Abstract
Iron is essential to virtually all living organisms and is integral to multiple metabolic functions. The most important function is oxygen transport in hemoglobin. Iron deficiency anemia in dogs and cats is usually caused by chronic blood loss and can be discovered incidentally as animals may have adapted to the anemia. Severe iron deficiency is characterized by a microcytic, hypochromic, potentially severe anemia with a variable regenerative response. Iron metabolism and homeostasis will be reviewed, followed by a discussion of diagnostic testing and therapeutic recommendations for dogs and cats with iron deficiency anemia.
Résumé
Anémie ferriprive. Le fer est essentiel pour presque tous les organismes vivants et il fait partie intégrante de multiples fonctions métaboliques. La fonction la plus importante est le transport de l’oxygène dans l’hémoglobine. L’anémie ferriprive chez les chiens et les chats est habituellement causée par une perte de sang chronique et peut être découverte incidemment car les animaux peuvent s’être adaptés à l’anémie. Une carence en fer grave est caractérisée par une anémie microcytique, hypochromique et potentiellement grave avec une réponse régénérative variable. Le métabolisme du fer et l’homéostase seront examinés, suivis d’une analyse des épreuves diagnostiques et des recommandations thérapeutiques pour les chiens et les chats atteints d’anémie ferriprive.
(Traduit par Isabelle Vallières)
Introduction
Iron deficiency anemia in dogs and cats most commonly occurs secondary to chronic external blood loss and does not occur until tissue stores of iron have been depleted. Treatment consists of addressing the underlying syndrome causing blood loss and restoring iron stores.
Iron metabolism and homeostasis
Iron in the form of heme is vital to many metabolic functions including oxygen transportation in hemoglobin. Iron is also a component of multiple enzymes, including cytochromes, necessary for energy generation and drug metabolism (1). Through the donation or acceptance of an electron, iron exists in either a reduced ferrous (Fe2+) or an oxidative ferric (Fe3+) state. The majority of functional iron is contained in hemoglobin, with smaller quantities found in myoglobin and cytochromes (1,2). The liver, which is the site of production of iron transport proteins, contains the largest non-functional iron stores either as ferritin or hemosiderin (1,3). Ferritin is both diffuse and soluble, and is the primary iron storage protein (1,4). Hemosiderin is similar in structure, but has more iron relative to protein and is insoluble (3). Iron is also stored in reticuloendothelial cells of the bone marrow and spleen, but is not commonly stored in the bone marrow of cats (1–3).
Dietary iron is absorbed mainly in the duodenum (1,2). Only ferrous iron is absorbed, and it is transported across the apical membrane of the enterocyte by divalent metal transporter 1 (1,2). It is then transferred across the enterocyte to the basolateral membrane by an unknown mechanism (1,2). Iron is exported across the basolateral membrane of enterocytes by ferroportin, then bound to transferrin in the plasma and transported for use in target organs and/or storage (1,2,4).
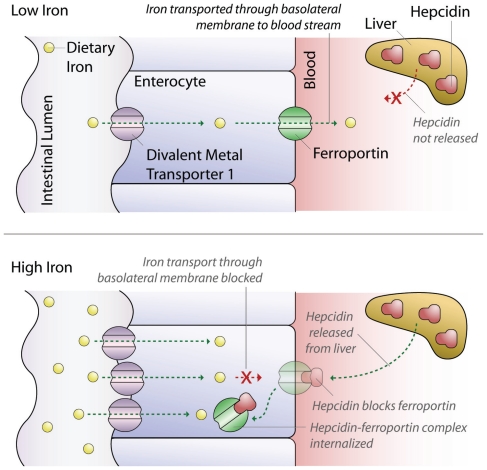
Body stores of iron are tightly regulated to provide adequate iron for cellular needs without developing toxicity from excess. Because the body lacks a mechanism to excrete excessive iron, homeostasis is tightly controlled by limiting enteric iron uptake through impaired efflux from enterocytes. Iron efflux is regulated by hepcidin, a recently discovered hormone produced by hepatocytes. When iron stores are adequate or high, hepcidin is released and binds to intestinal ferroportin causing internalization and destruction of ferroportin. The reduction in ferroportin causes absorbed dietary iron to remain in the enterocyte, where it is lost by enterocyte shedding. Conversely, when iron stores are low, hepcidin production and secretion are suppressed, increasing iron efflux from enterocytes into the blood (Figure 1) (1,2).
Figure 1.
Mechanism of intestinal iron absorption at low and high serum iron levels.
Tight homeostasis of iron is critical, as excessive iron accumulation in hepatocytes can cause pathologic damage, termed hemochromatosis (5). Subsequently, increased fibrosis and cirrhosis can occur (2). In contrast, iron deficiency leads to depletion of body iron stores, and ultimately, iron deficiency anemia and other metabolic dysfunctions. The duodenum’s ability to absorb dietary iron is very limited, but can be upregulated. However, the upregulation in iron absorption secondary to chronic blood loss and resulting iron deficiency may be insufficient to restore adequate iron homeostasis, even after blood loss has been arrested.
Causes of iron deficiency anemia
Iron deficiency results when either dietary intake does not meet the body’s requirement or when there is chronic external (non-resorptive) blood loss. The dietary iron requirement for adult dogs and cats is estimated at 80 mg/kg dry matter and is higher in puppies and kittens due to their rapid growth (6). Inadequate intake is rare except in nursing animals due to the low concentration of iron in milk (5). Inadequate dietary iron intake does not occur in dogs and cats fed commercial pet foods, but can rarely occur with home-cooked and vegetarian diets without appropriate iron supplementation (7,8). High iron content is found in meat products (such as liver, heart, and muscle), but also in brewer’s yeast, wheat germ, egg yolks, oysters, some dried beans, and some fruits. Green vegetables, cereals, fish, and fowl have an intermediate amount of iron. Foods low in iron include milk, milk products, and most non-green vegetables (3).
During acute blood loss, body iron stores are generally sufficient for accelerated erythropoiesis and subsequent iron uptake is adequate for restoring iron homeostasis. Iron deficiency anemia only develops over weeks to months of chronic or recurrent blood loss in both juvenile and adult animals (9). Causes of chronic external blood loss include ectoparasitism, endoparasitism, hematuria, epistaxis, hemorrhagic skin pathology, coagulopathy, thrombocytopenia, thrombocytopathia, and gastrointestinal hemorrhage (1,2,10,11). Gastrointestinal hemorrhage can result from primary gastrointestinal disease (benign or malignant neoplasm, ulceration, arteriovenous fistula), ulcerogenic drugs (most commonly non-steroidal anti-inflammatory agents and corticosteroids) or secondary to systemic diseases such as renal and hepatic diseases, bleeding disorders, and hypoadrenocorticism (11–16). Nursing animals are particularly prone to developing iron deficiency anemia due to lower body iron stores, larger requirements, and decreased intake from a milk-based diet (12,13). Surgical resection of the entire duodenum will result in iron malabsorption. Iron deficiency anemia can be induced iatrogenically through excessive phlebotomies of blood donor animals, as a regular blood donation of 450 mL removes approximately 200 mg of iron from the body (9). Finally, iron deficiency anemia can also be induced by repeated phlebotomies for diagnostic and monitoring purposes in smaller animals; the phlebotomy volume should not exceed 1% of the animal’s body weight per week (9).
Pathogenesis of iron deficiency anemia
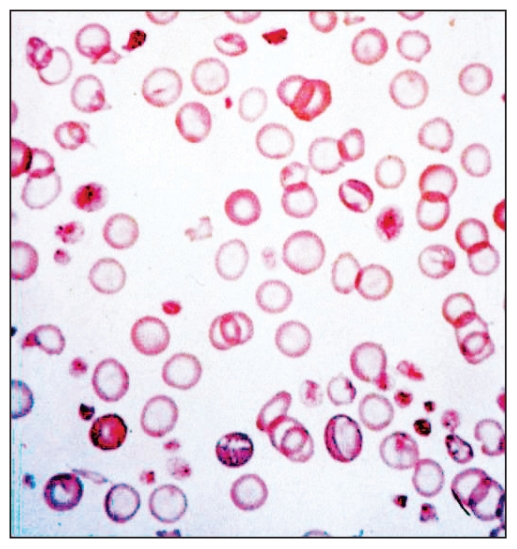
Iron deficiency anemia may be classified into 3 stages: storage iron deficiency, iron deficient erythropoiesis, and iron deficiency anemia (1,2). Initially during blood loss, iron body stores are preferentially utilized for accelerated erythropoiesis. After depletion of body iron stores, erythropoiesis and production of other iron-containing proteins (such as myoglobin) become limited, leading to an overt iron deficiency anemia (1,2). Anemia is exacerbated as iron-deficient erythrocytes have a shortened survival due to their fragility, which accelerates reticuloendothelial cell sequestration and destruction (17). The observed erythrocyte morphologic changes with the underlying iron deficiency reflect the severely hampered hemoglobin synthesis and are characterized by hypochromasia and microcytosis (12) (Figure 2). Furthermore, the hemoglobin-deficient erythroid precursors are thought to undergo additional mitoses while attempting to achieve ideal cytoplasmic hemoglobin levels, thereby exaggerating the microcytosis (12).
Figure 2.
Blood smear of a dog with severe iron deficiency.
While normocytic normochromic erythrocytes contain approximately 1/3 hemoglobin, red blood cell indices of animals with iron deficiency anemia demonstrate progressive decreases in mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and mean corpuscular volume (3,12). Early iron deficiency states may not be suspected as the anemia may be initially normocytic and normochromic. However, evaluation of the erythrogram and reticulocyte count, along with novel parameters such as reticulocyte hemoglobin content, may provide an earlier indication of iron deficiency anemia once these assays are available in canine and feline commercial laboratories (18). Initially, reticulocytosis is present due to increased production and release of reticulocytes secondary to anemia. However, as iron stores are depleted and the iron deficiency becomes more severe, the absolute reticulocyte count becomes inadequate for the degree of anemia (3,5). Furthermore, due to the lack of heme and reduced hemoglobin synthesis, the red blood cells become more fragile which can result in mild hemolysis, worsening the anemia.
Disease states with functional iron deficiency can occur when iron is not available for heme synthesis despite normal to increased body stores of iron (5). One example is anemia of inflammatory disease, which can be mistaken for iron deficiency anemia based on the hemogram. In this condition, serum iron levels are decreased secondary to iron sequestration in the liver, spleen, and bone marrow (1,2), which results in a functional iron deficiency, defective heme synthesis, and the formation of some microcytic and possibly hypochromic erythrocytes despite adequate body iron stores (2). Animals with chronic renal disease develop anemia, which is most commonly normocytic, normochromic, and non-regenerative (13). This anemia is mostly due to decreased renal erythropoietin synthesis, but chronic low-grade gastrointestinal hemorrhage with loss of iron and anemia of inflammatory disease can also contribute (11,13). Following treatment with recombinant human erythropoietin, the iron reserves can become limited and thus impair erythropoietin-induced erythropoiesis (19). Microcytosis, hypochromasia, and low serum iron concentrations have been reported in dogs with congenital portosystemic shunts but not other hepatic diseases (20). The pathogenesis of this apparent functional iron deficiency is not well understood, but thought likely to be a direct consequence of the portosystemic shunt as these features normalize following surgical intervention (9,20). Rare genetic defects in ferroportin and hepcidin regulation have been reported to cause iron refractory iron deficiency anemia in humans but these have not been reported in animals. However, a single currently unpublished case of iron refractory iron deficiency anemia in a cocker spaniel has recently been identified by the authors involving a defect in the hepcidin regulator, Tmprss6.
Physical examination findings
Clinical signs are variable and may be due to the underlying disease process, anemia, or both. However, since iron deficiency anemia develops gradually, many dogs and cats adapt and compensate for even the most severe anemia and do not demonstrate major clinical signs beyond pallor. Clinical signs solely due to anemia do not occur until anemia is severe, and can include lethargy, decreased exercise tolerance, weakness, weight loss, retarded growth, and generalized malaise (2). While these signs are typical for any anemia, the development of pica is unique to iron deficiency anemia (9). Clinical signs appear to arise from not only the anemia, but from a lack of other iron-containing proteins such as myoglobin, cytochrome c, and metabolic enzymes (5). Evidence of blood loss, such as melena, hematuria, or bleeding from other sites may be noted by the owners or at the time of the examination.
Physical examination results may be normal with the exception of pallor, or may reflect the underlying disease process. The presence of fleas, other ectoparasites, or hemorrhagic dermal pathology may be seen. If the anemia is severe, bounding pulses, arrhythmia, and a systolic heart murmur may be noted. Melena or hematochezia may be noted on examination of feces, on digital rectal examination, or on the thermometer but may only be visible intermittently. Animals are usually normovolemic to even slightly hypervolemic. While animals can develop a severe compensatory cardiomegaly to increase cardiac output, tachypnea and tachycardia are unusual with iron deficiency anemia (9).
Diagnostic approach to iron deficiency anemia
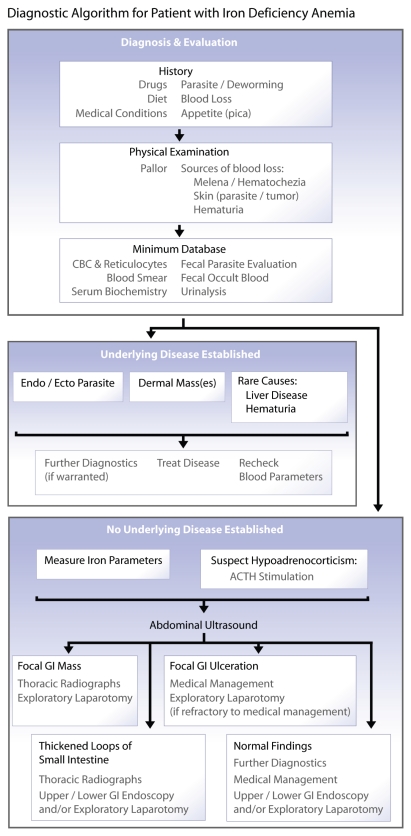
The diagnostic approach to iron deficiency anemia includes identifying the underlying disease or trigger with a thorough history, physical examination, and diagnostic evaluation (Figure 3). The history should include a thorough review of medications, diet, concurrent medical conditions, fecal characteristics, flea and tick exposure, and careful questioning of the owner for possible sources of blood loss.
Figure 3.
Diagnostic algorithm for a patient with iron deficiency anemia.
Minimum diagnostics, such as fecal examination for color and endoparasites, microscopic blood smear evaluation, packed cell volume, and total protein may be sufficient in juvenile anemic animals with suspected ectoparasite and/or endoparasite infestation. However, in many cases, further diagnostic evaluation is necessary, including complete blood (cell) count (CBC) with reticulocyte count, fecal occult blood test, serum iron parameters, coagulation parameters, biochemical profile (including albumin, globulins, and hepatic and renal parameters), urinalysis and abdominal imaging. Animals with chronic blood loss frequently have a marked reactive thrombocytosis which may exceed 1 × 106/μL; the mechanism causing the thrombocytosis is still undetermined (5,9). Furthermore, decreased neutrophil production due to iron deficiency may lead to neutropenia; the mechanism for this is also unknown (21).
If melena or hematochezia is not evident and no blood loss can be identified, fecal occult blood tests should be performed. Various commercial tests are available to detect occult blood in feces that may not be visible on visual inspection. However false positive results are possible with oral iron supplementation, meat diets, and much less likely with commercial animal diets due to the presence of dietary myoglobin, hemoglobin, or plant peroxidases (22,23). Withdrawal of iron supplementation and meat-based diet for 3 d may be required to accurately interpret results. Fecal occult blood test may also be negative if gastrointestinal hemorrhage occurs intermittently. Thus, repeated testing and color assessment by the owner are indicated in cases with elusive iron deficiency anemia. In some cases, the serum total protein values are also low due to the concomittent loss of plasma.
Iron status is further investigated by measuring serum iron parameters. Typically serum iron concentration is very low in animals with iron deficiency anemia. However, mildly low to low normal serum iron values can also be observed with anemia of inflammatory disease (Table 1) (2,12). Serum iron can be transiently elevated by intravascular red blood cell lysis, recent blood transfusion, and iron supplementation, which can complicate interpretation of laboratory data. Exogenously administered corticosteroids have also been shown to increase serum iron levels by an undetermined mechanism (24). The total iron binding capacity (TIBC) is a measure of the plasma’s ability to carry iron and represents the maximum concentration of iron that can be bound by plasma transferrin. This test is often performed as part of an iron panel, but is of limited clinical value in small animals, as it does not assess serum or tissue iron levels and does not change dramatically in disease. Iron saturation (IS) reflects the amount of iron bound to transferrin and is low (< 20%) in cases of iron deficiency anemia. Finally, the unsaturated iron binding capacity (UIBC) measures transferrin’s open iron binding sites and is elevated in iron deficiency anemia (24).
Table 1.
Expected serum parameters in iron deficiency anemia and anemia of inflammatory disease
| Iron deficiency anemia | Anemia of inflammatory disease | |
|---|---|---|
| Hematocrit | ↓ to ↓ ↓ ↓ | ↓ to ↓ ↓ |
| MCV | ↓ to ↓ ↓ ↓ | Normal to ↓ |
| MCHC | ↓ | Normal |
| Serum iron | ↓ to ↓ ↓ ↓ | Normal to ↓ ↓ |
| Serum TIBC | Normal to ↑ | Normal to ↓ |
| Serum ferritin | ↓ to ↓ ↓ | Normal to ↑ ↑ |
| Stainable iron in marrow | Absent | Normal to ↑ |
| Reticulocytes | Normal to ↓ | ↓ |
MCV — mean corpuscular volume, MCHC — mean corpuscular hemoglobin concentration, TIBC — total iron binding capacity.
Ferritin can be detected in serum and correlates well with body iron stores. However, the assay for ferritin is species- specific and therefore not widely available (26). Ferritin is decreased with iron deficiency anemia and is increased with elevated total body stores of iron (2,12). Ferritin is also an acute phase protein, and hyperferritinemia can occur with underlying disease, such as inflammatory disease, neoplasia, liver disease, or hemolytic disease (2,12). Nevertheless, low serum ferritin concentrations can be helpful in differentiating iron deficiency anemia from anemia of inflammatory disease (Table 1) (2).
Body iron stores can be qualitatively assessed by staining aspirates, impression smears, or biopsy sections of liver, spleen or bone marrow (or both) with Prussian blue stain. Iron concentration can be quantified in biopsied tissues such as liver and spleen (27). These methods are invasive and usually not performed in cases of known iron deficiency anemia. If iron is visualized in these samples, iron deficiency can be ruled out. It should, however, be noted that healthy, non-anemic cats with adequate iron homeostasis normally lack stainable iron in their bone marrow (2).
Diagnostic imaging may be warranted to further investigate iron deficiency anemia. Abdominal ultrasonography is recommended to visualize abdominal organs and to assess the gastrointestinal tract for evidence of ulceration, wall thickening, or masses. Examples of typical gastrointestinal tumors that can cause ulceration and chronic blood loss include leiomyoma, leiomyosarcoma, carcinoma, and round cell tumors. If primary gastrointestinal disease is suspected, and no abnormalities are noted with abdominal imaging, gastroduodenal or colonic endoscopy (or both), or exploratory laparotomy may be indicated to assess for ulceration and to obtain biopsies (3).
Therapeutic approach to iron deficiency anemia
The general principles of treating animals with iron deficiency anemia include preventing further blood loss, correcting the anemia if severe, initiating iron supplementation, and addressing the underlying disease. Animals with severe anemia may not show significant clinical signs but may rapidly decompensate shortly after presentation in the clinic, thus they should be handled carefully.
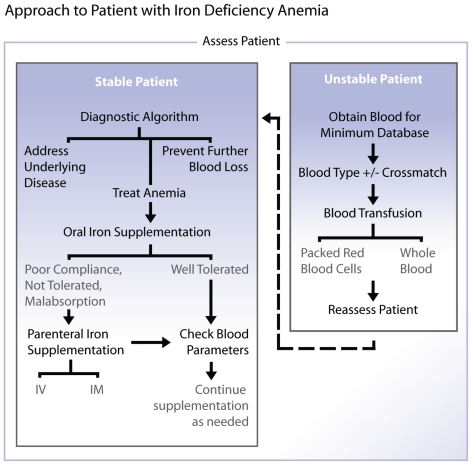
A blood transfusion may be necessary prior to receiving results from diagnostic evaluation if the animal is severely anemic and demonstrating signs of hypoxemia (Figure 4). Blood samples for CBC, and ideally serum biochemical profile, coagulation profile, and iron parameters should be obtained prior to the administration of a blood transfusion. Ideally stored compatible packed red blood cell products are slowly administered but fresh or stored whole blood may also be slowly transfused. Fluid overload secondary to rapid or overzealous blood transfusion or intravenous fluid administration can readily occur in these animals as they are usually normovolemic or even hypervolemic (28). This is in contrast to patients with acute to peracute blood loss anemia, where fluid resuscitation is appropriate.
Figure 4.
Diagnostic approach to a patient with iron deficiency anemia.
Transfusion with red blood cell products addresses the anemia and provides an excellent source of bioavailable iron. There is no specific transfusion trigger and in comparison to many other causes of anemia, iron deficient animals tolerate even severe anemia well unless challenged or stressed. Thus, blood transfusions are only indicated in cases of severe anemia with resulting tissue hypoxia, to stabilize a decompensated patient, or prior to performing general anesthesia and surgery on an animal with moderate to severe anemia. The amount of packed red blood cells transfused may be calculated as:
with an approximate target packed cell volume of 20%. Blood transfusions bear inherent risks including acute hemolytic and hypersensitivity reactions, hemolysis, infectious disease transmission, and volume overload (29). Prior to a first transfusion, dog erythrocyte antigen (DEA) 1.1 or AB blood typing should be performed in dogs and cats, respectively, and appropriate blood type compatible donors should be selected. If the animal has previously been transfused, a major and minor blood cross-match test should also be performed to assure blood compatibility. There are no specific guidelines that indicate when a transfusion is appropriate; clinical and laboratory assessments of the patient are used to make that decision.
Iron supplementation is generally needed to restore iron homeostasis and should be based on the degree of anemia, underlying pathology, red blood cell count, serum iron panel, and erythrocyte morphology. These same parameters are used to monitor further iron supplementation needs. Supplemental iron is beneficial in treating iron deficiency anemia but is not recommended for other forms of anemia and in fact may be harmful, as iron overload may occur (9). Iron can be delivered to animals through supplemental iron products administered either orally or parenterally, including blood transfusions.
In stable patients, oral iron therapy is usually preferred over parenteral iron administration in small animals due to its low cost and higher safety (9). Both ferrous and ferric forms are available but only the ferrous form is recommended due to superior absorption (9). Ferrous sulfate is used most frequently but ferrous gluconate and fumarate can also be used (30). Care must be taken when determining the dosage to be administered, as published doses are expressed as either milligrams of iron salt or elemental iron (30). Ferrous sulfate can be administered at a dose of 15 mg iron salt per kg body weight (5 mg elemental iron per kg) divided every 8 to 12 h (3). One of the more common side effects of oral iron supplemenation is gastrointestinal irritation, which can be minimized by dividing the dose several times a day. Interaction with other drugs is recognized and should be avoided (9,30). For instance iron can bind tetracycline and other drugs thereby decreasing the efficacy of both; these and other drugs should be administered several hours apart if given concurrently (30). Moreover, the bioavailability of iron is decreased if administered concurrently with antacids, eggs, or milk (30).
Parenteral forms of iron other than red blood cell products can be administered if oral supplementation causes side effects, is ineffective due to malabsorption, if the animal is vomiting, or if compliance is poor (9). A single dose of iron can also be administered parenterally prior to initiation of oral supplementation. Iron dextran is absorbed primarily by the lymphatic system following intramuscular administration, and approximately 70% of the iron is absorbed from the injection site within days (9). Iron dextran can be given at a dose of 10 mg elemental iron per kg body weight weekly to dogs, and at a dose of 50 mg per cat once every 3 to 4 wk (3,30). A small dose should be administered first as hypersensitivity reactions can occur (9). Other side effects of intramuscular iron include irritation and pain at the injection site (30,31).
Intravenous iron administration in dogs and cats is rarely performed, but is more common in humans. Several intravenous iron preparations are available in humans, including iron gluconate, iron sucrose, iron dextran, and ferric carboxymaltose; iron sucrose is considered the safest of the preparations (32). In humans, an initial test dose is given; if no adverse effects are noted, the remainder of the dose is administered over several hours. Adverse reactions from rapid infusion can include hypotension, tachycardia, dyspnea, and phlebitis (32). Currently, there is no reported dose of intravenous iron for dogs and cats; however, a weight-proportional dose similar to that used in humans (approximately 10 mg elemental iron per kg body weight) approximately once every 3 wk will likely be effective and safe based on anecdotal unpublished accounts.
Several months of iron supplementation may be necessary for red blood cell parameters to return to normal, and therapy should be continued beyond normalization of red blood cell parameters as body iron stores take much longer to be replenished (13). While serum iron would be expected to be normal or even high when being actively supplemented with iron, red blood cell indices should be monitored closely to gauge response to therapy and resolution of functional iron deficiency. Body iron stores are rarely assessed post-treatment, but measurement of serum ferritin and other iron parameters is warranted after termination of iron supplementation to ensure normalization.
In conclusion, iron is a vital element for multiple metabolic functions, most notably oxygen transport by hemoglobin. Iron deficiency anemia typically develops following chronic blood loss after iron body stores have been exhausted. Iron deficiency anemia is characterized by microcytosis and hypochromasia with inadequate regeneration, and low serum iron, iron saturation, and ferritin. If iron deficiency is not resolved, animals will often progress to a severe anemia, which is surprisingly well-tolerated unless the animal is stressed. Samples for diagnostic testing should be obtained prior to treatment. Therapy for iron deficiency anemia includes preventing further blood loss, oral and/or parenteral iron supplementation, and treating the underlying disease. With appropriate therapy, patients with iron deficiency anemia can have a good prognosis as long as the underlying disease can be addressed.
Acknowledgment
The authors thank Dr. Hans Christoffersen for the graphic art provided for this manuscript. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Crichton R. Iron Metabolism: From Molecular Mechanisms to Clinical Consequences. 3rd ed. 17–56. West Sussex, UK: John Wiley and Sons; 2009. pp. 141–325. [Google Scholar]
- 2.Harvey JW. Iron metabolism and its disorders. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical Biochemistry of Domestic Animals. 6th ed. Burlington, Massachusetts: Elsevier; 2008. pp. 259–285. [Google Scholar]
- 3.Harvey JW. Microcytic anemia. In: Feldman BF, Zinkl JG, Jain MC, editors. Schalm’s Veterinary Hematology. 5th ed. Philadephia, Pennsylvania: Lippincott, Williams and Wilkins; 2000. pp. 200–204. [Google Scholar]
- 4.Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss DJ. Iron and copper deficiencies and disorders of iron metabolism. In: Weiss DJ, Wardrop KJ, editors. Schalm’s Veterinary Hematology. 6th ed. Ames: Blackwell Publishing; 2010. pp. 167–171. [Google Scholar]
- 6.Dzanis DA. The Association of American Feed Control Officials dog and cat food nutrient profiles: Substantiation of nutritional adequacy of complete and balanced pet foods in the United States. J Nutr. 1994;124(12 Suppl):2535S–2539S. doi: 10.1093/jn/124.suppl_12.2535S. [DOI] [PubMed] [Google Scholar]
- 7.Gross Kl, Wedekind KJ, Cowell CS, et al. Nutrients. In: Hand MS, Thatcher CD, Remillard RL, et al., editors. Small Animal Clinical Nutrition. 4th ed. Marceline, Missouri: Walsworth Publishing Company; 2000. pp. 75–77. [Google Scholar]
- 8.Michel KE. Unconventional diets for dogs and cats. Vet Clin North Am Sm Anim Practice. 2006;36:1269–1281. doi: 10.1016/j.cvsm.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Giger U. Regenerative anemias caused by blood loss or hemolysis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, Missouri: Elsevier Saunders; 2005. pp. 1886–1908. [Google Scholar]
- 10.Chulilla JAM, Colas MSR, Martin MG. Classification of anemia for gastroenterologists. World J Gastroenterol. 2009;15:4627–4637. doi: 10.3748/wjg.15.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White C, Reine N. Feline nonregenerative anemia: Pathophysiology and etiologies. Compend Contin Educ Vet. 2009;31:E1–E19. [PubMed] [Google Scholar]
- 12.Stockholm SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2002. pp. 105–150. [Google Scholar]
- 13.Abrams-Ogg T. Nonregenerative anemia. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 788–797. [Google Scholar]
- 14.Waldrop JE, Rozanski EA, Freeman LM, et al. Packed red blood cell transfusions in dogs with gastrointestinal hemorrhage: 55 cases (1999–2001) J Anim Hosp Assoc. 2003;39:523–527. doi: 10.5326/0390523. [DOI] [PubMed] [Google Scholar]
- 15.Klein SC, Peterson ME. Canine hypoadrenocorticism: Part I. Can Vet J. 2010;51:63–69. [PMC free article] [PubMed] [Google Scholar]
- 16.Gelens HC, Moreau RE, Stalis IH, et al. Arteriovenous fistula of the jejunum associated with gastrointestinal hemorrhage in a dog. J Am Vet Med Assoc. 1993;202:1867–8. [PubMed] [Google Scholar]
- 17.Anderson C, Aronson I, Jacobs P. Erythropoiesis: Erythrocyte deformability is reduced and fragility increased by iron deficiency. Hematol. 2000;4:457–460. [PubMed] [Google Scholar]
- 18.Moritz A, Becker M. Automated hematology systems. In: Weiss DJ, Wardrop KJ, editors. Schalm’s Veterinary Hematology. 6th ed. Ames, Iowa: Blackwell; Publ: 2010. pp. 1054–1066. [Google Scholar]
- 19.Polzin DJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 1990–2021. [Google Scholar]
- 20.Simpson KW, Meyer DJ, Boswood A, et al. Iron status and erythrocyte volume in dogs with congenital portosystemic vascular anomalities. J Vet Intern Med. 1997;11:14–19. doi: 10.1111/j.1939-1676.1997.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Lima CS, Paula EV, Takahasi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol. 2006;85:705–709. doi: 10.1007/s00277-006-0150-0. [DOI] [PubMed] [Google Scholar]
- 22.Cook AK, Gilson SD, Fischer WD, Kass PH. Effect of diet on results obtained by use of two commercial test kits for detection of occult blood in feces of dogs. Am J Vet Res. 1992;53:1749–1751. [PubMed] [Google Scholar]
- 23.Duncan JR, Prasse KW, Mahaffey EA. Veterinary Laboratory Medicine Clinical Pathology. 3rd ed. Ames, Iowa: Iowa State Univ; Press: 1994. p. 160. [Google Scholar]
- 24.Harvey JW, French TW, Meyer DJ. Chronic iron deficiency anemia in dogs. J Am Anim Hosp Assoc. 1982;18:946–960. [Google Scholar]
- 25.Harvey JW, Levin DE, Chen CL. Potential effects of glucocorticoids on serum iron concentration in dogs. Vet Clin Path. 1987;16:46–50. doi: 10.1111/j.1939-165x.1987.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 26.Weeks BR, Smith JE, Phillips RM. Enzyme-linked immunosorbent assay for canine serum ferritin, using monoclonal anti-canine ferritin immunoglobulin G. Am J Vet Res. 1988;49:1193–1195. [PubMed] [Google Scholar]
- 27.Schultheiss PC, Bedwell CL, Hamar DW, et al. Canine liver iron, copper and zinc concentrations and association with histologic lesions. J Vet Diagn Invest. 2002;14:396–402. doi: 10.1177/104063870201400506. [DOI] [PubMed] [Google Scholar]
- 28.Yaphe W, Giovengo S, Moise NS. Severe cardiomegaly secondary to anemia in a kitten. J Am Vet Med Assoc. 1993;202:961–964. [PubMed] [Google Scholar]
- 29.Prittie JE. Triggers for use, optimal dosing, and problems associated with red cell transfusions. Vet Clin North Am Sm Anim Practice. 2003;33:1261–1275. doi: 10.1016/s0195-5616(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 30.Plumb DC. Plumb’s Veterinary Drug Handbook. 6th ed. Ames, Iowa: Blackwell Publ; 2008. pp. 329–331.pp. 424–425. [Google Scholar]
- 31.Hayat A. Safety issues with intravenous iron products in the management of anemia in chronic kidney disease. Clin Med Res. 2008;6:93–102. doi: 10.3121/cmr.2008.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz M, Gomez-Ramirez S, Barcia-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15:4666–4674. doi: 10.3748/wjg.15.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]