Abstract
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse serves as a model of basal ganglia injury and Parkinson’s disease. The present study investigated the effects of MPTP-induced lesioning on associative memory, conditioned fear, and affective behavior. Male C57BL/6 mice were administered saline or MPTP and separate groups were evaluated at either 7 or 30 days post-lesioning. In the social transmission of food preference test, mice showed a significant decrease in preference for familiar food 30 days post-MPTP compared to controls. Mice at both 7 and 30 days post-MPTP-lesioning had increased fear extinction compared to controls. HPLC analysis of tissues homogenates showed dopamine and serotonin were depleted in the striatum, frontal cortex, and amygdala. No changes in anxiety or depression were detected by the tail suspension, sucrose preference, light-dark preference, or hole-board tests. In conclusion, acute MPTP-lesioning regimen in mice causes impairments in associative memory and conditioned fear, no mood changes, and depletion of dopamine and serotonin throughout the brain.
Keywords: affective behavior, MPTP, Parkinson’s disease, anxiety, depression, conditioned fear
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by motor impairment including slowness of movement, rigidity, balance dysfunction, and resting tremor. However, disabling non-motor symptoms are seen in 30 to 60% of patients and include semantic and episodic memory loss, impairment of executive function, depression, and anxiety (Cummings, 1992; Hornykiewicz, 1963; Pillon et al., 1989a; Walsh and Bennett, 2001). A number of brain regions have been implicated in influencing non-motor behavioral symptoms including the basolateral amygdala, nucleus accumbens, frontal cortex, and the raphe nucleus (Ressler and Nemeroff, 2000) (Walsh and Bennett, 2001). Together with dopamine, serotonin from the dorsal and medial raphe nuclei is thought to play a central role in regulating affective behavior. Perturbation of serotonin neurotransmission in normal individuals can lead to depression, anxiety, and memory impairment (Mann and Yates, 1986; Mann, 1999; Pillon et al., 1989b). Patients with PD develop central serotonergic dysfunction, such as low cortical serotonin levels and degeneration of the dorsal raphe nucleus (Agid et al., 1989; Cummings, 1992; Gotham et al., 1986; McCance-Katz et al., 1992; Scatton et al., 1983). 1-methyl-4-phenyl-1,2,3,6,-tetrahydropyridine (MPTP) neurotoxicity in the substantia nigra pars compacta (SNpc) produces severe dopamine depletion in mice and nonhuman primates, and causes significant decrease of serotonin across multiple brain regions. For example, chronic MPTP-treatment in monkeys decreases levels of serotonin in the caudate nucleus, putamen, nucleus accumbens, hypothalamus, and cortical areas (Frechilla et al., 2001; Perez-Otano et al., 1991; Pifl et al., 1991; Russ et al., 1991). In mice, acute administration of MPTP leads to serotonin loss in the striatum and frontal cortex one week after lesioning (Rousselet et al., 2003).
The purpose of the current study was to evaluate the effects of MPTP-lesioning on associative memory, conditioned fear, depression, and anxiety, since this neurotoxic injury leads to depletion of dopamine and serotonin in brain regions important for these behaviors. After acute MPTP-lesioning, mice were tested at 7 days (greatest dopamine depletion) and 30 days (partial recovery of striatal dopamine). We used established mouse tests for associative memory (social transmission of food preference), fear conditioning, anxiety (light-dark preference, hole board), and depression (tail suspension, sucrose preference). Brain regions involved in control of affective behavior (frontal cortex, amygdala, and the raphe nucleus), as well as the basal ganglia (ventral mesencephalon, striatum) were examined for levels of dopamine, serotonin and their metabolites. We observed impairment in associative memory in mice at 30 days post-MPTP-lesioning and increased fear extinction at both 7 and 30 days post-MPTP. Despite significant depletion of both dopamine and serotonin at these time points, there was no significant increase in depression and anxiety compared to control mice. Overall, these results indicate that the acute MPTP-lesioned mouse model manifests some but not all non-motor behaviors seen in patients with PD.
MATERIALS AND METHODS
Animals, Treatment Groups and MPTP Administration
Male C57BL/6 mice, 8 to 10 weeks of age (Charles River Laboratories, Wilmington, MA) and weighing between 25 and 30g were group-housed in a temperature-controlled room under a 12h light/12h dark cycle with free access to water and standard rodent food. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at the University of Southern California. A total of 42 mice were used in this study. For lesioning, mice received 4 i.p. injections of 20 mg/kg MPTP (free-base; Sigma-Aldrich, St. Louis, MO) in saline at 2h intervals or 4 injections of 0.1 ml 0.9 % NaCl as control. Mice were tested either at (i) 7 days post-saline (n=20), (ii) 7 days post-MPTP (n=10) or (iii) 30 days post-MPTP (n=12). The degree of lesioning was determined at both 7 and 30 days post-lesioning using unbiased stereological counting of the remaining dopaminergic neurons in the SNpc as well as analysis of the immunoreactivity for tyrosine hydroxylase protein within the striatum. These methods are outlined below and the degree of lesioning was in agreement with previous reports using the same MPTP-lesioning regimen (Jackson-Lewis et al., 1995; Jakowec et al., 2001; Petzinger et al., 2001; Petzinger et al., 2007; Przedborski et al., 2001).
Behavioral Testing
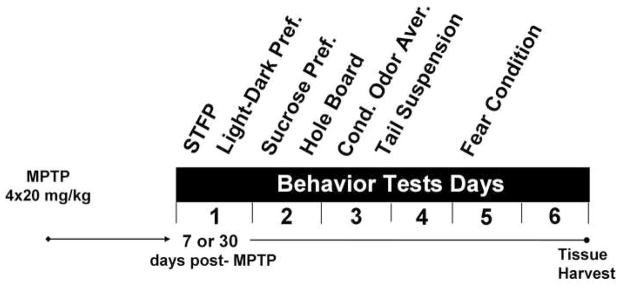
After MPTP-lesioning, mice were subjected to a series of behavioral tests for anxiety (light-dark exploration, hole-board), depression (sucrose preference, tail suspension), associative memory (social transmission of food preference [STFP]), and conditioned fear. Tests were conducted over 6 days with the following order: (1) STFP, (2) light-dark exploration, (3) sucrose preference, (4) hole-board, (5) conditioned odor aversion, (6) tail suspension, and (7) conditioned fear (Fig 1). The design of the behavior testing battery took into account starting with the least stressful test and progressing to the most stressful (Crawley, 2008). The tests which are known to induce the most acute stress response (tail suspension and fear conditioning) (Brown et al., 1984; Liu et al., 2003; Pugh et al., 1997) were conducted at the end of the battery. The order of tests was not randomized. Any potential carry-over effects were equal between the groups as all mice were tested in the same order. Tests occurring on the same day were conducted at least 3h apart. Each behavioral test was administered once to each mouse. All tests were performed in a darkened room with dim red lights and animals were allowed to habituate to the testing room for 1h prior to each test. Details for each test are presented in the following sections.
Fig. 1.
Diagram representing the timeline and order of behavioral tests following acute MPTP-lesioning (4 × 20 mg/kg, 2h apart) or saline injections (4 × 0.1 ml, 2h apart). The order of behavioral tests was designed according to increased stress. Separate groups of mice were tested at 7 or 30 days post-MPTP-lesioning.
Social transmission of food preferences (STFP) for olfactory memory was conducted as described previously (Holmes et al., 2002; Kogan et al., 1997; Wrenn et al., 2003). Briefly, a demonstrator mouse was randomly chosen from each home cage prior to MPTP administration. Initially, all mice were habituated for 18h to powdered chow presented in two 125 g food jar assemblies (Dyets, Inc., Bethlehem, PA) in the opposite corners of the home cage. During this time, standard food pellets were unavailable. One day later, demonstrator mice were removed from their home cages, individually housed, and food-deprived overnight with free access to water. The next day, each demonstrator mouse received powdered chow mixed with either 1% cinnamon or 2% cocoa (w/w) for 1h, or until at least 0.2 g of powdered food was consumed. To avoid a bias in the cued flavor, half of the demonstrators randomly received cinnamon- and the other half cocoa-flavored food. Immediately afterwards, demonstrator mice were returned to their home cages to interact with observer mice for 30 min. At the end of the interaction period, demonstrator mice were removed. Testing of food preference in observer mice took place 24h later, following overnight food deprivation with free access to water. During the test, observer mice were caged individually and were given a free choice of food flavored with 1% cinnamon or 2% cocoa. To control for possible place preference, the position of the food jar assemblies with the cued flavor was balanced between cages. Observer mice were allowed to eat for 1h and food consumption from each jar was determined by weight. The percent of total food intake consumed as the cued flavor was determined.
The light-dark exploration test for anxiety was conducted as previously described (Holmes et al., 2002). The test uses the ethological conflict between the tendencies of mice to explore a novel environment and to avoid a brightly lit open area. This test has been shown to be sensitive to changes in serotonergic tone (Holmes et al., 2002). A standard polypropylene mouse cage (30 × 19 × 13 cm) was divided with an opaque partition containing a small opening at the bottom (8 × 5 cm) into a larger light chamber and a smaller dark chamber. The light chamber (20 × 19 × 13 cm) was transparent and brightly illuminated by a 60 watt bulb placed 40 cm above the cage top. The dark chamber (10 × 19 × 13 cm) was black and closed at the top with a black Plexiglas lid. The test was conducted in a soundproof room and the apparatus was cleaned with warm water and 70% ethanol between each mouse. Each mouse was placed in the lighted chamber facing away from the entrance to the dark chamber, and its behavior was recorded on video for 5 min. Measurements were obtained for: (i) latency to first enter the dark chamber, (ii) time spent in the dark, and (iii) number of transitions between the two compartments. A transition was considered only when a mouse entered into a compartment with 3 or more paws.
The sucrose preference test for depression was performed as a modification of the 2-bottle preference test for mice (Strekalova et al., 2004). Mice were deprived of food and water overnight and placed in separate cages 1h before the start of testing. Mice were offered solutions of 1% sucrose (Sigma-Aldrich, MO) or tap water for 1h. Fluid consumption was determined by weight and expressed as percent of total fluid intake consumed from the sucrose solution. The positions of the water and sucrose bottles were alternated to control for side preferences.
The hole-board test for anxiety based on exploratory behavior was performed as previously described (Boissier and Simon, 1962; do-Rego et al., 2006). This test is frequently used as an indicator of directed exploratory behavior in rodents (Crawley, 1985). The testing apparatus consisted of a 2 cm-thick square plastic board, 40 × 40cm, with 16 holes (2 cm diameter) regularly spaced 7cm apart over the surface and 3.5 cm from the edges (Ugo Basile, Italy). The board was positioned 50 cm above floor level. Each animal was placed at the corner of the board and allowed to freely explore for 5 min. The number and location of head dips was recorded using a video camera and videotapes were scored by a trained observer blinded to the treatment group. A head dip was considered when a mouse placed its head into a whole up to the neck. Between testing of each mouse, the board surface was cleaned with water and 70% ethanol.
The conditioned odor aversion with isoamyl-acetate was used as a rapid assessment of odor detection in mice. The one bottle test was performed as previously described (Passe and Walker, 1985; Wright and Harding, 1982) with minor modifications. Mice were deprived of food and water overnight and housed separately 1h before the start of testing. Testing consisted of four 10 min trials. Breaks between the trials lasted 30 min. During the first two trials, mice were allowed to drink water containing both 0.1% (v/v) isoamyl acetate (artificial banana aroma) and 0.5% (w/v) quinine hydrochloride (bitter taste) (Sigma-Aldrich, MO). During the third trial, mice were tested for avoidance of isomayl acetate odorized water without quinine hydrochloride. The last trial consisted of tap water. Fluid consumption was determined by weight. Preference ratio for isoamyl acetate was determined from the last two trials as follows: odorized water (g)/odorized water + tap water (g). Preference ratio of below 0.5 indicates aversion, and therefore detection of the odorant.
The tail suspension test for depression was preformed as previously described (Steru et al., 1985). This test relies on immobility as a measure of “behavioral despair” once the mouse perceives that the escape from the apparatus is impossible. Mice were individually suspended by their tails at a height of 20cm using a piece of adhesive tape wrapped around the tail, 2 cm from the tip. Behavior was videotaped for 6 min. The duration of immobility was measured using a stopwatch. Mice were considered immobile only when hanging completely motionless. Mice that climbed up their tails were excluded from analysis. Results were expressed as percent of time spent immobile.
Auditory conditioned fear response was assessed as previously described (LeDoux, 2000). The test consisted of an 8 min acquisition phase on the first day and an 8 min extinction phase on the next day. Training was conducted in a soundproof room with dim red light and background noise level of 50dB. Each mouse was placed in the middle of a testing chamber (23 × 20 × 20 cm) with an electrified metal rod floor (2 mm diameter, 6 mm separation). The chamber was cleaned with water and 70% ethanol before testing each mouse. The fear acquisition consisted of a 3 min acclimation period, 3 pairings of tone/foot shock separated with 1 min quiet intervals, and 1 min quiet consolidation period at the end of testing. For each pairing of tone/foot shock, mice were presented tone (30 s of 80dB, 1000Hz/8000Hz continuous alternating sequence of 250 ms pulses) generated using LabView 7.1 software (National Instruments Corporation, Austin, TX) and delivered through speakers on the top of the testing chamber. Each tone was immediately followed by a mild foot shock (2 s, 0.6 mA). Freezing behavior (no visible movement except for respiration) was recorded on videotape during the test. The extinction phase was conducted the next day in a different room illuminated with blue indirect light. Each mouse was placed in a cylindrical Plexiglas observation chamber (diameter 28 cm) with a smooth Plexiglas floor. Following an initial 2 min acclimation period, recall and extinction of freezing in response to the tone (presented continuously for 6 min) was monitored in the absence of foot shock. The duration of the freezing response in seconds was measured within each 1-minute interval of both acquisition and extinction phases. Freezing behavior was manually scored using Observer XT version 6.1.35 software (Noldus Information Technology, San Diego, CA).
Tissue Preparation
Brains were collected 24h after the last behavior test. For immunohistochemistry, a subset of mice (n = 4 per group), were sacrificed by pentobarbital overdose and transcardially perfused with 4% paraformaldehyde, post-fixed in the perfusion fixative for 48h at 4°C, cryoprotected in 20% sucrose for 24h, frozen in isopentane on dry ice, and stored at −80°C. For HPLC analysis, another subset of mice (n = 5 per group) were killed by cervical dislocation. Brains were quickly removed and regions of interest identified using a standard mouse brain atlas (Paxinos and Franklin, 2001). Frontal cortex (rostral to Bregma +2.50), dorsal and ventral regions of mid-striatum (including the nucleus accumbens), amygdala, ventral mesencephalon (containing substantia nigra and VTA) and the raphe nucleus (dorsal and medial part) were rapidly dissected, immediately frozen in isopentane on dry ice and stored at −80°C.
Neurochemical Analysis
Neurotransmitter concentrations of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin, and 5-hydroxyindoleacetic acid (5-HIAA) were determined by HPLC with electrochemical detection as previously described (Irwin et al., 1992; Kilpatrick et al., 1986; Petzinger et al., 2007). The system consisted of an ESA auto-sampler (ESA Inc., Chelmsford, MA) equipped with a 150 × 3.2 mm reverse phase C-18 column (3 μm diameter) regulated at 28°C and a CoulArray 5600A (ESA Inc, Chelmsford, MA), equipped with a 4-channel analytical cell with potentials set at −100 mV, −30 mV, 220 mV and 350 mV. The HPLC was integrated with a DellGX-280 computer with CoulArray analytical program for Windows (ESA Inc, Chelmsford, MA). Mobile phase consisted of acetonitrile in phosphate buffer and an ion-pairing agent and was delivered at a rate of 0.6ml/min. Fresh frozen tissue was homogenized in 0.4 M HClO4, and centrifuged to separate precipitated protein. The pellet was resuspended in 0.5 M NaOH and used to determine total protein concentration with the CoomassiePlus protein assay (Pierce, Rockford, IL) and microplate reader ELx800 (BioTek Instruments Inc., Winooski, VT) equipped with KCjunior software.
Immunohistochemical Staining
Analysis of relative expression of striatal tyrosine hydroxylase (TH) immunoreactivity was carried out as previously described (Jakowec et al., 2004; Petzinger et al., 2006; Petzinger et al., 2007). Briefly, coronal brain sections were cut at 25 μm thickness through the mid-striatum and collected in phosphate-buffered saline (PBS, pH 7.2). Sections were exposed to rabbit polyclonal anti-tyrosine hydroxylase antibody (1:5000, Chemicon, Temecula, CA) for 24h at 4°C followed by 2h incubation in IRDye700 conjugated goat anti-rabbit IgG (1:2500, Molecular Probes, Eugene, OR). Following extensive washing, sections were mounted on gelatin-coated slides and scanned using LI-COR Odyssey near infrared imaging platform (LI-COR Biotechnology, Lincoln, NE). Multiple brain sections at the mid-striatum (4 to 5 sections per mouse) from 4 mice per group were prepared and analyzed in parallel. Fluorescence intensity within an oval shaped region of interest (1mm2) in dorsal striatum was measured and corrected for background by subtracting the adjacent corpus callosum. Values for treatment groups were normalized to saline animals prior to statistical analysis.
Unbiased Stereological Counting of SNpc Dopaminergic Neurons
The degree of MPTP-lesioning at 7 and 30 days post-MPTP lesioning was determined by unbiased stereological counting of dopaminergic neurons in the SNpc. For this purpose, coronal sections were collected starting rostral to the substantia nigra at Bregma −2.50 mm before the closure of the third ventricle through to the prominence of the pontine nuclei at Bregma −4.24 mm according to the stereotaxic atlas of the mouse brain (Paxinos and Franklin, 2001). Every sixth section from 4 mice per group was included in the analysis. Sections were exposed to rabbit polyclonal anti-tyrosine hydroxylase antibody (1:5000, Chemicon, Temecula, CA) for 24h at 4°C followed by and avidin-biotin complex (AB C elite Kit, Vector Labs, Burlingame, CA). Staining was visualized by exposure to 3,3′diaminobenzidine tetrahydrochloride (Pierce, Rockford, IL), after which sections were mounted on gelatin-coated slides, and cover-slipped. Cell nuclei were visualized by cresyl-violet staining. The SNpc was delineated from the rest of the brain based on TH-ir. Sections were examined using an Olympus BX-50 microscope (Olympus Optical, Tokyo, Japan) equipped with a motorized stage and digital Retiga cooled CCD camera (Q-Imaging, Burnaby, British Columbia, Canada). Each stained ventral mesencephalon section was viewed at low magnification (4x) and the SNpc outlined and delineated from the ventral tegmental-immunoreactive neurons using the third nerve and cerebral peduncle as landmarks. Neurons were viewed at higher magnification (40x) and counted if they displayed TH-ir and had a clearly defined nucleus, cytoplasm, and nucleolus. Analysis was performed with the computer-imaging program BioQuant Nova Prime (BioQuant Imaging, Nashville, TN). The total number of SNpc dopaminergic neurons was determined based on the method of Gundersen and Jensen (1987).
Statistical Analysis
With the exception of fear-conditioning, all results were evaluated by a one-way analysis of variance (ANOVA) and Bonferroni multiple comparison test when appropriate, or by one-way ANOVA on ranks followed by Dunn’s post-hoc test when the normality test or equal variances test failed. Software used for statistical analysis was Prism5 for Windows (Graph Pad Software Inc., San Diego, CA). A repeated measures ANOVA was used to analyze freezing behavior from the fear-conditioning test. Data from all experiments are presented as mean ± SEM and p<0.05 was considered significant.
RESULTS
Social Transmission of Food Preference
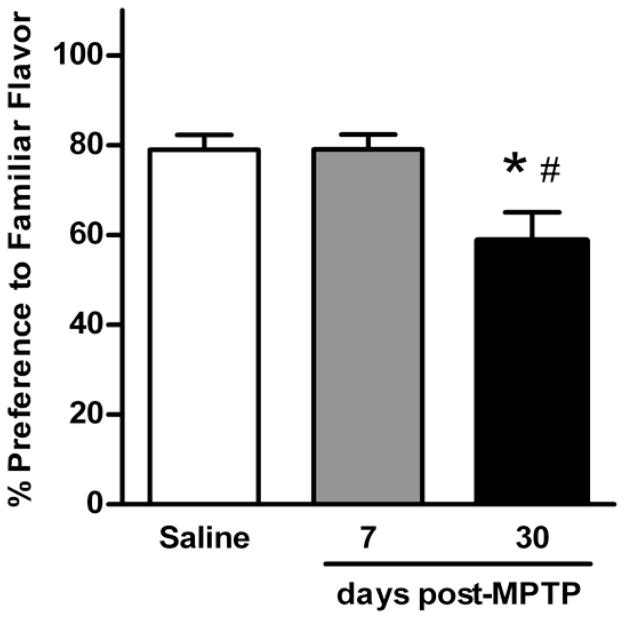
Associative olfactory memory was assessed using the STFP test (Fig. 2). When presented with 2 unfamiliar flavors of powdered food, control mice strongly preferred the flavor previously consumed by the demonstrator mouse (79.0 ± 3.7% of total food intake). Lesioned mice tested 7 days after MPTP showed a similar preference (79.0 ± 3.3%). However, preference for the demonstrated flavor declined significantly in mice tested 30 days post-MPTP (58.7 ± 6.3%) (F(2,41)=5.614; p<0.05). As the STFP test relays on the ability of mice to discriminate odors, it was important to test if all mice had similar olfactory function. For this purpose, the conditioned odor aversion test with isoamyl acetate was used. Mice from all three groups avoided water odorized with isoamyl acetate (preference ratio for saline: 0.4 ± 0.1; 7 days post-MPTP: 0.2 ± 0.1; 30 days post-MPTP: 0.3 ± 0.1). Preference ratio below 0.5 indicates the ability to discriminate odors. Based on these results, mice in this study did not display detectable impairment in olfactory function.
Fig. 2.
Associative memory impairment in MPTP-lesioned mice measured by the social transmission of food preference test. The preference for familiar food was tested in control (n = 20) and lesioned mice at 7 days (n = 10) and 30 days post-MPTP (n = 12). Data are presented as mean ± SEM of percent preference for familiar food. The symbols “ * ” and “ # ” represent statistically significant differences compared to the saline control and the 7 days post-MPTP groups, respectively (p < 0.05).
Light-Dark Preference and Hole-Board
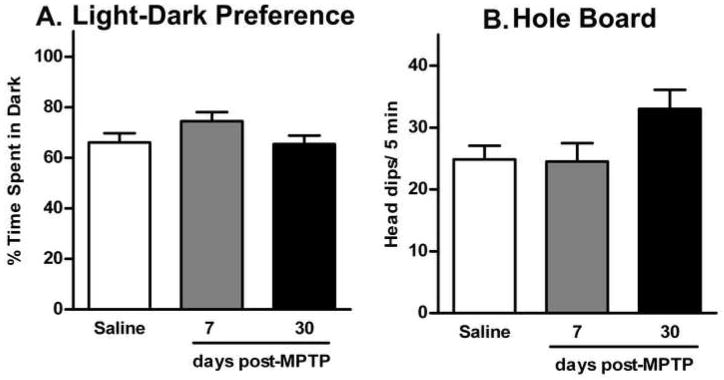
Light-dark preference and the hole-board tests were used to determine levels of anxiety in mice 7 and 30 days post-MPTP-lesioning (Fig. 3). Mice from all three groups showed a similar preference for the dark compartment (F(2,39) = 1.428; p>0.05) during the light-dark exploration test (Figure 3A). Saline-treated mice spent 66.1 ± 3.7% of the time in the dark. Likewise, mice at 7 days post-MPTP-lesioning spent 74.5 ± 3.6% of time in the dark and those tested at 30 days post-MPTP-lesioning spent 65.4 ± 3.3% of the time in the dark. The average number of transitions between the light and dark compartments was similar in all three groups (13.1 ± 1.6 for saline mice; 12.6 ± 1.3, and 16.0 ± 1.9 for mice 7 days and 30 days post-MPTP-lesioning, respectively; F(2,39) = 1.246; p>0.05). There was no significant difference between the latency to first enter the dark compartment between groups (26.7 ± 5.3 s for saline mice; 15.9 ± 4.2 s and 25.6 ± 5.6 s for mice 7 days and 30 days post-MPTP-lesioning, respectively; F(2,39) = 0.9846; p>0.05). Exploratory behavior was measured in the hole-board test as a second test of anxiety (Fig. 3B). As measured by head dipping, saline treated mice visited 24.8 ± 2.2 holes during the 5 min test, similar to mice at 7 days post-MPTP-lesioning (24.5 ± 3.0 holes). Mice tested at 30 days post-MPTP-lesioning had 33.0 ± 3.1 head dips. The difference in the total number of head dips between groups was not statistically significant (F(2,33) = 3.053; p>0.05).
Fig. 3.
Anxiety in mice 7 and 30 days post-MPTP-lesioning measured in light-dark preference and the hole-board tests. Data are presented as mean ± SEM from control (n = 20), 7 days (n = 10) and 30 days post-MPTP (n = 12). (A) Time spent in the dark compartment during the 5 min light-dark preference test (in percent of total time). (B) The total number of head dips during the 5 min hole-board test.
Sucrose Preference and Tail Suspension
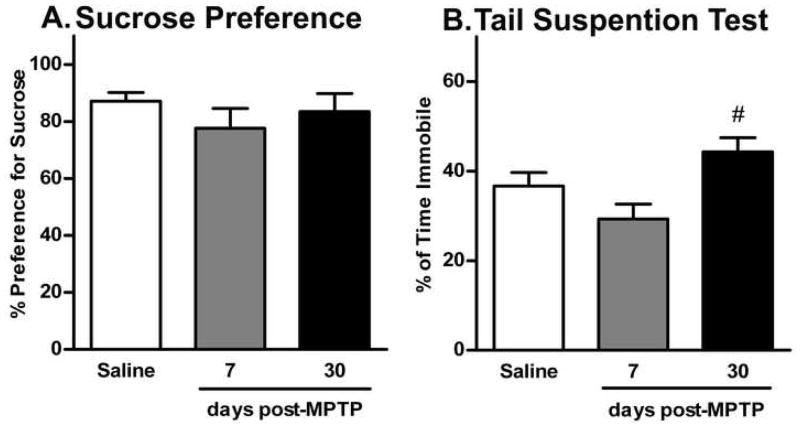
Sucrose preference and the tail suspension tests were used to determine levels of depression in mice after MPTP-lesioning (Fig. 4). In particular, the sucrose preference test measures anhedonia following overnight water deprivation (Strekalova et al., 2004). Mice from all three groups had high preference for a 1% sucrose solution (80–85%) compared to tap water (15–20%) (Fig. 4A). There was no significant difference in the amount of sucrose consumed (F(2,38) = 0.968; p>0.05). Furthermore, all mice had similar fluid intake during the 1h testing period (saline mice: 0.9 ± 0.1g; 7 days post-MPTP-lesioning: 0.8 ± 0.1g; and 30 days post-MPTP-lesioning: 1.1 ± 0.1g; F(2,37) = 1.268; p>0.05). The tail suspension test measures behavioral despair (Steru et al., 1985). Saline-treated mice spent 36.6 ± 3.1% of total time in passive immobility (Fig. 4B). Similarly, mice at 7 days post-MPTP-lesioning spent 29.3 ± 3.3% of total time in immobility. Interestingly, mice tested at 30 days post-MPTP-lesioning spent significantly more time immobile (44.3 ± 3.2%) compared to the 7 days post-MPTP group (F(2,39) = 4.372; p<0.05), however, this was not statistically different compared to saline-treated mice. Only one mouse in the control group was excluded form the test because it climbed up its tail. None of the MPTP-lesioned mice were excluded from the test.
Fig. 4.
The effect of acute MPTP-lesioning on depression in mice 7 and 30 days post-treatment measured using sucrose preference and tail suspension tests. Data are presented as mean ± SEM of (A) preference for 1% sucrose and (B) time spent in immobility (in percent of total time) for control (n = 18), 7 days (n = 10) and 30 days post-MPTP (n = 12). The symbol “ # ” represents statistically significant difference compared to the 7 days post-MPTP group (p < 0.05).
Conditioned Fear
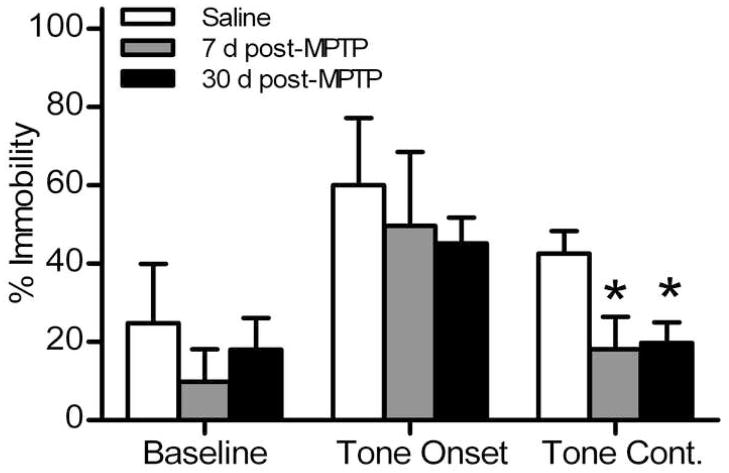
Acquisition and extinction of conditioned fear response was measured using the auditory fear conditioning test. All mice showed little or no freezing during the baseline period of acquisition session (percent time freezing for saline: 1.4 ± 0.4%; for 7 days post-MPTP: 0.2 ± 0.1%; and for 30 days post-MPTP: 0.7 ± 0.4%). During subsequent pairings of tone and foot shock, all mice showed increased freezing behavior. Mice in all three groups had similar levels of freezing after the third foot shock (saline: 54.9 ± 10.7%; 7 days post-MPTP: 46.2 ± 10.5%; and 30 days post-MPTP: 49.4 ± 4.1%; F(2,16) = 0.249; p>0.05). The next day, (Fig. 5), the baseline freezing response of all mice was similar (percent freezing for saline: 23.7 ± 6.2%; 7 days post-MPTP: 9.6 ± 2.4%; and 30 days post-MPTP: 18.5 ± 8.3%). At the onset of auditory stimulus (without foot shock), all mice showed a robust increase in the freezing response (saline: 68.8 ± 3.1%; 7 days post-MPTP: 56.8 ± 6.7%; and 30 days post-MPTP: 51.0 ± 6.2%). However, after 6 minutes of continuous tone exposure, both groups of MPTP-lesioned mice spent significantly less time freezing (7 days post-MPTP: 9.5 ± 3.2%; and 30 days post-MPTP: 17.5 ± 8.2%) compared to saline controls (43.7 ± 6.0%). Repeated measures ANOVA followed by Bonferroni post-test showed a significant difference in freezing response over time between saline-treated and MPTP-lesioned mice at both 7 and 30 days (F(2,17) = 23.08; p<0.05).
Fig. 5.
Acquisition and extinction of fear response in MPTP-lesioned mice measured in the fear conditioning test. Fear-induced immobility was measured in control (n = 5), 7 days (n = 6), and 30 days post-MPTP mice (n = 8). Data are presented as mean ± SEM of the freezing response (percent of 2 or 4 min periods). Following 2 min baseline, continuous tone was played for 6 min without foot shock (tone onset: min 3–4; tone continuation: min: 5–8). The symbol “ * ” indicates statistically significant difference compared to the saline control group (p < 0.05).
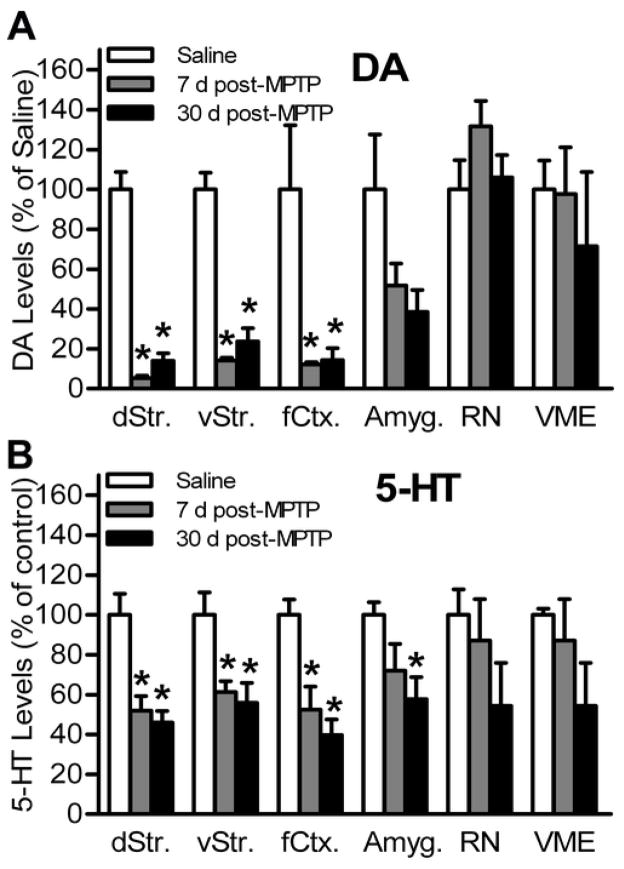
HPLC Analysis of Dopamine, Serotonin, and their Metabolites
HPLC analysis was used in tissue homogenates to determine the levels of dopamine and its metabolites (DOPAC and HVA), as well as serotonin and its metabolite 5-HIAA. The turnover ratio for dopamine was determined as ([DOPAC] + [HVA])/[dopamine] and for serotonin as [5-HIAA]/[serotonin]. Six brain regions were analyzed including the frontal cortex (rostral to the motor cortex), dorsal striatum, ventral striatum (including the nucleus accumbens), ventral mesencephalon (VME) (including the substantia nigra and VTA), amygdala, and the raphe nucleus (including the dorsal and medial raphe) (Table 1 and Fig. 6). Taken together, MPTP-lesioned mice had severe dopamine depletion in the dorsal and ventral striatum and frontal cortex, and no significant loss in the amygdala, VME, or the raphe nucleus. The greatest depletion of dopamine was measured at 7 days post-MPTP. Serotonin was significantly depleted in the dorsal and ventral striatum, frontal cortex, and amygdala. The greatest loss of serotonin was measured at 30 days post-MPTP-lesioning. There was no significant change in the level of the serotonin metabolite 5-HIAA in any of the examined brain regions.
Table 1.
Dopamine and serotonin levels measured by HPLC and their calculated turnover ratios in six brain regions of the MPTP-lesioned and control mice.
HPLC analysis of dopamine, serotonin, and their calculated turnover ratios in the dorsal striatum, ventral striatum, frontal cortex, amygdala, ventral mesencephalon (VME), and raphe nucleus from control, 7 days, and 30 days post-MPTP-lesioned mice (n = 5/group).
| Treatment | DA | DA Turnover | 5-HT | 5-HT Turnover | |
|---|---|---|---|---|---|
| Dorsal Striatum | Control | 141.3 ± 12.4 | 0.2 ± 0.0 | 7.1 ± 0.8 | 1.0 ± 0.1 |
| 7 d post-MPTP | 7.5 ± 1.6* | 1.1 ± 0.5 | 3.7 ± 0.5* | 2.4 ± 1.0 | |
| 30 d post-MPTP | 19.9 ± 5.2* | 1.4 ± 0.3 | 3.3 ± 0.4* | 2.9 ± 0.5 | |
| Ventral Striatum | Control | 91.1 ± 7.7 | 0.2 ± 0.0 | 16.4 ± 1.8 | 0.7 ± 0.1 |
| 7 d post-MPTP | 13.0 ± 1.3* | 0.6 ± 0.2 | 10.0 ± 0.9* | 1.1 ± 0.4 | |
| 30 d post-MPTP | 21.6 ± 6.1* | 1.2 ± 0.4 | 9.1 ± 1.6* | 1.9 ± 0.6 | |
| Frontal Cortex | Control | 4.0 ± 1.5 | 0.8 ± 0.2 | 12.8 ± 1.1 | 0.5 ± 0.0 |
| 7 d post-MPTP | 0.5 ± 0.1* | 1.4 ± 0.2 | 6.7 ± 1.5* | 0.8 ± 0.3 | |
| 30 d post-MPTP | 0.6 ± 0.2* | 4.2 ± 1.3* | 5.1 ± 1.0* | 2.5 ± 1.0 | |
| Amygdala | Control | 9.2 ± 2.5 | 0.7 ± 0.1 | 11.9 ± 0.8 | 1.2 ± 0.2 |
| 7 d post-MPTP | 4.8 ± 1.0 | 0.8 ± 0.3 | 8.6 ± 1.6 | 1.9 ± 0.8 | |
| 30 d post-MPTP | 3.5 ± 1.0 | 2.1 ± 1.0 | 6.8 ± 1.3* | 3.2 ± 1.2 | |
| VME | Control | 5.2 ± 0.8 | 1.1 ± 0.0 | 23.7 ± 0.7 | 1.1 ± 0.0 |
| 7 d post-MPTP | 5.1 ± 1.2 | 1.8 ± 0.6 | 20.7 ± 4.9 | 2.8 ± 1.2 | |
| 30 d post-MPTP | 3.8 ± 2.0 | 4.6 ± 1.4* | 12.9 ± 5.2 | 6.6 ± 2.4 | |
| Raphe Nucleus | Control | 1.4 ± 0.2 | 1.0 ± 0.0 | 15.8 ± 2.0 | 1.6 ± 0.4 |
| 7 d post-MPTP | 1.9 ± 0.2 | 0.8 ± 0.1 | 12.5 ± 3.5 | 3.2 ± 1.5 | |
| 30 d post-MPTP | 1.5 ± 0.2 | 1.2 ± 0.3 | 11.5 ± 2.9 | 3.3 ± 1.1 |
Concentrations are expressed as ng/mg of protein. Data are presented as mean ± SEM.
Abberviations: DA: dopamine; 5-HT: serotonin; VME: ventral mesencephalon.
DA turnover: ([DOPAC] + [HVA])/[DA]; 5-HT turnover: [5-HIAA]/[5-HT]
p < 0.05 compared to the saline group.
Fig. 6.
Dopamine and serotonin levels in MPTP-lesioned mice. Twenty four hours following behavioral testing, the dorsal striatum (dStr), ventral striatum (vStr), frontal cortex (fCtx), amygdala, ventral mesencephalon (VME), and the raphe nucleus (RN) tissue homogenates were analyzed for monoamine concentrations (n= 5/group) using HPLC. Data are presented as mean ± SEM. (A) Dopamine and (B) serotonin loss relative to control mice in brain regions regulating associative memory and affective behavior at 7 and 30 days post-MPTP-lesioning. The symbol “ * ” indicates statistically significant differences compared to the saline control group (p < 0.05).
Among all six regions investigated, the dorsal striatum of saline-treated mice contained the highest concentration of dopamine (141.3 ± 12.4 ng dopamine/mg protein). Here, acute MPTP-lesioning caused a significant loss of dopamine that persisted for at least 30 days (F(2,14) = 89.00; p<0.05). There was 95% depletion in mice at 7 days post-MPTP (7.5 ± 1.6 ng dopamine/mg protein) and 86% depletion at 30 days post-MPTP-lesioning (19.9 ± 5.2 ng dopamine/mg protein). The change in dopamine turnover ratio did not reach significance at 7 days (1.1 ± 0.4) nor at 30 days post-MPTP-lesioning (1.4 ± 0.3) compared to controls (0.2 ± 0.0) (F(2,14) = 3.654; p>0.05). The ventral striatum of saline-treated mice contained the second highest concentration of dopamine (91.0 ± 7.7 ng dopamine/mg protein). In this region MPTP caused an 86% depletion at 7 days post-MPTP-lesioning (13.0 ± 1.3 ng dopamine/mg protein) and 77% depletion at 30 days post-MPTP (21.6 ± 6.1 ng dopamine/mg of protein) (F(2,14) = 56.63; p<0.05). However, differences in dopamine turnover ratio were not statistically significant (F(2,14) = 3.654; p>0.05), similar to dopamine turnover in the dorsal striatum.
In the frontal cortex of control mice the dopamine concentration (4.0 ± 1.5 ng dopamine/mg protein) was low compared to the striatum. Nonetheless, MPTP-lesioning caused significant dopamine loss (88% depletion) at 7 days after MPTP (0.5 ± 0.1 ng dopamine/mg protein) and at 30 days post-MPTP (0.6 ± 0.2 ng dopamine/mg protein; 86% depletion) (F(2,14) = 5.43; p<0.05). Furthermore, dopamine turnover ratio was significantly increased at 7 days (1.4 ± 0.2) and 30 days (4.2 ± 1.3) post-MPTP-lesioning, compared to controls (0.8 ± 0.2) (F(2,14)= 5.863; p < 0.05).
In the amygdala of saline-treated mice dopamine levels (9.2 ± 2.5 ng dopamine/mg protein) were about one-tenth of those in the striatum. MPTP-induced dopamine loss in amygdala did not reach statistical significance (F(2,14) = 3.132; p>0.05), and dopamine turnover ratio remained similar between the groups (Table 1). Cell bodies of dopamine and serotonin-producing neurons are located in VME and the raphe nucleus, respectively. These two regions had low detectable levels of dopamine in control mice (Table 1) but MPTP-lesioning did not cause statistically significant dopamine loss in the VME nor the raphe nucleus (Fig. 6A). The fact that the VME also contained the VTA, which is less affected by MPTP-lesioning, accounts for the fact changes in dopamine within this region were not statistically different from saline control at 7 days post-lesioning but did show a non-statistical difference at 30 days post-lesioning.
HPLC analysis of serotonin in tissue homogenates showed that loss of this neurotransmitter was modest compared to dopamine loss following MPTP-lesioning. In the dorsal striatum of saline control mice, serotonin was low (7.1 ± 0.8 ng serotonin/mg protein) but nonetheless, was significantly depleted at 7 days (3.7 ± 0.5 ng serotonin/mg protein, 48% loss) and at 30 days post-MPTP-lesioning (3.3 ± 0.4 ng serotonin/mg protein, 64% loss) (F(2,14) = 13.10; p<0.05). The ventral striatum of control mice contained twice as much serotonin compared to the dorsal striatum (16.4 ± 1.8 ng serotonin/mg protein). Here, the serotonin loss was moderate but still significant: 10.0 ± 0.9 ng/mg protein or 39% depletion at 7 days and 9.1 ± 1.6 ng serotonin/mg protein or 44% depletion at 30 days post-MPTP-lesioning respectively (F(2,14) = 6.80; p<0.05). The serotonin turnover ratio in both the dorsal and ventral striatum did not change significantly following MPTP-lesioning (Table 1).
Serotonin concentrations in the frontal cortex (12.8 ± 1.1 ng serotonin/mg protein) and amygdala (11.9 ± 0.7 ng serotonin/mg protein) were similar in control mice. MPTP-lesioning caused a 48% depletion in the frontal cortex at 7 days (to 6.7 ± 1.5 ng serotonin/mg protein) and 60% depletion at 30 days post MPTP-lesioning (to 5.1 ± 1.0 ng serotonin/mg protein). This decrease was statistically significant compared to controls (F(2,14) = 11.28; p<0.05). There was no change in serotonin turnover ratio in the frontal cortex (Table 1). In the amygdala, serotonin remained unchanged at 7 days post-MPTP (8.5 ± 1.6 ng serotonin/mg protein); however, it was significantly depleted at 30 days post-MPTP-lesioning (6.9 ± 1.3 ng serotonin/mg protein) compared to control (F(2,14) = 4.03; p<0.05).
The raphe nucleus (containing both the dorsal and medial aspects) and VME (containing the substantia nigra and VTA) had high average serotonin concentrations in all mice (Table 1) and MPTP-lesioning did not cause a significant depletion of serotonin in these regions.
TH-Immunoreactivity in Dorsal Striatum and Dopaminergic Cell Loss in SNpc
TH immunoreactivity is often used as a marker of the integrity of dopaminergic axons in the striatum (Jakowec et al., 2004; Petzinger et al., 2007). There was significant reduction of TH-immunireactivity (TH-ir) in dorsal striatum of mice 7 and 30 days post-MPTP-lesioning (F(2,9) = 45.41; p<0.05). MPTP caused a 58% reduction of TH-ir in the dorsal striatum examined at 7 days post-lesioning and 45% loss at 30 days post-MPTP-lesioning.
The number of surviving TH-positive neurons in the SNpc was used as an additional measure of the integrity of midbrain dopaminergic system 7 and 30 days post-MPTP-lesioning. MPTP-lesioning caused 68% loss of TH-ir SNpc neurons in 7 days post-MPTP and 66% loss at 30 days post-MPTP.
DISCUSSION
The MPTP-lesioned mouse serves as a model of basal ganglia injury and Parkinson’s disease. While the majority of studies focus specifically on motor deficits, few studies have addressed the non-motor features including affective behavior. The purpose of this study was to examine non-motor behaviors (associative memory, conditioned fear, anxiety, and depression) in the C57BL/6 mouse following an standardized acute lesioning regimen with MPTP (Jackson-Lewis et al., 1995). We report associative memory impairment measured by STFP evident only at 30 days post-MPTP. In addition, mice had increased fear extinction at both 7 days and 30 days post-MPTP. In contrast, there were no significant changes in anxiety (measured by the hole-board and light-dark preference tests), or depression (measured by the sucrose preference and tail-suspension tests). Dopamine and serotonin levels in brain homogenates were depleted in the striatum, frontal cortex, and amygdala.
Dopaminergic neurotransmission within the basal ganglia has been implicated in cognitive processes, and specifically, in associative learning (Alcaro et al., 2007). However, only a few studies have examined memory function after basal ganglia injury in rodent models. A previous study reported that MPTP-lesioned CD-1 mice had impaired social memory and recognition behavior (Dluzen and Kreutzberg, 1993). In our study, the STFP test revealed impaired associative memory in mice only at 30 days post-MPTP-lesion compared to saline-treated mice. At this time point, there was still an 86% depletion of dopamine and a 60% depletion of serotonin within the frontal cortex. This is consistent with other studies using 6-OHDA-lesioning in rats where performance in the STFP test depends on an intact frontal cortex (Ross et al., 2005) and basal forebrain (Berger-Sweeney et al., 2000). Taken together, these data support the importance of the frontal cortex and the role of dopamine and serotonin in the associative learning processes.
The fear conditioning response is mediated by the basolateral amygdala, hippocampus, medial prefrontal cortex, and nucleus accumbens (Davis and Whalen, 2001; Helmstetter, 1992; LeDoux, 2000; Maren and Quirk, 2004). The extinction of conditioned fear is a progressive decrease of the fear response generated by repeated presentation of the conditioned stimulus (tone) without any unconditioned stimulus (foot-shock). Changes in the extinction of conditioned fear can be influenced by either glutamate or dopamine neurotransmission particularly in the frontal cortex and amygdala (Falls et al., 1992; Guarraci et al., 1999; Ledgerwood et al., 2003; Walker et al., 2002). For example, dopamine D1 and D2 receptor antagonists targeting the amygdala can lead to potentiated extinction of fear response (Greba et al., 2001; Greba and Kokkinidis, 2000; Nader and LeDoux, 1999; Ponnusamy et al., 2005). In our study, MPTP-lesioning had no effect on the acquisition of the fear response; instead we observed increased fear extinction at both early and late time points after MPTP-lesioning. Interestingly, a recent study in patients with PD reported decrease of the startle response to aversive stimuli and this behavior was linked to dopamine dysfunction in the amygdala and frontal cortex (Bowers et al., 2006). Although dopamine loss in the amygdala did not reach statistical significance in our MPTP-lesioned mice, altered cortical input to the amygdala could be responsible for observed behavioral responses. It is possible that significant loss of dopamine in the frontal cortex in mice at 7 and 30 days post-MPTP could be involved in this process. It is know that GABAergic interneurons surrounding the basolateral amygdala receive cortical and mesolimbic dopaminergic input (Marowsky et al., 2005). Modulation of dopamine gate in these neurons may modulate stress-induced behavioral responses (Bowers et al., 2006; Marowsky et al., 2005). Dopamine loss in the frontal cortex, which we observed in MPTP-lesioned mice, could cause disinhibition of these interneurons and block the output of the amygdala in response to aversive stimuli, thus preventing the normal fear induced freezing response.
Dopamine, serotonin, and other neurotransmitter system perturbations are involved in anxiety disorders and may account for the clinical anxiety seen in more than 40% of patients with PD (Walsh and Bennett, 2001; Wood and Toth, 2001). In our study, using light-dark exploration and the hole-board tests we found no increase in anxiety at either 7 or 30 days post-MPTP-lesioning. These results are in agreement with others who also showed no difference in anxiety using the light-dark preference test 7 days after MPTP-lesioning in the mouse (Rousselet et al., 2003). Depression is the most common co-morbid disorder affecting up to 45% of patients with PD (Slaughter et al., 2001). We examined depression in our model using both the tail suspension and sucrose preference tests, since both may be influenced by serotonin dysfunction (Jones and Lucki, 2005; Lira et al., 2003; Mayorga et al., 2001; Steru et al., 1985). We did not observe increased depression in MPTP-lesioned mice using these tests. Interestingly, a recent study using the bilateral 6-OHDA-lesioned rat reported an increase in depression-like behavior that was measured using the forced swim test, with no changes in serotonin levels (Branchi et al., 2008). It is possible that the neurotoxicant lesioning paradigm, time after lesion, and species used could underlie differences in observed behaviors in these two animal models.
Lesioning of the dopaminergic system, using 6-OHDA or MPTP, also leads to perturbations of the serotonergic system. However, the extent and nature of these perturbations depends on a number of factors including animal age, toxin used, lesioning regimen, and lesion severity. For example, rats and mice lesioned with 6-OHDA in early postnatal life develop serotonergic hyper-innervation within the striatum and frontal cortex and elevated levels of serotonin (Avale et al., 2004; Berger et al., 1985; Snyder et al., 1986; Yamazoe et al., 2001). This is in contrast to lesioning in adults where serotonin levels remain unchanged (Branchi et al., 2008; Snyder et al., 1986; Stachowiak et al., 1986). On the other hand, MPTP-lesioning in adult animals causes a significant decrease of striatal and extra-striatal serotonin levels. For example, in the nonhuman primate, MPTP-lesioning causes decreased levels of serotonin in multiple brain regions including the caudate nucleus, putamen, nucleus accumbens, hypothalamus, and cerebral cortex (Frechilla et al., 2001; Perez-Otano et al., 1991; Pifl et al., 1991; Russ et al., 1991). In our studies, we found significant depletion of serotonin following MPTP-lesioning in the mouse, a similar effect reported by others (Rousselet et al., 2003) but not all (Sedelis et al., 2000). Despite serotonin depletion in brain regions important for affective behavior, our MPTP-lesioned mouse did not show significant changes in anxiety and depression. The lack of behavioral effect could be explained by (i) the level of serotonin depletion may not be severe enough to manifest elevated anxiety, or (ii) the affected neurotransmitter systems may compensate to overcome perturbation. The serotonergic system following MPTP-lesioning may compensate in an analogous fashion to that of the dopaminergic system where studies in our lab have shown recovery of dopamine function due to increased evoked release of dopamine and altered dopamine receptor expression (Petzinger et al., 2007). On the other hand, studies in PD patients have shown neuronal loss in the dorsal raphe nucleus which could cause mood disorders (Agid and Blin, 1987; Paulus and Jellinger, 1991; Scatton et al., 1983). However, these abnormalities in humans are thought to develop at later stages of the disease, following dysfunction of dopaminergic system (Dauer and Przedborski, 2003). With this in mind, there is a possibility that behavioral changes in our acute MPTP-lesioned mouse may develop at later time points after treatment. Future studies could be designed to examine different lesioning regimens (acute versus chronic), different time points after lesioning (early versus late), and could include pharmacological challenges to the serotonergic system to reveal dysfunction in this neurotransmitter system. In addition, our initial analysis of neurotransmitter changes involved tissue sections such as the ventral striatum that did not delineate potentially interesting anatomical sites such as the core and shell of the nucleus accumbens. Future studies using higher resolution techniques such as molecular imaging can be designed to examine altered innervation and synaptogenesis within these regions thought to influence non-motor behaviors.
The MPTP-lesioned mouse is commonly used as an animal model of PD. Specific motor functions such as skilled forepaw use, balance, and coordination have been consistently reported to be impaired following MPTP-lesioning (Meredith and Kang, 2006; Meredith et al., 2008; Rozas et al., 1998; Sedelis et al., 2000; Tillerson et al., 2002; Tillerson and Miller, 2003). The behavioral tests used in our study do not rely on any of these specific motor skills. For example, saline control and MPTP-lesioned mice showed similar levels of ambulatory activity in the hole-board and light-dark preference tests. We therefore conclude that MPTP-lesioned mice in the present study did not exhibit obvious impairment of spontaneous locomotor activity that could influence their performance in affective behavior tests used in these studies.
In conclusion, our data showed impairment in associative memory at 30 days and increased fear extinction at both 7 and 30 days post-MPTP-lesioning, but no significant increase in depression or anxiety. Impairments in memory and fear conditioning were accompanied by severe depletion in dopamine and serotonin levels in the amygdala, frontal cortex, and striatum. It is possible that the emergence of depression and anxiety in this mouse model depends upon greater serotonin loss in critical brain regions. The findings from this study suggest that mood disorders in patients with PD with may not develop before extensive damage in multiple brain regions occurs.
Acknowledgments
The authors would like to acknowledge the generous support of Team Parkinson, The Parkinson Alliance, and the Whittier PD Education Group. This study was funded by NINDS RO1 NS44327-1, NIDCD K18 DC009125, and U.S. Army NETRP (Grant # W81XWH-04-1-0444). We are thankful to Friends of the USC Parkinson’s Disease Research Group including George and MaryLou Boone and Walter and Susan Doniger for their generous support. Authors would like to thank Susan Geffen for help with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid Y, Blin J. Nerve cell death in degenerative diseases of the central nervous system: clinical aspects. Ciba Found Symp. 1987;126:3–29. doi: 10.1002/9780470513422.ch2. [DOI] [PubMed] [Google Scholar]
- Agid Y, Cervera P, Hirsch E, Javoy-Agid F, Lehericy S, Raisman R, Ruberg M. Biochemistry of Parkinson’s disease 28 years later: a critical review. Mov Disord. 1989;4(Suppl 1):S126–44. doi: 10.1002/mds.870040514. [DOI] [PubMed] [Google Scholar]
- Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, Nemirovsky SI, Raisman-Vozari R, Rubinstein M. Elevated serotonin is involved in hyperactivity but not in the paradoxical effect of amphetamine in mice neonatally lesioned with 6-hydroxydopamine. J Neurosci Res. 2004;78:289–96. doi: 10.1002/jnr.20245. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Stearns NA, Frick KM, Beard B, Baxter MG. Cholinergic basal forebrain is critical for social transmission of food preferences. Hippocampus. 2000;10:729–38. doi: 10.1002/1098-1063(2000)10:6<729::AID-HIPO1010>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Berger TW, Kaul S, Stricker EM, Zigmond MJ. Hyperinnervation of the striatum by dorsal raphe afferents after dopamine-depleting brain lesions in neonatal rats. Brain Res. 1985;336:354–8. doi: 10.1016/0006-8993(85)90667-5. [DOI] [PubMed] [Google Scholar]
- Boissier JR, Simon P. [The exploration reaction in the mouse. Preliminary note.] Therapie. 1962;17:1225–32. [PubMed] [Google Scholar]
- Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, Foote K, Okun M. Startling facts about emotion in Parkinson’s disease: blunted reactivity to aversive stimuli. Brain. 2006;129:3356–65. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Armida M, Cassano T, Pezzola A, Potenza RL, Morgese MG, Popoli P, Alleva E. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J Neurosci Res. 2008 doi: 10.1002/jnr.21642. [DOI] [PubMed] [Google Scholar]
- Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114:1546–9. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–18. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry. 1992;149:443–54. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Kreutzberg JD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) disrupts social memory/recognition processes in the male mouse. Brain Res. 1993;609:98–102. doi: 10.1016/0006-8993(93)90860-p. [DOI] [PubMed] [Google Scholar]
- do-Rego JC, Viana AF, Le Maitre E, Deniel A, Rates SM, Leroux-Nicollet I, Costentin J. Comparisons between anxiety tests for selection of anxious and non anxious mice. Behav Brain Res. 2006;169:282–8. doi: 10.1016/j.bbr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–63. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechilla D, Cobreros A, Saldise L, Moratalla R, Insausti R, Luquin M, Del Rio J. Serotonin 5-HT(1A) receptor expression is selectively enhanced in the striosomal compartment of chronic parkinsonian monkeys. Synapse. 2001;39:288–96. doi: 10.1002/1098-2396(20010315)39:4<288::AID-SYN1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Depression in Parkinson’s disease: a quantitative and qualitative analysis. J Neurol Neurosurg Psychiatry. 1986;49:381–9. doi: 10.1136/jnnp.49.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–26. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114:262–72. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Young SL, Kapp BS. A functional role for dopamine transmission in the amygdala during conditioned fear. Ann N Y Acad Sci. 1999;877:732–6. doi: 10.1111/j.1749-6632.1999.tb09312.x. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51:1271–6. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. [The tropical localization and content of noradrenalin and dopamine (3-hydroxytyramine) in the substantia nigra of normal persons and patients with Parkinson’s disease.] Wien Klin Wochenschr. 1963;75:309–12. [PubMed] [Google Scholar]
- Irwin I, Finnegan KT, Delanney LE, Di Monte D, Langston JW. The relationships between aging, monoamine oxidase, striatal dopamine and the effects of MPTP in C57BL/6 mice: a critical reassessment. Brain Res. 1992;572:224–31. doi: 10.1016/0006-8993(92)90473-m. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegen. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Donaldson DM, Barba J, Petzinger GM. Postnatal expression of alpha-synuclein protein in the rodent substantia nigra and striatum. Dev Neurosci. 2001;23:91–9. doi: 10.1159/000048700. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J Neurosci Res. 2004;76:539–50. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30:1039–47. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–76. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–9. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–71. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Peprah D, Gershenfeld HK. Tail-suspension induced hyperthermia: a new measure of stress reactivity. J Psychiatr Res. 2003;37:249–59. doi: 10.1016/s0022-3956(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Mann DM, Yates PO. Neurotransmitter deficits in Alzheimer’s disease and in other dementing disorders. Hum Neurobiol. 1986;5:147–58. [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–37. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–7. [PubMed] [Google Scholar]
- McCance-Katz EF, Marek KL, Price LH. Serotonergic dysfunction in depression associated with Parkinson’s disease. Neurology. 1992;42:1813–4. doi: 10.1212/wnl.42.9.1813. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson’s disease progression. Acta Neuropathol. 2008;115:385–98. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, LeDoux J. The dopaminergic modulation of fear: quinpirole impairs the recall of emotional memories in rats. Behav Neurosci. 1999;113:152–65. doi: 10.1037//0735-7044.113.1.152. [DOI] [PubMed] [Google Scholar]
- Passe DH, Walker JC. Odor psychophysics in vertebrates. Neurosci Biobehav Rev. 1985;9:431–67. doi: 10.1016/0149-7634(85)90021-1. [DOI] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–55. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Perez-Otano I, Herrero MT, Oset C, De Ceballos ML, Luquin MR, Obeso JA, Del Rio J. Extensive loss of brain dopamine and serotonin induced by chronic administration of MPTP in the marmoset. Brain Res. 1991;567:127–32. doi: 10.1016/0006-8993(91)91444-6. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Fisher B, Hogg E, Abernathy A, Arevalo P, Nixon K, Jakowec MW. Behavioral motor recovery in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned squirrel monkey (Saimiri sciureus): changes in striatal dopamine and expression of tyrosine hydroxylase and dopamine transporter proteins. J Neurosci Res. 2006;83:332–47. doi: 10.1002/jnr.20730. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Quik M, Ivashina E, Jakowec MW, Jakubiak M, Di Monte D, Langston JW. Reliability and validity of a new global dyskinesia rating scale in the MPTP-lesioned non-human primate. Mov Disord. 2001;16:202–7. doi: 10.1002/mds.1075. [DOI] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vuckovic M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Bonnet AM, Esteguy M, Guimaraes J, Vigouret JM, Lhermitte F, Agid Y. Cognitive slowing in Parkinson’s disease fails to respond to levodopa treatment: the 15-objects test. Neurology. 1989a;39:762–8. doi: 10.1212/wnl.39.6.762. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Cusimano G, Bonnet AM, Lhermitte F, Agid Y. Does cognitive impairment in Parkinson’s disease result from non-dopaminergic lesions? J Neurol Neurosurg Psychiatry. 1989b;52:201–6. doi: 10.1136/jnnp.52.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–74. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–11. [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ross RS, McGaughy J, Eichenbaum H. Acetylcholine in the orbitofrontal cortex is necessary for the acquisition of a socially transmitted food preference. Learn Mem. 2005;12:302–6. doi: 10.1101/lm.91605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC. Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis. 2003;14:218–28. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83:165–75. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- Russ H, Mihatsch W, Gerlach M, Riederer P, Przuntek H. Neurochemical and behavioural features induced by chronic low dose treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the common marmoset: implications for Parkinson’s disease? Neurosci Lett. 1991;123:115–8. doi: 10.1016/0304-3940(91)90171-o. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–8. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000;30:171–82. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:187–96. doi: 10.1176/jnp.13.2.187. [DOI] [PubMed] [Google Scholar]
- Snyder AM, Zigmond MJ, Lund RD. Sprouting of serotoninergic afferents into striatum after dopamine-depleting lesions in infant rats: a retrograde transport and immunocytochemical study. J Comp Neurol. 1986;245:274–81. doi: 10.1002/cne.902450209. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Stricker EM, Jacoby JH, Zigmond MJ. Increased tryptophan hydroxylase activity in serotonergic nerve terminals spared by 5,7-dihydroxytryptamine. Biochem Pharmacol. 1986;35:1241–8. doi: 10.1016/0006-2952(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–17. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J Neurosci Methods. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–51. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J. 2001;77:89–93. doi: 10.1136/pmj.77.904.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Toth M. Molecular pathways of anxiety revealed by knockout mice. Mol Neurobiol. 2001;23:101–19. doi: 10.1385/MN:23:2-3:101. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Wright JW, Harding JW. Recovery of olfactory function after bilateral bulbectomy. Science. 1982;216:322–4. doi: 10.1126/science.7063891. [DOI] [PubMed] [Google Scholar]
- Yamazoe I, Takeuchi Y, Matsushita H, Kawano H, Sawada T. Serotonergic heterotypic sprouting in the unilaterally dopamine-depleted mouse neostriatum. Dev Neurosci. 2001;23:78–83. doi: 10.1159/000048698. [DOI] [PubMed] [Google Scholar]