Abstract
Four cases of Columbid herpesvirus-1 infection in great horned owls (Bubo virginianus) were identified in Calgary, Alberta. Necropsy findings included severe multifocal hepatic and splenic necrosis, pharyngeal ulceration and necrosis, and gastrointestinal necrosis. Occasional eosinophilic intranuclear viral inclusion bodies were associated with the foci of necrosis and polymerase chain reaction (PCR) testing confirmed a diagnosis of herpesvirus-induced disease. The sequence of a PCR amplicon had 99.7% homology to Columbid herpesvirus-1.
Résumé
Mortalité causée par le Columbid herpesvirus-1 chez les grands-ducs d’Amérique (Bubo virginianus) de Calgary, en Alberta. Quatre cas d’infection par le Columbid herpesvirus-1 chez les grands-ducs d’Amérique (Bubo virginianus) ont été identifiés à Calgary, en Alberta. Les constatations à la nécropsie incluaient une nécrose hépatique et splénique multifocale grave, une ulcération et une nécrose pharyngées et une nécrose gastro-intestinale. Des corps d’inclusion virale intranucléaires éosinophiles occasionnels ont été associés aux foyers de nécrose et des tests de réaction en chaîne par la polymérase (PCR) ont confirmé un diagnostic de maladie induite par le virus herpétique. La séquence d’un amplicon de PCR avait une homologie de 99,7 % avec Columbid herpesvirus-1.
(Traduit par Isabelle Vallières)
The International Union for the Conservation of Nature (IUCN) has reported the extinction of 833 animal species over the past 500 years, only 3.7% of which are attributed primarily to infectious disease (1). Today, infectious disease is recognized as an important factor influencing the health status of wildlife populations. Examples include white-nose disease in North American bats (2), chytridiomycosis in amphibian species (3), and morbillivirus in Baikal seals (Phoca sibirica) (4). In addition, pathogens may be able to accelerate the decline of a wildlife population when working with other driving factors including climate change, invasive species, and environmental pollution (1). Therefore, understanding infectious disease in wildlife species has become an important aspect of understanding the overall health status of a given wildlife population.
The great horned owl (Bubo virginianus) (GHO) is one of the most common large birds of prey found in North America, yet, little is known about the major causes of morbidity and mortality in this species (5). One study involved postmortem examinations on 132 GHO to determine the cause of death, and concluded that the majority of mortalities were attributed to human-related trauma. The second most common cause of mortality was toxicity from exposure to environmental toxins such as chlorinated hydrocarbons and organophosphates. Only 6 owls (4%) in this study died as a result of infectious disease and only 2 of these deaths were diagnosed as the result of a herpesvirus infection (6).
Herpesvirus in owls was first reported in North America in 1932 (7). Since then herpesvirus has been reported in various species of birds of prey including the prairie falcon (Falco mexicanus), the American kestrel (Falco sparverius) and the peregrine falcon (Falco peregrinus) (8–11). In 1996, the Canadian Cooperative Wildlife Health Centre diagnosed 3 cases of herpesvirus infection in owl species from the Toronto area (12). Although the disease and the lesions have been well-described (9–12), very little is known about the prevalence of this virus in the North American GHO population and whether or not it is the same species of herpesvirus involved in all cases of GHO herpesvirus infections.
More recent research has revealed a specific herpesvirus species, Columbid herpesvirus-1 (CoHV-1), that has killed a number of raptors (13). Sequencing has shown that the strain is identical in pigeons, owls, falcons, and hawks (13). Since the discovery of CoHV-1, DNA sequencing has been performed in more recent studies and the strain CoHV-1 has been confirmed in 2 Cooper’s hawks (Accipiter cooperii) in North America and 3 species of Australian birds (14,15).
Because of the costs associated with diagnostic testing and the need for access to pathology expertise, few wildlife rehabilitaion centers perform regular postmortem examinations and thus diseases go undiagnosed. A summer research project involving postmortem examination of wild birds from the Calgary area revealed herpesvirus infection in 4 GHOs. Herpesvirus infection was confirmed by polymerase chain reaction (PCR), and DNA sequencing showed 99.7% homology to CoHV-1. This paper summarizes the findings of this outbreak and examines the potential for long-term surveillance at local rehabilitation centres.
Case descriptions
During the summer of 2010, and as part of a larger postmortem surveillance project involving 62 birds, 16 GHO and 20 rock pigeons (Columba livia) were necropsied. No lesions were noted in the rock pigeons; however, 4 GHO had gross and histopathologic lesions consistent with herpesvirus infection. All owls were presented dead to the Calgary Wildlife Rehabilitation Society (CWRS) and later referred to the University of Calgary Faculty of Veterinary Medicine (UCVM) Diagnostic Services Unit. The owls were kept frozen until they were thawed 2 d prior to the necropsy. A complete gross necropsy examination was performed, with samples of the heart, liver, lung, kidney, spleen, pectoral muscle, thyroid glands, proventriculus, ventriculus, duodenum with pancreas, ileum, jejunum, the large colon with the paired ceca, cloaca (including the bursa of fabricius) and the brain collected from each owl and fixed in 10% neutral-buffered formalin for a minimum of 2 d. Separate samples of liver, lung, heart, kidney, spleen, and bursa of fabricius were frozen in a −80°C freezer for virology and toxicology testing, as necessary. Formalin-fixed tissues were processed routinely for light microscopic examination, sectioned at 4 to 5 μm and stained with hematoxylin and eosin (H & E).
Case 1
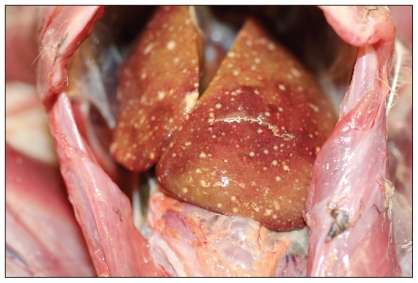
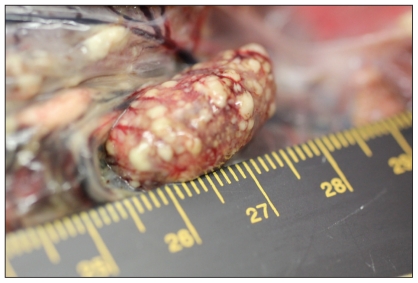
Case 1 was an adult male GHO found dead in Edworthy Park in northwest Calgary on May 15, 2010. The owl was in thin body condition with minimal food in the gastrointestinal tract. Both the liver and the spleen were peppered with hundreds of pinpoint white foci on the surface and throughout the parenchyma (Figures 1 and 2). On histopathologic examination, approximately 40% of the liver had multifocal to coalescing areas of coagulative necrosis, randomly scattered throughout the parenchyma. The inflammatory response surrounding foci of necrosis was minimal with a few macrophages present. In occasional hepatocytes bordering areas of necrosis, there were eosinophilic intranuclear viral inclusion bodies and chromatin margination. The spleen had similar areas of coagulative necrosis and eosinophilic intranuclear inclusion bodies present in surrounding splenic cells.
Figure 1.
Necrotizing hepatitis in an adult female great horned owl. Small slightly raised multifocal areas of necrosis are scattered throughout the hepatic surface.
Figure 2.
Necrotizing splenitis in a juvenile male great horned owl. Slightly elevated, variably sized areas of pale discoloration represent foci of necrosis.
Case 2
Case 2 was an adult male GHO found dead in the same park on May 20, 2010. The bird was emaciated and mildly dehydrated. Gross lesions in the liver and spleen were similar to those in case 1. On histopathology, both the spleen and liver had multifocal areas of necrosis with approximately 90% of the tissue affected. Eosinophilic intranuclear viral inclusion bodies were observed in both organs within cells adjacent to necrotic foci. The thyroid gland contained 1 well-demarcated area of coagulative necrosis and eosinophilic intranuclear inclusion bodies in bordering intact cells. The parathyroid also had a focal area of coagulative necrosis but no intranuclear inclusion bodies were detected in this lesion.
Cases 3 and 4
Case 3, an adult female, and case 4, a fledgling male GHO were found dead side-by-side on May 26, 2010 at the Veterinary Science Research Station in northwest Calgary, and were presumed to be related. These birds were in adequate body condition. The liver, spleen, and pharynx showed similar gross lesions to those in case 1. In addition, case 3 (adult) had a pale tan nodule approximately 2 mm in diameter within the lung parenchyma. Similar to case 1, necrotizing splenitis, hepatitis, and pharyngitis with intralesional, intranuclear, eosinophilic viral inclusion bodies were noted microscopically in both cases (Figure 3). The small focal lesion observed in the lungs of case 3 represented a focal area of granulomatous inflammation, necrosis, and vascular thrombosis with abundant fungal hyphae and occasional conidiophores (morphology consistent with Aspergillus spp.). Foci of coagulative necrosis containing occasional intranuclear viral inclusion bodies, similar to those in the spleen and liver, were observed in the lungs and in the wall of the large intestine of the young fledgling male (case 4).
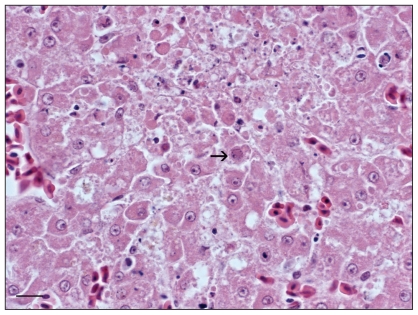
Figure 3.
Focus of hepatocellular necrosis containing eosinophilic intranuclear inclusion bodies (arrow) within hepatocytes (60× magnification). H & E stain.
Frozen liver or spleen samples (or both) from the 4 cases were sent to the Animal Health Centre, Abbottsford, British Columbia. Nested PCR was performed using the protocol published by VanDevanter et al (16), using the published primers to target herpesvirus gene: forward primer DFA (+) (5′-GAY TTY GCN AGY YTN TAY CC-3′), forward primer ILK (+) (5′-TCC TGG ACA AGC AGC ARN YSG CNM TNA A-3′), and reverse primer KG1 (−) (5′-GTC TTG CTC ACC AGN TCN ACN CCY TT-3′), and nested primers TGV (+) (5′-TGT AAC TCG GTG TAY GGN TTY ACN GGN GT-3′) and IYG (−) (5′-CAC AGA GTC CGT RTC NCC RTA DAT-3′. The DNA sequence determined for 1 amplicon had 99.7% homology to the published CoHV-1 sequence.
In order to gauge possible changes in the health status of the GHO population in the Calgary area, the number admitted to the Calgary Wildlife Rehabilitation Society during the summer months (May–July) for the last 3 y were compared. The numbers of GHO admitted to the rehabilitation were 7, 10, and 20 in 2008, 2009, and 2010, respectively. In addition, the number of GHO presented dead to the center increased from zero in 2008 and 2009 to 5 in 2010.
Discussion
Herpesvirus infection in birds of prey is caused by an alpha-herpesvirus (17). Initially the virus was given its own name in birds of prey such as Strigid herpesvirus-1 in owls and Falconid herpesvirus-1 in falcons (18,19). However recent research showed that certain birds of prey died from infection with Columbid herpesvirus-1 (CoHV-1), which is believed to be transmitted through the consumption of infected pigeons (13). Herpesvirus is prevalent in the pigeon population and has little to no effect on the health of this species (13). The genome of the virus in owls is indistinguishable from that of CoHV-1. Pigeons, especially those kept in captivity, are common subclinical carriers of the virus (13,17). Some species of birds of prey are very susceptible to the virus and become extremely weak, anorexic, depressed, and die within 7 to 12 d post-infection (20). In our study, the herpesviral DNA had 99.7% homology to CoHV-1. In owls, falcons, and eagles, the disease is known as hepatosplenitis since the classical and most consistent lesions in these birds include multifocal necrosis of the spleen and liver (17,20). The disease may be characterized by an acute or per-acute course, and necrotizing lesions in these cases may induce little or no concomitant inflammatory response (20). Intranuclear inclusion bodies, an important diagnostic feature of the alpha herpesvirus infection, can be observed in parenchymal or inflammatory cells bordering foci of necrosis (20).
Evaluation of population data (passive surveillance) of GHO in the greater Calgary area revealed 2 peaks of mortality, in 1999 and 2010. In both these events, there was evidence of herpesvirus infection. In 1999, herpesvirus was diagnosed by necropsy and histopathology, as a cause of mortality in GHO by Dr. Sandie Black from the Calgary Zoo. In March of that year, 3 wild GHO were found dead on zoo property. On postmortem examination, all 3 owls had multifocal areas of hepatic and splenic necrosis (Dr. Sandie Black, Head Veterinarian, Calgary Zoo, Calgary, Alberta, personal communication, June, 2010). Low numbers of eosinophilic intranuclear inclusion bodies were seen within the hepatic parenchyma of 1 owl, consistent with an alphaherpesvirus infection, possibly CoHV-1.
Peaks of mortality due to CoHV-1 infection in GHO have several plausible explanations. Since the virus is acquired from the rock pigeon, it is likely that circumstances that force owls to consume more pigeons would correlate with increased incidence of CoHV-1 infection in owls (13). When food is scarce, particularly in late spring, GHO are more likely to hunt pigeons. During these times of food scarcity, the number of GHO infected with Trichomonas gallinae, a protozoan parasite harbored by pigeons, is elevated (21). It is possible that similar environmental circumstances could explain CoHV-1 infection in these GHO. Increases in the pigeon population may also increase the likelihood of pigeons transmitting CoHV-1 to one another and the likelihood of an owl acquiring the disease. The rock pigeons necropsied as part of the larger scale project had no lesions consistent with a herpesvirus infection. CoHV-1 infection in pigeons is usually subclinical and unlikely to manifest in gross or histopathologic lesions, hence an increase in herpesvirus infection within the pigeon population may go unnoticed. We did not perform PCR for alpha-herpesvirus on pigeon tissue; however, this may be an avenue to explore in future outbreaks.
Examination of birds that die at the CWRS or other rehabilitation centers throughout Canada can contribute to better understanding of the dynamics of herpesvirus infection in owls and of other infectious diseases affecting a wide range of wild birds.
Acknowledgments
The authors acknowledge the pathology staff at the UCVM. Specifically, we thank Jim Carlsen, Murray Cumming, Maureen Bukhari, Mel Nicolas, and Elizabeth Pollock. We thank the staff at the CWRS for keeping records and bird samples for the project and Adrien Daniel who acted as a contact throughout the project. Finally we thank Morris Animal Foundation, the Canadian Cooperative for Wildlife Health, and the UCVM Summer Undergraduate Research Experience program for funding this project. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
The study was supported by Morris Animal Foundation, Canadian Cooperative Wildlife Health Centre, and a University of Calgary Studentship.
References
- 1.Smith K, Acevedo-Whitehouse K, Pedersen A. The role if infectious diseases in biological conservation. Animal Conserv. 2009;12:1–12. [Google Scholar]
- 2.Frick W, Pollock J, Hicks A, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- 3.Schloegel L, Hero J, Speare R, McDonal K, Daszak P. The decline of the sharp-snouted day frog (Taydactylus acutirostris): The first documented case of extinction by infection in a free-ranging wildlife species? Ecohealth. 2006;3:35–40. [Google Scholar]
- 4.Osterhaus A, Groen J, UytdeHaag F, et al. Distemper virus in Baikal seals. 1989;338:209–210. doi: 10.1038/338209a0. [DOI] [PubMed] [Google Scholar]
- 5.Hinterland’s who’s who [homepage on the Internet] Environment Canada and Canadian Wildlife Federation; c2011. [Last accessed January 24, 2012]. Available from http://www.hww.ca. [Google Scholar]
- 6.Franson J, Little E. Diagnostic findings in 132 Great horned owls. J Raptor Res. 1996;30:1–6. [Google Scholar]
- 7.Errington P. Great horned owls dying in the wild from diseases. Wilson Bill. 1932;44:180. [Google Scholar]
- 8.Sileo L, Carlson C, Crumley S. Inclusion body disease in a great horned owl. J Wildl Dis. 1975;11:92–96. doi: 10.7589/0090-3558-11.1.92. [DOI] [PubMed] [Google Scholar]
- 9.Ward F, Fairchild D, Vuicich J. Inclusion body hepatitis in prairie falcons. J Wildl Dis. 1971;7:120–124. doi: 10.7589/0090-3558-7.2.120. [DOI] [PubMed] [Google Scholar]
- 10.Potgieter LN, Kocan AA, Kocan KM. Isolation of herpesvirus from an American Kestrel. J Wildl Dis. 1979;15:143–149. doi: 10.7589/0090-3558-15.1.143. [DOI] [PubMed] [Google Scholar]
- 11.Mozos E, Hervas J, Moyano T, Diaz J, Gomez-Villamandos J. Inclusion body disease in a peregrine falcon (Falco peregrinus): Histological and ultrastructural study. Avian Pathol. 1994;23:175–181. doi: 10.1080/03079459408418986. [DOI] [PubMed] [Google Scholar]
- 12.Canadian Cooperative Wildlife Health [homepage on the Internet] Newsletter. c2011. 4-2. Winter. 1996. [Last accessed January 24, 2012]. Available from http://www.ccwhc.ca/newsletters/newsletter4-2.pdf.
- 13.Gailbreath K, Oaks L. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (Columbid herpesvirus 1) J Wildlife Dis. 2008;44:427–433. doi: 10.7589/0090-3558-44.2.427. [DOI] [PubMed] [Google Scholar]
- 14.Pinkerton M, Wellehan J, Johnson A, Childress A, Fitzgerald S, Kinsel M. Columbid herpesvirus-1 in two Cooper’s hawks (Accipiter cooperii) with fatal inclusion body disease. J Wildlife Dis. 2008;44:622–628. doi: 10.7589/0090-3558-44.3.622. [DOI] [PubMed] [Google Scholar]
- 15.Phalen D, Holz P, Rasmussen L, Bayley C. Fatal Columbid herpesvirus-1 infection in three species of Australian birds of prey. Aust Vet J. 2011;89:193–196. doi: 10.1111/j.1751-0813.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 16.VanDevanter P, Warrener P, Bennett L, et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaleta E, Docherty D. Avian herpesviruses. In: Thomas N, Hunter D, Atkinson C, editors. Infectious Disease of Wild Birds. Ames, Iowa: Blackwell; Publ: 2007. pp. 63–86. [Google Scholar]
- 18.Ramis A, Majo N, Pumarola M, Fondevila D, Ferrer L. Herpesvirus hepatitis in two eagles in Spain. Avian Dis. 1994;38:197–200. [PubMed] [Google Scholar]
- 19.Burtscher H, Sibalin M. Herpesvirus strigis: Host spectrum and distribution in infected owls. J Wildl Dis. 1975;11:164–169. doi: 10.7589/0090-3558-11.2.164. [DOI] [PubMed] [Google Scholar]
- 20.Wheler C. Herpesvirus disease in raptors: A review of the literature. In: Redig P, Cooper J, Remple D, Hunter B, editors. Raptor Biomedicine. Minneapolis, Minnesota: University of Minnesota Press; 1993. pp. 105–140. [Google Scholar]
- 21.Redig P, Cruz-Martinez L. Chapter 9: Raptors. In: Tully T, Dorrestein G, Jones A, editors. Avian Medicine. 2nd ed. St. Louis, Missouri: Elsevier; 2009. pp. 209–242. [Google Scholar]