailing point of view, the formation of the pore is initiated by the CypD translocation from the mitochondrial matrix to the IMM to bind with ANT. CypD binding to ANT results in the formation of an ANT channel in IMM. The ANT formed channel together with the channel formed by VDAC in OMM constitutes a tunnel-like structure crossing the mitochondrial membranes, thus connecting mitochondrial matrix with the cytosol [49, 51]. Studies in animal models have shown that the mPTP formation can be efficiently blocked by the addition of a cyclophilin D inhibitor, cyclosporine A (CSA) or by ablation of Cyp D [40, 51, 52].
Despite this considerable evidence supporting the molecular basis of mPTP, the roles of VDAC and ANT in pore formation have been challenged in recent studies. In their investigation of VDAC knockout mice, Krauskopf et al did not find decreased mPTP formation in the presence of calcium [53]. Baines et al observed an indispensable role of VDAC [54] in the formation of mPTP. Their data suggesting that channel in OMM formed by VDAC allows nonspecific transport of substances below a certain size. Another study performed by Kokoszka et al on ANT gene ablation mice suggests that ANT deficiency does not contribute significantly to prevention of mPTP formation [55]. Considering the impermeability of IMM, it is important to clarify the mPTP compartment in IMM. A recent study performed by Leung et al proposes a candidate molecule in IMM, called phosphate carrier (PiC) [56]. This group found that PiC can form a pore either by itself or in association with ANT, suggesting that PiC but not ANT is the necessary component of mPTP. Intriguingly, CypD binding is also a sufficient, but not necessary initiating step for PiC associated pore opening. In fact, high concentrations of calcium alone can trigger PiC. This data may provide an explanation for the failure of VDAC or ANT ablation to prevent mPTP. In addition, these results implicate the presence of independent non-CypD mPTP formation which can be triggered by high concentrations of calcium without the facilitation of CypD. Whereas there are disagreements about whether VDAC and ANT are necessary, it is generally accepted that CypD is a necessary compartment for mPTP formation, especially in the most commonly occurring scenarios when calcium levels are not high enough to trigger the CypD independent mPTP formation.
Consequences of mPTP formation
Once formed, mPTP constitutes a non-selective, high conductance pore allowing transport of not only calcium but any solute below the pore size. This results in mitochondrial osmotic swelling and dissipation of mitochondrial membrane potential as well as damage to the mitochondrial respiratory chain thereby reducing mitochondrial oxidative phosphorylation process that then results in decreased ATP production and increased ROS generation. Mitochondrial swelling leads to ruptures in the OMM, which in turn allows the release of apoptogenic factors from the mitochondria into the cytosol. It can therefore be concluded that massive formation of mPTP under pathological conditions causes severe mitochondrial injury and cell death. Several agents such as CSA [18, 57], Sanglifehrin A (SfA) [58], and ADP [50] are reported to have the potential to inhibit mPTP formation and ameliorate the consequences of mPTP formation.
Based on the findings in the preceding paragraphs, one may conclude that CypD is the most important initiating molecule for mPTP, and that mPTP formation results in severe mitochondrial dysfunction, irreversible cell damage and finally cell death.
2. Amyloid beta (Aβ)and mPTP
Aβ is the product of amyloid precursor protein [59] cleaved by β and γ secretase. Massive deposition of Aβ is a hallmark change in AD brain; extracellular and intracellular accumulation of Aβ as well as its aggregated forms renders severe damages to neurons. Results of studies regarding Aβ neurotoxicity show that Aβ affects cellular damages primarily through inducing free radicals and calcium dysregulation, both of which have been widely reported in the literature in both in vitro and in vivo studies. The synergistic impacts of increased ROS and calcium perturbation lead to fatal cell damage. Aβ generates oxidizing products during its aggregation [28]. These oxidizing products as well as Aβ itself affect the functions of sodium-potassium ATPase and calcium ATPase [60, 61], which in turn causes dysregulation of L type voltage sensitive calcium channel (LVSCC) [62, 63], α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) [64], N-methyl D-aspartate receptor (NMDAR) [65], and inositol 1,4,5-trisphosphate receptors [66, 67] that then mediates significantly increased calcium flux into the cytosol. This increased intracellular calcium causes mitochondrial calcium overloading resulting in an elevation of mitochondrial ROS production. Both exogenous ROS induced by Aβ and mitochondrial originated ROS act upon mitochondria mediating further mitochondrial calcium perturbation and ROS generation.
Several lines of investigation have indicated that free radicals and calcium are potent inducers of mPTP formation. Based on the significant Aβ induced ROS formation and calcium perturbations as noted above, the conditions for mPTP formation are ideal in an Aβ rich environment; it is therefore logical to hypothesize that massive mPTP formation occurs in Aβ challenged mitochondria. Indeed, several studies [18, 19, 29, 41, 68–70] suggest the involvement of mPTP formation in Aβ-induced neuronal and mitochondrial stress as evidenced by disrupted mitochondrial membrane potential, increased production of ROS generation and mitochondrial swelling, and increased cytochrome C release. Aβ-mediated abnormalities in mitochondrial function are rescued by the addition of the mPTP inhibitor, CsA, or by genetic blockade of CypD, which confirms the role that mPTP in this pathological process and stimulates interest in elucidating the exact mechanism of Aβ stimulated mPTP formation.
As discussed above, the role of Aβ in mediating ROS generation, calcium dysregulation and the resultant mitochondrial respiration failure establishes mitochondria as the ‘victim’ in mPTP formation. Studies by Parks et al adopting in vivo investigation indicate that Aβ induces free radicals through mechanisms involving the NMDA receptor and nitric oxide synthase [41]. Our own studies found increased mitochondrial ROS, decreased cytochrome c oxidase activity and declined mitochondrial respiration control ratio starting at the age of 6 months old in an AD-type animal model as compared with their wild type littermates (controls) [20]. Notably, mitochondrial function is largely recovered in CypD-deficient AD-type mice overexpressing human mutant APP/Aβ. Neurons lacking CypD or CSA-treated neurons are quite resistant to Aβ insults, showing fewer apoptotic cells, improved mitochondrial membrane potential and decreased cytochrome c release in comparison with cultured neurons from nontransgenic littermate controls [18, 22]. Taken together, these studies offer strong evidence that ROS and calcium dysregulation induced by Aβ is involved in mPTP formation.
3. Cyclophilin D involves in Aβ-mediated mPTP perturbation
Expression of CypD in AD brain and from mAPP mice
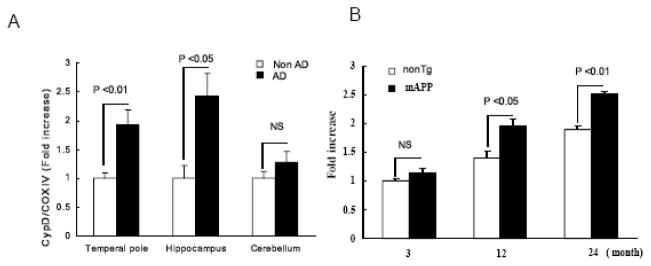
In view of the known key role of CypD in mPTP function, investigation was undertaken to assess whether alternations in CypD expression are associated with AD pathology. Findings revealed that expression of CypD was significantly elevated in Aβ-containing cortical mitochondria from AD-affected regions (temporal pole and hippocampus) as compared to non-AD cortical mitochondria of age-matched non-AD patients. There was not a significant difference in CypD expression in cerebellum between human AD and non-AD brains (Fig. 1A). Further, increased level of CypD in AD correlate to the presence of mitochondrial Aβ in AD brains [18]. Consistent with the observations of human AD brain, transgenic (Tg) mice expressing a mutant form of human amyloid precursor protein (mAPP mice) displayed age-dependent elevated level of CypD in the cerebral cortex (including the hippocampus) as compared with non transgenic (nonTg) littermate controls (Fig. 1B) [18, 22]. These results suggest that alternations in CypD expression may impact the pathogenesis of AD in addition to its known role in age-related cellular perturbation.
Figure 1.
Cyclophilin D expression levels. Mitochondria were prepared from indicated brain regions of human AD/Non- AD (n = 9 – 12 per group) (Panel A) or from cortices of the indicated mice at different ages (n = 6 –10 per group) (Panel B). Isolated mitochondria were then subjected to SDS PAGE followed by the addition of anti cyclophilin D Ig G. The cyclophilin D expression level was normalized with cytochrome C oxidase. * P < 0.05. Figures are cited from [18].
Given the fact that Cyp D is the critical molecule in mPTP formation and CypD ablation prevents mPTP formation, it has been proposed that increased CypD favors the opening of mPTP[18, 22, 71, 72]. Recent studies indicate that high level of CypD in neuronal mitochondria increase vulnerability to mPTP and require higher level of CSA to inhibit mPTP opening [73]. In contrast, neuronal cells with decreasing level of CypD become less sensitive to mPTP induction. Previous studies from our laboratory demonstrate that mAPP cortical mitochondria have a higher level of CypD as well as increased CypD translocation to the IMM, calcium-induced mitochondrial swelling, and decreased calcium buffering capacity. These deleterious effects on mitochondrial properties are largely blocked by CypD deletion and the addition of CSA [18, 22]. This indicates that increased CypD in mAPP mice leads to decreased mPTP formation threshold, although these data should be interpreted with reservation since existing mitochondrial dysfunction in mAPP mice may be a factor in mitochondrial response to calcium. Furthermore, neuronal cells stably overexpressing CypD demonstrate exacerbated mitochondrial malfunction caused by oxidative stress and Aβ insults (unpublished observations, Du. H and Yan, SD) compared to mock-transfected cells. These data indicate that modulation of CypD expression may lead to the functional changes in mPTP under pathological conditions. Aβ- or oxidative stress-induced increases in CypD expression may therefore contribute to impaired mPTP function/formation in an environment enriched for amyloid pathology, such as AD.
Aβ interacts with CypD
Results of in vitro binding assays [using surface Plasmon resonance (SPR) and recombinant human CypD protein] undertaken to determine if Aβ binds to CypD, revealed that oligomeric Aβ40 and Aβ42, has higher affinity for binding to CypD than does monomeric Aβ. It is now known that interactions between Aβ and CypD are specific because sequence reversed Aβ peptide showed no binding with CypD and antibodies against either Aβ or CypD inhibited the binding [18, 22]. To determine if CypD and Aβ interact in pathologically relevant settings, we performed immunoprecipitation-immunoblotting studies. The data suggested the Aβ-CypD complex in AD brain tissues and mAPP mice brains; whereas, as expected in age-matched non-AD brains with undetectable or low levels of cerebral Aβ, there was virtually no detectable or very low level of CypD-Aβ complex. Formation of Aβ-CypD complex is associated with mitochondrial Aβ level in the AD brain [18].
Colocalization of CypD and Aβ in mitochondria has been confirmed by confocal and electron microscopy by showing that α-Aβ and α-CypD are extensively colocalized in the cerebral cortex of AD patients or mAPP mice [18]. These results provide further evidence that the CypD-Aβ interaction occurs within mitochondria in vivo.
Blockade of CypD protects against Aβ-induced mitochondrial and neuronal toxicity
CypD plays a key role in stabilizing mitochondrial permeability transition (mPT) and serves to open the mitochondrial permeability transition pore (mPTP), thereby allowing the diffusion of contents such as calcium and cytochrome C out of mitochondria matrix to the cytoplasm where they may induce cell death via necrosis and apoptosis. It was therefore important to examine the effect of CypD deletion on mitochondrial properties in an Aβ-rich environment, including the capacity for Ca2+ uptake, mPT, membrane potential and ROS generation.
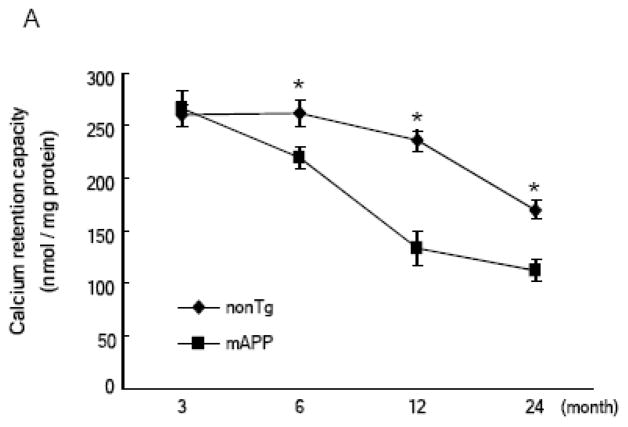
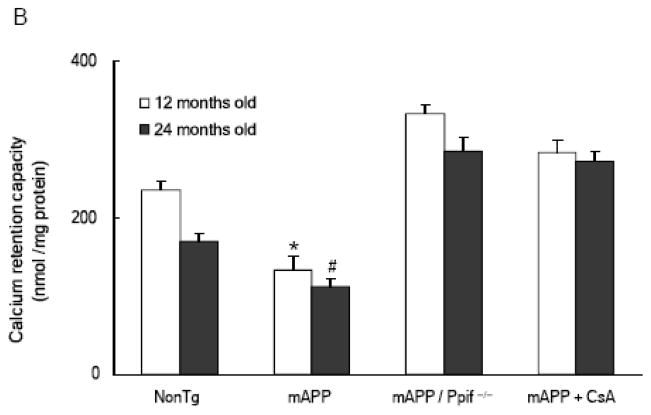
Assessment of Ca2+ uptake capacity of cortical mitochondria by measuring the decrease in extra-mitochondrial free Ca2+ from the medium after the addition of CaCl2 pulses revealed that changes in calcium uptake capacity occurred in an age-dependent manner in both nonTg and mAPP mice. When compared to mice aged 3 to 6 months, nonTg mice showed a trend towards reduction of calcium uptake capacity by up to 22–24 months old; of note, however, mAPP mice showed a decreased brain mitochondrial calcium uptake capacity as compared to nonTg mice (Fig. 2A). At 3 months of age, both nonTg and mAPP mice possess adequate calcium buffering capacity. In contrast, impaired capacity for Ca2+ uptake begins at 6 months of age and progressively decreased in 12 and 22–24 months old mAPP mice with reductions of ~18%, ~50%, and ~58% respectively, as compared to the 3 months old mAPP mice (Fig. 2A). Importantly, cortical mitochondria from CypD-deficient mAPP mice (mAPP/Ppif−/−) demonstrate relatively more Ca2+ uptake at the mice ages of 6, 12, and 22–24 months, respectively, when compared to mAPP mitochondria. Similarly, mAPP cortical mitochondria treated with CSA demonstrate a protection on mitochondrial Ca2+ uptake capacity (Fig. 2B).
Figure 2.
Age dependent mitochondrial calcium retention capacity (CRC) change: (Panel A) Brain mitochondrial CRC was compared between nonTg and mAPP mice at 3, 6, 12 and 24 months of age (n = 4–6). Substantially decreased CRC of mAPP mitochondria in comparison to other genotypes was detected after the age of 6 months. * P < 0.05. (Panel B) CRC was compared between brain mitochondria from the indicated mice at the ages of 12 and 24 months respectively (n = 4– 6 for each group). mAPP mitochondria were treated with cyclosporine A (1 μM). *, #: P < 0.05 vs other genotypes of mice. Figures are cited and conflated from [18, 22].
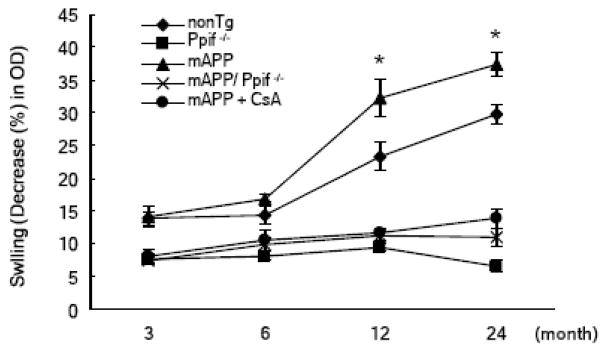
To determine the effect of mPTP on calcium handling ability of Aβ-rich mitochondria from mAPP mice, the next logical step includes measurement of mitochondrial swelling in response to Ca2+. Cortical mitochondria from transgenic and nonTg mice showed swelling in response to Ca2+, whereas mAPP mitochondria displayed increased swelling compared to nonTg mitochondria at the mice ages of 12 and 22–24 months although both cortical mitochondria of nonTg and mAPP mice exhibited an age-dependent increased swelling in response to Ca2+ (Fig. 3) [18, 22]. Importantly, CypD-deficient mAPP mitochondria (mAPP/Ppif−/−) were more resistant to swelling and permeability transition induced by Ca2+ than mAPP mitochondria (Fig. 3) [18, 22]. The addition of CSA to the mAPP mitochondria also attenuated swelling in response to Ca2+ (Fig 3) [18, 22]. The inhibitory effect of CSA on Ca2+-induced swelling was comparable to those of the mAPP/Ppif−/− mice. Taken together, these results indicate that Aβ-rich cortical mitochondria are more susceptible to Ca2+-induced mPTP, and that blockade of CypD protects against Aβ-mediated dysfunction of mPTP, suggesting a role for Aβ-CypD interaction in mPTP.
Figure 3.
Age dependent mitochondrial swelling change in response to Calcium. Brain mitochondria isolated from the indicated mice at 3, 6 12 and 24 months old (n = 4–10 for each group) were subjected to measured swelling induced by calcium (500 nmol/mg mitochondrial fraction). Decreased amplitude (percentage of initial OD at 540 nm) at the end of the test was compared among groups. 1 μM cyclosporine A was used in the treatment of mAPP mitochondria. * P < 0.05. Figures are cited and conflated from [18, 22].
To fully evaluate mitochondrial function, we measured the inner mitochondrial membrane potential (ΔΨm) in brain slices in situ. Brain slices from Tg mice were loaded with tetramethylrhodamine methyl ester (TMRM) to assess inner mitochondrial ΔΨm. The intensity of TMRM staining was significantly decreased in the cerebral cortices and hippocampi from 12 months old mAPP mice compared to nonTg mice. In contrast, mitochondria from CypD-deficient mAPP mice were largely resistant to the loss of ΔΨm demonstrating a higher TMRM staining intensity compared to mitochondria from single mAPP mice [18]. Thus, the ΔΨm of mitochondria lacking CypD is protected from Aβ-mediated swelling and opening of the membrane permeability transition pore.
As mitochondria are the principal sites of generation of ROS, and Aβ is known to trigger oxidative stress, we tested whether CypD-Aβ interaction correlates with ROS generation in mitochondria. To evaluate mitochondrial ROS generation, mice were injected intravenously with MitoSox Red, a novel fluorogenic dye for highly selective detection of superoxide production in the mitochondria of live cells. The percentage of area occupied by MitoSox Red staining was increased 2–3 fold in the cerebral cortices and hippocampi (CA1 to CA3) of mAPP mice compared to other groups (nonTg, Ppif−/−, and mAPP/Ppif−/− mice) [18]. These data indicate that the absence of CypD protects from Aβ-mediated mitochondrial ROS generation.
Assessments of mitochondrial function by examining oxygen consumption, the activity of cytochrome c oxidase, a key enzyme in the respiratory chain, and level of ATP in the brain of various Tg mice were then undertaken. The mitochondrial respiratory rate, as assessed by the respiratory control ratio (ratio of oxygen consumption in state III/state IV), was significantly reduced in brain mitochondria of mAPP mice as compared to their nonTg littermates, whereas CypD-deficient mAPP mice showed completely or largely rescued reduction of oxygen consumption at the ages of 6 to 24 months [18, 22]. Similar as previously reported [16, 20] that mAPP mice had depressed respiratory function deteriorating with age, we found that mAPP mice showed decreased cytochrome c oxidase activity and impaired energy metabolism as shown by a reduction in ATP levels compared to nonTg controls at 12 to 24 months of age; while the cytochrome c oxidase activity and ATP level in nonTg mice were comparable to those found in CypD-deficient mice, suggesting that deletion of CypD does not interfere with mitochondrial function under physiologic conditions. Importantly, double mAPP/Ppif−/− mice showed significantly increased mitochondrial enzyme activity (up to 40–50%) and abrogated reduction of ATP as compared to mAPP mice [18, 22], indicating that in an Aβ-rich environment, blockade of CypD attenuates or protects against Aβ-mediated mitochondrial dysfunction.
Blockade of CypD improves synaptic function and learning memory in an AD mouse model
Alterations in mitochondrial properties and perturbation of energy metabolism and free radical generation in mAPP mice may correlate with synaptic and neuronal dysfunction. There are strong protective effects of CypD deficiency on Aβ-mediated mitochondrial and neuronal toxicity. It was therefore important to determine whether CypD deficiency improves synaptic function, learning and memory. In the radial arm water maze test that detects hippocampus-dependent learning and memory deficits, CypD-deficient and nonTg mice showed strong learning and memory capacity at the ages of 6, 12 and 24 months [18, 22], suggesting there are no deleterious effects of CypD deficiency on normal physiological function. In contrast, mAPP mice displayed impaired spatial learning memory for platform location between trials (average of about 5–6 errors by trials 4 and retention test). Spatial learning memory was significantly improved in double mAPP/Ppif−/− mice (~2–3 errors) compared to mAPP mice (~ 5–6 errors by trials 4 or 5) [18, 22]. The four groups of transgenic mice showed no difference in swimming speed or in the time required to reach the platform in the visible platform test (not shown). These results indicate that improvement in learning and memory is a consequence of CypD deficiency in mAPP mice.
Cognitive abnormalities in AD are thought to be linked to synaptic dysfunction [74]. As mAPP/Ppif−/− mice showed improvement in learning and memory, studies were then undertaken to determine whether these mice also showed improvement of long term potentiation (LTP), a form of synaptic plasticity that is widely studied as a cellular model for learning and memory. Slices from 12–13 months old mAPP mice showed a reduction in LTP compared to slices from non Tg littermates [18], while slices from mAPP/Ppif−/− mice displayed improved LTP, thereby indicating that depletion of CypD may protect against the Aβ synaptotoxicity.
4. Targeting cypD inhibition is a potential approach to prevent and treat AD
Increasing evidence indicates that Aβ-mediated mitochondrial dysfunction plays a critical role in the pathogenesis of AD. Aβ has global effects on mitochondrial function, including decreased mitochondrial respiratory function, diminished mitochondrial key enzymes activities, induced mtDNA mutants, and ROS generation, causing calcium perturbation, triggering mPTP formation and eventually neuronal damage. Therefore, defeating Aβ at mitochondrial site is a practical preventive as well as therapeutic strategy for Alzheimer’s disease. However, it should be mentioned that current knowledge about Aβ mediated mitochondrial dysfunction still quite limits the ability to elucidate an effective and efficient strategy to fully prevent Aβ toxicity on mitochondria. Many approaches used to date have been developed to prevent mitochondria from Aβ-induced damage, including eliminating ROS [59, 75], enhancing Aβ clearance [76, 77] and stabilizing calcium homeostasis [78, 79]. Compared to those strategies that mainly focus on keeping mitochondria from insults by eliminating damage causative factors, studies about blocking massive mPTP formation are at an advantage as they focus on increasing mitochondrial resistance to existing harmful insults. New insights into mitochondrial permeability transition pore and Aβ enables us delving into AD pathogenesis to find an efficient ways to protect mitochondria. CypD inhibition is a feasible and possibly significant therapeutic approach. Cyclosporine A, an inhibitor of CypD broadly used in a clinical application, might be a potential therapeutic option for the treatment of AD. However, in terms of the potential toxic effects of CSA, such as the immunosuppressive effect [80], the usage of CSA in the treatment of AD would be likely limited. Searching for a novel agent that selectively targets CypD, mPTP, or a mimetic of CSA without toxic effects is still a rational approach to AD therapy.
5. Conclusions
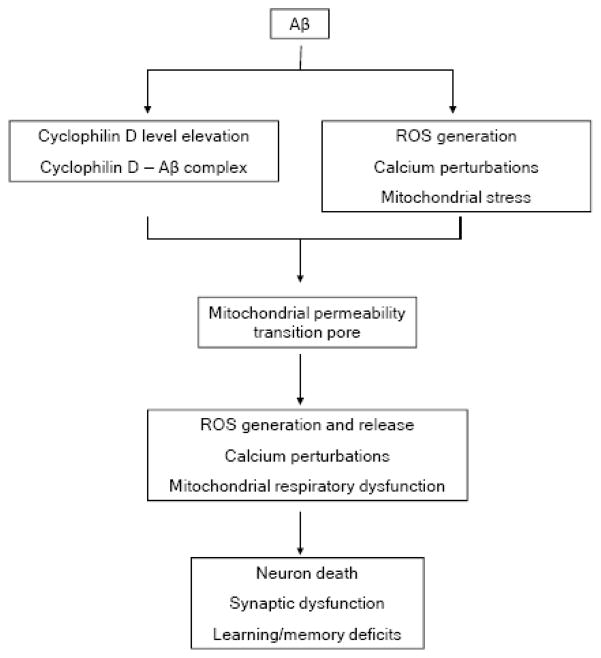
Aβ mitochondrial toxicity has been intensively studied, whereas the precise mechanism(s) is largely unknown. The development of research on the involvement of Aβ in mPTP formation enhances our knowledge regarding Aβ associated mitochondrial toxicity and adds to our knowledge base aforementioned mechanism(s) of Alzheimer’s disease. Aβ-CypD interaction promotes the formation of mPTP, leading to mitochondrial and neuronal stresses. The absence of CypD protects against Aβ-induced mPTP formation and the resultant mitochondrial/cellular stresses, as well as impaired learning memory (Figure 4). Thus, the blockade of CypD or mPTP formation may be a candidate approach for prevention and treatment of AD.
Figure 4.
Schematic figure: Aβ increases cyclophilin D expression level and interacts with cyclophilin D. Further, Aβ is involved in intracellular calcium and ROS perturbations and mitochondrial respiration dysfunction. By decreasing the threshold of mPTP due to these effects, Aβ facilitates the formation of mPTP resulting in mitochondrial perturbations including increased ROS generation, exacerbated perturbation of calcium metabolism, and mitochondrial membrane potential collapse. These severe mitochondrial perturbations lead to multiple cellular stresses, neuron death and finally deficits in learning/memory ability.
Acknowledgments
This work was supported by the USPHS (PO1 AG17490) and Alzheimer Association.
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- mPTP
mitochondrial permeability transition pore
- cyp D
cyclophilin D
- VDAC
voltage dependent anion channel
- ANT
adenine nucleotide translocator
- CSA
cyclosporine A
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Katzman R. Senile dementia of the Alzheimer type. Ann Neurol. 1983;14:497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Aging, amyloid, and Alzheimer’s disease. N Engl J Med. 1989;320:1484–1487. doi: 10.1056/NEJM198906013202209. [DOI] [PubMed] [Google Scholar]
- 5.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Spillantini MG. Molecular neuropathology of Alzheimer’s disease: in situ hybridization studies. Cell Mol Neurobiol. 1990;10:159–174. doi: 10.1007/BF00733642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Alzheimer’s disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets CNS Neurol Disord. 2005;4:383–403. doi: 10.2174/1568007054546117. [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, Drew KL, Nunomura A, Takeda A, Hirai K, Zhu X, Atwood CS, Raina AK, Rottkamp CA, Sayre LM, Friedland RP, Perry G. Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochem Int. 2002;40:527–531. doi: 10.1016/s0197-0186(01)00123-1. [DOI] [PubMed] [Google Scholar]
- 9.Thangavel R, Sahu SK, Hoesen GW, Zaheer A. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in alzheimer’s disease. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball MJ. Neurofibrillary tangles in the dementia of “normal pressure” hydrocephalus. Can J Neurol Sci. 1976;3:227–235. doi: 10.1017/s0317167100025348. [DOI] [PubMed] [Google Scholar]
- 11.Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ, Lemere CA. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- 12.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Serrano J, Bentura ML, Martinez-Murillo R, Martinez A, Rodrigo J. Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer’s disease. Histol Histopathol. 2004;19:823–844. doi: 10.14670/HH-19.823. [DOI] [PubMed] [Google Scholar]
- 13.Trimmer PA, Keeney PM, Borland MK, Simon FA, Almeida J, Swerdlow RH, Parks JP, Parker WD, Jr, Bennett JP., Jr Mitochondrial abnormalities in cybrid cell models of sporadic Alzheimer’s disease worsen with passage in culture. Neurobiol Dis. 2004;15:29–39. doi: 10.1016/j.nbd.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, Davis RE, Parker WD., Jr Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 15.Carrieri G, Bonafe M, De Luca M, Rose G, Varcasia O, Bruni A, Maletta R, Nacmias B, Sorbi S, Corsonello F, Feraco E, Andreev KF, Yashin AI, Franceschi C, De Benedictis G. Mitochondrial DNA haplogroups and APOE4 allele are non-independent variables in sporadic Alzheimer’s disease. Hum Genet. 2001;108:194–198. doi: 10.1007/s004390100463. [DOI] [PubMed] [Google Scholar]
- 16.Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. Faseb J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 17.Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 18.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Wei Xu H, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. Faseb J. 2005 doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 21.Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 22.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001;15:1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan V, Chauhan A. Oxidative stress in Alzheimer’s disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Hosoda T, Nakajima H, Honjo H. Estrogen protects neuronal cells from amyloid beta-induced apoptotic cell death. Neuroreport. 2001;12:1965–1970. doi: 10.1097/00001756-200107030-00038. [DOI] [PubMed] [Google Scholar]
- 26.Pereira C, Agostinho P, Oliveira CR. Vinpocetine attenuates the metabolic dysfunction induced by amyloid beta-peptides in PC12 cells. Free Radic Res. 2000;33:497–506. doi: 10.1080/10715760000301041. [DOI] [PubMed] [Google Scholar]
- 27.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 28.Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morais Cardoso S, Swerdlow RH, Oliveira CR. Induction of cytochrome c-mediated apoptosis by amyloid beta 25–35 requires functional mitochondria. Brain Res. 2002;931:117–125. doi: 10.1016/s0006-8993(02)02256-4. [DOI] [PubMed] [Google Scholar]
- 30.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 31.Chin JH, Tse FW, Harris K, Jhamandas JH. Beta-amyloid enhances intracellular calcium rises mediated by repeated activation of intracellular calcium stores and nicotinic receptors in acutely dissociated rat basal forebrain neurons. Brain Cell Biol. 2006;35:173–186. doi: 10.1007/s11068-007-9010-7. [DOI] [PubMed] [Google Scholar]
- 32.Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res. 2004;76:872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 37.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 38.Rosenstock TR, Carvalho AC, Jurkiewicz A, Frussa-Filho R, Smaili SS. Mitochondrial calcium, oxidative stress and apoptosis in a neurodegenerative disease model induced by 3-nitropropionic acid. J Neurochem. 2004;88:1220–1228. doi: 10.1046/j.1471-4159.2003.02250.x. [DOI] [PubMed] [Google Scholar]
- 39.Galindo MF, Jordan J, Gonzalez-Garcia C, Cena V. Reactive oxygen species induce swelling and cytochrome c release but not transmembrane depolarization in isolated rat brain mitochondria. Br J Pharmacol. 2003;139:797–804. doi: 10.1038/sj.bjp.0705309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 41.Parks JK, Smith TS, Trimmer PA, Bennett JP, Jr, Parker WD., Jr Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem. 2001;76:1050–1056. doi: 10.1046/j.1471-4159.2001.00112.x. [DOI] [PubMed] [Google Scholar]
- 42.Kroner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys. 1986;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths EJ. Mitochondrial calcium transport in the heart: physiological and pathological roles. J Mol Cell Cardiol. 2009;46:789–803. doi: 10.1016/j.yjmcc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Prentki M, Janjic D, Wollheim CB. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983;258:7597–7602. [PubMed] [Google Scholar]
- 45.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 46.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J. 2002;367:541–548. doi: 10.1042/BJ20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 48.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem Soc Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 49.Szabo I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 50.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997;272:3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 51.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- 52.Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur J Neurosci. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 53.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem. 2008;283:26312–26323. doi: 10.1074/jbc.M805235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansson MJ, Persson T, Friberg H, Keep MF, Rees A, Wieloch T, Elmer E. Powerful cyclosporin inhibition of calcium-induced permeability transition in brain mitochondria. Brain Res. 2003;960:99–111. doi: 10.1016/s0006-8993(02)03798-8. [DOI] [PubMed] [Google Scholar]
- 58.Clarke SJ, McStay GP, Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem. 2002;277:34793–34799. doi: 10.1074/jbc.M202191200. [DOI] [PubMed] [Google Scholar]
- 59.Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, Perry G. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bores GM, Smith CP, Wirtz-Brugger F, Giovanni A. Amyloid beta-peptides inhibit Na+/K+-ATPase: tissue slices versus primary cultures. Brain Res Bull. 1998;46:423–427. doi: 10.1016/s0361-9230(97)00382-1. [DOI] [PubMed] [Google Scholar]
- 61.Mark RJ, Hensley K, Butterfield DA, Mattson MP. Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price SA, Held B, Pearson HA. Amyloid beta protein increases Ca2+ currents in rat cerebellar granule neurones. Neuroreport. 1998;9:539–545. [PubMed] [Google Scholar]
- 63.Ekinci FJ, Malik KU, Shea TB. Activation of the L voltage-sensitive calcium channel by mitogen-activated protein (MAP) kinase following exposure of neuronal cells to beta-amyloid. MAP kinase mediates beta-amyloid-induced neurodegeneration. J Biol Chem. 1999;274:30322–30327. doi: 10.1074/jbc.274.42.30322. [DOI] [PubMed] [Google Scholar]
- 64.Cowburn RF, Wiehager B, Trief E, Li-Li M, Sundstrom E. Effects of beta-amyloid-(25–35) peptides on radioligand binding to excitatory amino acid receptors and voltage-dependent calcium channels: evidence for a selective affinity for the glutamate and glycine recognition sites of the NMDA receptor. Neurochem Res. 1997;22:1437–1442. doi: 10.1023/a:1021942109490. [DOI] [PubMed] [Google Scholar]
- 65.Hiruma H, Katakura T, Takahashi S, Ichikawa T, Kawakami T. Glutamate and amyloid beta-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J Neurosci. 2003;23:8967–8977. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez JR, Lyckman A, Oddo S, Laferla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem. 2008;105:262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 67.Kim JH, Rah JC, Fraser SP, Chang KA, Djamgoz MB, Suh YH. Carboxyl-terminal peptide of beta-amyloid precursor protein blocks inositol 1,4,5-trisphosphate-sensitive Ca2+ release in Xenopus laevis oocytes. J Biol Chem. 2002;277:20256–20263. doi: 10.1074/jbc.M108326200. [DOI] [PubMed] [Google Scholar]
- 68.Moreira PI, Santos MS, Moreno A, Rego AC, Oliveira C. Effect of amyloid beta-peptide on permeability transition pore: a comparative study. J Neurosci Res. 2002;69:257–267. doi: 10.1002/jnr.10282. [DOI] [PubMed] [Google Scholar]
- 69.Moreira PI, Santos MS, Moreno A, Oliveira C. Amyloid beta-peptide promotes permeability transition pore in brain mitochondria. Biosci Rep. 2001;21:789–800. doi: 10.1023/a:1015536808304. [DOI] [PubMed] [Google Scholar]
- 70.Shevtzova EF, Kireeva EG, Bachurin SO. Effect of beta-amyloid peptide fragment 25–35 on nonselective permeability of mitochondria. Bull Exp Biol Med. 2001;132:1173–1176. doi: 10.1023/a:1014559331402. [DOI] [PubMed] [Google Scholar]
- 71.Eliseev RA, Filippov G, Velos J, VanWinkle B, Goldman A, Rosier RN, Gunter TE. Role of cyclophilin D in the resistance of brain mitochondria to the permeability transition. Neurobiol Aging. 2007;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 72.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 73.Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 75.Yang X, Yang Y, Li G, Wang J, Yang ES. Coenzyme Q10 attenuates beta-amyloid pathology in the aged transgenic mice with Alzheimer presenilin 1 mutation. J Mol Neurosci. 2008;34:165–171. doi: 10.1007/s12031-007-9033-7. [DOI] [PubMed] [Google Scholar]
- 76.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18:240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D. Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci. 2008;28:6787–6793. doi: 10.1523/JNEUROSCI.2377-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chipana C, Camarasa J, Pubill D, Escubedo E. Memantine prevents MDMA-induced neurotoxicity. Neurotoxicology. 2008;29:179–183. doi: 10.1016/j.neuro.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Diehl-Schmid J, Forstl H, Perneczky R, Pohl C, Kurz A. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008;23:754–759. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 80.Fujisaki C, Utsuyama M, Kuroda Y, Watanabe A, Seidler H, Watanabe S, Kitagawa M, Hirokawa K. An immnosuppressive drug, cyclosporine-A acts like anti-depressant for rats under unpredictable chronic stress. J Med Dent Sci. 2003;50:93–100. [PubMed] [Google Scholar]