Abstract
A 6-year-old neutered male domestic shorthair cat was presented for acute onset of vomiting. Exploratory laparotomy identified a duplex gallbladder and left cholecystectomy was performed. Histopathology confirmed biliary mucocele and hepatic cholestasis. While rare, biliary mucoceles should be considered as a differential diagnosis for feline extrahepatic bile duct obstruction.
Résumé
Vésicule biliaire double congénitale et mucocèles biliaires associées à une cholestase et à une cholélithiase hépatiques partielles chez un chat. Un chat domestique à poils courts, un mâle castré âgé de 6 ans, a été présenté pour l’apparition aiguë de vomissements. Une laparatomie exploratoire a identifié une vésicule biliaire double et une cholecystectomie gauche a été réalisée. L’histopathologie a confirmé des mucocèles biliaires et une cholestase hépatique. Quoique rares, les mucocèles biliaires devraient être considérées comme un diagnostic différentiel pour l’obstruction des canaux biliaires extrahépatiques félins.
(Traduit par Isabelle Vallières)
Intrahepatic cholestasis in cats occurs most commonly secondary to bile duct destruction and fibrosis as a part of the cholangitis cholangiohepatitis syndrome complex (1–3). In contrast, cholestasis secondary to other etiologies including extrahepatic biliary obstruction (EHBO) is uncommon in feline patients (1,4). Both inflammatory and space-occupying processes can obstruct the common bile duct. Since the feline common bile duct fuses with the major pancreatic duct prior to emptying into the duodenum, obstruction secondary to pancreatitis is common (2,4). Microlithiasis and sludged bile causing transient biliary obstruction and idiopathic pancreatitis have also been described in cats (1). Differential diagnoses for feline biliary obstruction include neoplasia, biliary cystadenomas, pancreatitis, cholelithiasis, parasitism, and foreign body obstruction (1,2,5). In cats, biliary mucoceles are extremely rare. There are only 2 previous reports of feline biliary mucoceles in the veterinary literature and treatment for only 1 of these cases was described (6,7). In that case report, the patient was managed with cholecystojejunostomy and gastrostomy tube placement (6). This case report describes partial intrahepatic biliary obstruction secondary to biliary mucocele in a cat with a congenital duplex gallbladder which was treated with left cholecystectomy and esophageal feeding tube placement.
Case description
A 6-year-old neutered male domestic shorthair cat was presented to the Ontario Veterinary College Health Sciences Center (OVC-HSC) for acute onset of vomiting, anorexia, and lethargy of 48 h duration. The cat had a previous history of upper respiratory tract infection, with the last recurrence 6 mo prior to presentation, and eosinophilic granuloma complex, which was well controlled with a hypoallergenic diet (Hypoallergenic HP dry; Medical-Royal Canin, Guelph, Ontario).
The cat had experienced a similar episode of vomiting and anorexia 4 mo prior to presentation to the OVC-HSC which had been investigated by the referring veterinary clinic. At that time, complete blood (cell) count (CBC) identified mild eosinophilia [eosinophils = 1.49 × 109/L, reference interval (RI): 0.0 to 0.79 × 109/L] and biochemical profile identified mild azotemia (creatinine = 239 μmol/L, RI: 71 to 212 μmol/L) with a urine specific gravity of 1.036. Urinalysis identified occasional struvite crystals and mild hematuria (red blood cells = 0 to 5 cells/400× field; trace hemolyzed blood). Feline pancreatic lipase immunoreactivity (Spec fPL® Test; Idexx Laboratories, performed by Idexx Reference Laboratories, Markham, Ontario) was within normal limits (0.8 μg/L, reference value: < 3.5 μg/L) making pancreatitis unlikely. Abdominal radiographs identified a moderate amount of fecal material in the colon and an area of small intestinal plication and gathering of the bowel loops in the caudal abdomen.
The cat was hospitalized for intravenous fluid therapy, analgesia [butorphanol tartrate (10 mg/mL injection; Torbugesic, Fort Dodge/Pfizer Animal Health, Kirkland, Quebec), 0.3 mg/kg body weight (BW), IV, q2 to 4h] and anti-emetic therapy [maropitant citrate (10 mg/mL injection; Cerenia, Pfizer Animal Health); 1 mg/kg BW, SQ, q24h]. Creatinine levels normalized after 24 h of fluid therapy (creatinine = 177 μmol/L, RI: 71 to 212 μmol/L); however, the cat did not respond to supportive care. Bedside abdominal ultrasound performed by the referring clinician on a portable ultrasound unit identified plication of the small intestines and exploratory laparotomy was performed due to a suspected linear foreign body. No gastrointestinal foreign body was identified during surgery and the liver, pancreas, kidneys, and urinary bladder appeared grossly normal. Intestinal biopsies were acquired. The cat did extremely well after surgery and resumed eating within 12 h of recovery. The cat was discharged from the referring clinic with oral buprenorphine hydrochloride (0.3 mg/mL injection; Chiron Compounding Pharmacy, Guelph, Ontario), 0.01 mg/kg BW, PO, q12h, maropitant citrate (16 mg tablets; Cerenia, Pfizer Animal Health), 1 mg/kg BW, PO, q24h, and cyproheptadine (94 mg tablets, Pharmascience, Montreal, Quebec), 2 mg total, PO, q12h. Intestinal biopsies were consistent with recent, mild mechanical abrasion and it was hypothesized that the cat had ingested a foreign object or toxic substance which had resulted in an acute gastroenteritis.
Four months later, upon presentation to the OVC-HSC, the cat was bright, alert, and responsive. Vital parameters were within normal limits. Mild weight loss of 0.3 kg was noted. Mucous membranes were pink, but tacky. Pigmented macules, consistent with Lentigo simplex of orange cats, were identified on oral and ocular mucous membranes. Mild abdominal pain was elicited on abdominal palpation and generalized inspiratory wheezes were identified bilaterally on thoracic auscultation. The remainder of the cat’s physical examination was unremarkable.
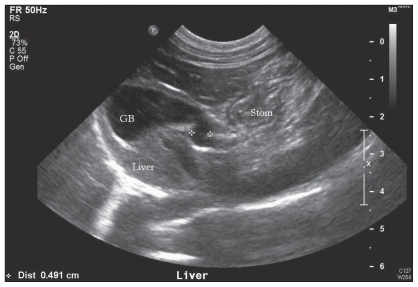
The CBC was within normal limits. Biochemical profile and urinalysis identified mild pre-renal azotemia [blood urea nitrogen (BUN) = 12.1 mmol/L, RI: 6.0 to 12.0 mmol/L] with urine specific gravity of 1.038. Urinalysis performed on a cystocentesis sample identified moderate hematuria (red blood cells = 20 to 30 cells/400×). Urine culture was negative for bacterial growth. Feline leukemia virus and feline immunodeficiency virus combination test (SNAP® FIV/FeLV Combo Test; Idexx Laboratories, Westbrook, Maine, USA) was negative and feline pancreatic lipase immunoreactivity (Spec fPL® Test; Idexx Laboratories, performed by Idexx Reference Laboratories) was within normal limits (1.0 μg/L, reference value: < 3.5 μg/L). Total T4 thyroid levels were normal (25 nmol/L, RI: 13 to 55 nmol/L). The cat was sedated with medetomidine hydrochloride (1.0 mg/mL injection; Domitor, Pfizer Animal Health), 22 μg/kg BW, IM for abdominal ultrasound. Ultrasonography, performed by a veterinary radiologist, identified diffuse hepatic hyperechogenicity and hyperechoic mottling of the right limb of the pancreas. Abdominal ultrasound was also suspicious of a bilobed or duplex gallbladder (Figure 1), although no distension of the common, extrahepatic, or intrahepatic bile ducts was noted.
Figure 1.
Abdominal ultrasound image of the liver and 1 of the gallbladders. The diameter of the cystic bile duct is demarcated by 2 white crosses.
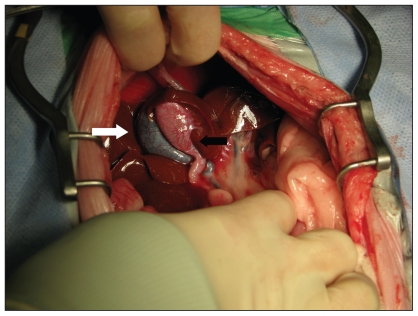
Exploratory laparotomy was recommended as the next diagnostic step and the cat was premedicated with hydromor-phone hydrochloride (2 mg/mL injection; Sandoz Canada, Boucherville, Quebec), 0.05 mg/kg BW, IV and induced with a combination of propofol emulsion (10 mg/mL injection, Diprivan; AstraZeneca, Mississauga, Ontario), 1 mg/kg BW, IV, and midazolam (1 mg/mL injection; Sandoz Canada), 0.2 mg/kg BW, IV. The cat was maintained under a stable plane of anesthesia with inhalant isoflurane (99.9% inhalant anesthesia; Abbott Laboratories, St-Laurent, Quebec). During surgery the cat became bradycardic within 30 min of induction and was administered glycopyrrolate (0.2 mg/mL injection USP; Sandoz Canada), 11 μg/kg BW, IV. A ventral midline exploratory laparotomy was performed. Two gallbladder structures were identified, each with a separate cystic duct that emptied into the common bile duct (Figure 2). The right gallbladder was moderately distended with bile of normal consistency and appeared grossly normal. Bile could be freely expressed through the right cystic duct. The left gallbladder was grossly abnormal with white intramural infiltrates. The left gallbladder contained a small amount of viscous bile and a 2-mm, freely moveable cholelith was present in the left cystic duct. Left cholecystectomy was performed. Despite lying in close proximity to each other, the left and right gallbladder walls were not associated. The entire left cystic duct was not resected due to its proximity to the right cystic duct. However, the remaining left cystic duct was flushed copiously with sterile saline and no EHBO was present. Sterile saline was injected into the right gallbladder following left cholecystectomy and no leakage or bile duct obstruction was noted. Biopsies were acquired from the liver, stomach, small intestines, and the right limb of the pancreas. Esophagostomy tube placement was recommended at the time of surgery, but was declined by the owner.
Figure 2.
Intraoperative photograph of the cranial abdomen. The right gallbladder (white arrow) is grossly normal. The left gallbladder (black arrow) is grossly abnormal with white intramural infiltrates.
Aerobic and anaerobic bacterial cultures of bile from the left gallbladder were negative. Histopathology of the left gall-bladder identified thickened mucosa with focal erosions and occasional epithelial-lined cysts. The left gallbladder submucosa contained inspissated mucus and the lumen contained a mixture of mucus, hemorrhage, and fibrin consistent with gallbladder mucocele. Histopathology of the liver identified expanded portal tracts with moderate numbers of hepatocytes and Kupffer cells containing golden intracytoplasmic pigment consistent with obstructive cholestasis. Intestinal and gastric biopsies were consistent with low-grade lymphocytic-plasmacytic inflammatory bowel disease. Histopathology of the pancreas was unremarkable.
Upon recovery, the cat was treated supportively in the OVC-HSC intensive care unit with intravenous fluid therapy; analgesia: fentanyl citrate constant rate infusion (50 μg/mL injection USP; Sandoz Canada), at 2 to 6 μg/kg BW per hour followed by buprenorphine hydrochloride (0.3 mg/mL injection, Chiron Compounding Pharmacy), at 0.01 mg/kg BW, IV, q6h; anti-emetic therapy: metoclopramide hydrochloride constant rate infusion (5 mg/mL injection; Sandoz Canada, at 2 mg/kg BW per day, and maropitant citrate (10 mg/mL injection; Cerenia, Pfizer Animal Health), at 1 mg/kg BW, SQ, q24h; and postoperative antimicrobials: amoxicillin-clavulanate (50/12.5 mg/mL oral suspension; Clavamox Drops, Pfizer Animal Health), 12.5 mg/kg BW, PO, q12h. Despite a stable clinical condition, the cat remained anorexic for 72 h after surgery. Repeat biochemical profile identified mild elevation in alanine aminotransferase (135 U/L, RI: 31 to 105 U/L), mild decreases in BUN (4.4 mmol/L, RI: 6.0 to 12.0 mmol/L), potassium (K+ = 3.5 mmol/L, RI: 3.6 to 5.2 mmol/L), and mild hypoalbuminemia (20 g/L, RI: 30 to 44 g/L) consistent with anorexia and early hepatic lipidosis. The CBC identified mild leukocytosis characterized by mild mature neutrophilia consistent with a stress leukogram (white blood cells = 22.7 × 109/L, RI: 4.2 to 13.0 × 109/L; segmented neutrophils = 17.03 × 109/L, RI: 2.1 to 8.3 × 109/L). Repeat abdominal ultrasound (by a second veterinary radiologist) identified a moderately distended right gallbladder; however, there was no distention of the common, extrahepatic, or intrahepatic bile ducts. Therefore, ongoing post-operative biliary obstruction was unlikely. Small intestines and stomach were normal and devoid of contents. No abdominal free fluid was identified. Mild pancreatic inflammation was identified surrounding the pancreatic biopsy site. Due to increased hepatic enzyme activity, maropitant citrate therapy was discontinued.
Hypotheses for the ongoing anorexia in this patient included clinical signs related to hepatic lipidosis or inflammatory bowel disease. As the cat had been inappetent for a total of 5 d (2 d prior to presentation followed by 72 h after surgery), the cat was re-anesthetized and an esophagostomy feeding tube was placed. The cat was discharged from hospital with ondansetron hydrochloride (0.8 mg/mL oral solution; Apotex, Toronto, Ontario), 0.1 mg/kg BW, PO, q12h, metoclopramide hydrochloride (1 mg/mL oral solution; Pharmascience), 0.2 mg/kg BW, PO, q8h, amoxicillin-clavulanate (50/12.5 mg/mL oral suspension; Clavamox Drops, Pfizer Animal Health), 12.5 mg/kg BW, PO, q12h, tramadol (10 mg capsules, compounded by the Ontario Veterinary College Pharmacy), 2 mg/kg BW, PO, q12h, and ursodiol (50 mg/mL oral suspension; compounded by the Ontario Veterinary College Pharmacy), 10 mg/kg BW, PO, q24h.
The cat’s appetite gradually improved following institution of enteral feeding via an esophagostomy feeding tube, which was removed 1 mo after surgery. At last follow-up, 7 mo after surgery, the cat was doing well on oral ursodiol and a hypoallergenic diet (Hypoallergenic HP dry; Medical-Royal Canin). The owner reported sporadic vomiting of bile (once every 3 wk), but declined further treatment for inflammatory bowel disease.
Discussion
This cat was a challenging case to diagnose as initial clinicopathologic testing and diagnostic imaging were largely unremarkable. The cat’s non-specific clinical signs and physical examination findings upon presentation were consistent with partial obstructive cholestasis, as well as many other intra-abdominal disorders such as pancreatitis, gastrointestinal foreign body, and neoplasia. We were initially suspicious of pancreatitis based on ultrasonographic changes of the right limb of the pancreas; however, feline pancreas specific lipase and pancreatic biopsy were not consistent with pancreatitis. It is possible that hyper-echoic pericholecystic fat, which has been reported secondary to canine biliary mucoceles (8), was mistaken for mottling of the pancreas. Partial biliary obstruction explains why pre-operative laboratory testing was inconsistent with changes expected in complete cholestasis (elevated alkaline phosphatase, gamma glutamyl-transferase, bilirubin, and cholesterol) (1,2,4).
Furthermore, abdominal ultrasound is limited for the diagnosis of feline gallbladder disease. Cats with hepatobiliary disease have variable sonographic findings and only a limited number of studies have assessed these changes objectively (9,10). For example, a gallbladder wall thickness > 1 mm is consistent with feline biliary disease, but a normal wall thickness does not rule out mild or chronic inflammation (11). A study by Gaillot et al (7) found that a common bile duct diameter of > 5 mm is consistent with feline EHBO, but gallbladder dilation was present in only half of the cats with EHBO (10). As the feline gallbladder wall can contract secondary to inflammation, this may mask distention of the gallbladder during an obstructive process (12). While it is possible to identify intraluminal gall-bladder debris and choleliths ultrasonographically, these findings can be misleading. Many small animal patients with choleliths are asymptomatic (1,2). For example, the cholelith identified in this cat’s left cystic duct passed freely through his cystic duct and common bile duct into the duodenum with digital manipulation and flushing at surgery. It is therefore possible that the cholelith was an incidental finding.
Ultrasonography is considered the gold standard for diagnosis of canine biliary mucoceles, which have a striated hyperechoic “kiwi fruit” appearance when complete (1,2), but there is limited information regarding the sonographic appearance of biliary mucoceles in feline patients. In both previous reports of feline biliary mucocele, the gallbladder content was described only as echoic and organized (6,7). These 2 reports do not match the classic striated pattern described in dogs, but they are similar to the appearance of “early” canine biliary mucoceles as described by Besso et al (8), who hypothesized a continuum from immobile echogenic bile to stellate pattern to finely striated “kiwi fruit” appearance. In contrast, no such gallbladder content was identified in our patient. As immobile hypoechoic mucus is better visualized ultrasonographically when surrounded by echogenic bile, the lack of biliary sludge in our patient may explain why the mucocele was not detected on ultrasound. Scintigraphy using 99m Tc-diisopropyl iminodiacetic acid radioisotope can be used to diagnose partial biliary obstruction (2) and could have been useful prior to surgery but was not performed. Given the lack of clinicopathologic changes, partial biliary obstruction was not being considered as a differential diagnosis before surgery and, therefore, scintigraphy was not considered as part of the diagnostic work-up. Ultimately, this case illustrates the benefits of exploratory laparotomy in cases with non-specific clinical findings when less invasive diagnostic tests do not provide a diagnosis.
Biliary mucoceles were once considered to occur exclusively in the dog and involve bile-laden, semisolid mucoid material within the gallbladder (1,2). They occur commonly in dogs, with a predisposition in Shetland sheepdogs, cocker spaniels, and miniature schnauzers (2). In contrast, biliary mucoceles are rare in feline patients, with only 2 previous reports in the veterinary literature (6,7). In the first paper, a cat with extra-hepatic bile duct obstruction and organized echoic non-mobile gallbladder content was diagnosed with biliary mucocele by necropsy (7). No further information regarding the clinical signs, course of disease or treatment was presented. In the second case report, the cat was anorexic and icteric upon presentation and was diagnosed with concurrent hepatic lipidosis (6). Despite experiencing hepatic encephalopathy secondary to hepatic lipidosis post-operatively, the cat survived cholecystojejunostomy and did well long-term (6).
Recommended treatment for canine biliary mucoceles is cholecystectomy followed by chronic choleretic therapy (1,2,13). Cholecystotomy with removal of inspissated bile is not recommended as biliary mucoceles can reoccur and both transmural ischemic necrosis of the gallbladder wall and cholelithiasis can occur secondary to bile stasis (1). We elected to perform cholecystectomy of the left gallbladder in our patient, while leaving the right gallbladder in place, for 3 reasons. First, risk of biliary mucocele recurrence is high in abnormal gallbladders (1), yet our patient’s right gallbladder appeared grossly normal. Second, right and left gallbladder cholecystectomy involved higher surgical risk due to the close anatomical connection of the right and left cystic duct to the common bile duct, and third, it would also result in lifelong post-operative choleretic treatment. Prognosis for cholecystectomy in dogs is guarded as there is high peri-operative mortality (22% to 40%), with higher risk if the dog has experienced biliary rupture (1,2,13). Similarly, prognosis for feline surgical biliary decompression (via cholecystectomy, cholecystojejunostomy, or choledochoduodenostomy) is also guarded, with 56% mortality reported in a retrospective review of feline EHBO (4). The mortality rate of cats with underlying neoplasia was 100% within 72 h of presentation (4). In cats without neoplasia, 40% died or were euthanized within 1 wk of surgery (4). Refractory hypotension was the most commonly reported adverse event, affecting 68% of cats, followed by cardiopulmonary arrest (4). It should be noted that this retrospective review excluded cases of partial biliary obstruction and did not compare complication rates of different surgical techniques for biliary decompression. Considering that our patient recovered from cholecystectomy of the left gallbladder without post-operative complications, it appears that further studies are required to adequately evaluate the morbidity and mortality rates of feline cholecystectomy.
Aberrant gallbladders are rarely described in the veterinary literature, although an anatomic review by Boyden (14) revealed that up to 1 in 8 cats have some type of gallbladder anomaly. The most commonly reported anomaly is a bilobed gallbladder, which is considered an inconsequential finding (1,15,16). True duplex gallbladders, as noted in this case, have separate cystic ducts which drain into the common bile duct and comprise 10% of feline congenital gallbladder anomalies (14). In the veterinary literature gallbladder anomalies described in association with clinical disease are even less common. Suppurative cholangitis has been described in 1 cat secondary to a bilobed gallbladder (17). There is also a report of a cat with a duplex gallbladder experiencing septic bile peritonitis following left gallbladder rupture (18). The description of the duplex gallbladder is similar to our case, except that in the previous report the right and left gallbladder walls were attached and communicated with each other (18). A choledochoduodenostomy was performed but the cat did not recover well from surgery and was euthanized 72 h after surgery (18). As it has been estimated that 12% of cats (14) have some form of aberrant gallbladder and that biliary disease is so infrequently reported with these congenital biliary abnormalities, it appears that feline gallbladder anomalies are not associated with increased risk of biliary disease.
Although triaditis of inflammatory bowel disease, pancreatitis, and cholangiohepatitis has been well-described in cats, the underlying etiology remains unknown (2,19). It is hypothesized that pancreatitis and cholangiohepatitis are sequelae to the inflammatory bowel disease. While the cat of this report was diagnosed with lymphocytic-plasmacytic inflammatory bowel disease, clinicopathological diagnostic testing and histology were not consistent with pancreatitis or cholangiohepatitis. Therefore, while inflammatory bowel may have played a role in the cat’s post-operative anorexia, we suspect that the diagnosis of inflammatory bowel disease was unrelated to the biliary disease and cholestasis.
This report details a novel situation in which a cat with duplex gallbladder experienced partial EHBO secondary to biliary mucocele in 1 gallbladder. While biliary mucoceles are rare in cats, they should be considered as a differential diagnosis for feline cholestasis. Cholecystectomy of the affected gallbladder led to resolution of the clinical signs without complications. It is important to note that congenital anomalous gallbladders do not appear to predispose cats to biliary disease and that the diagnosis of inflammatory bowel disease was likely an incidental finding in this case. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Center SA. Diseases of the gallbladder and biliary tree. Vet Clin N Am Small Anim Pract. 2009;39:543–598. doi: 10.1016/j.cvsm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger SJ, Feldman EC. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, Missouri: Saunders; 2010. [Google Scholar]
- 3.Kahn CM. The Merck Veterinary Manual. 9th ed. Philadelphia,Pennsylvania: National Publ; 2005. [Google Scholar]
- 4.Mayhew PD, Holt DE, McLear RC, et al. Pathogenesis and outcome of extrahepatic biliary obstruction in cats. J Small Anim Pract. 2002;43:247–253. doi: 10.1111/j.1748-5827.2002.tb00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier FG, Morato GS, Righi DA, et al. Cystic liver disease related to high Platynosomum fastosum infection in a domestic cat. J Feline Med Surg. 2007;9:51–55. doi: 10.1016/j.jfms.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett SL, Milne M, Slocombe RF, et al. Gallbladder mucocoele and concurrent hepatic lipidosis in a cat. Aust Vet J. 2007;85:397–400. doi: 10.1111/j.1751-0813.2007.00182.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaillot HA, Penninck DG, Webster CRL, et al. Ultrasonographic features of extrahepatic biliary obstruction in 30 cats. Vet Radiol Ultrasound. 2007;48:439–447. doi: 10.1111/j.1740-8261.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 8.Besso JG, Wrigley RH, Gliatto JM, et al. Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Vet Radiol Ultrasound. 2000;41:261–271. doi: 10.1111/j.1740-8261.2000.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 9.Newell SM, Selcer GA, Girard E, et al. Correlations between ultrasono-graphic findings and specific hepatic diseases in cats: 72 cases (1985–1997) J Am Vet Med Assoc. 1998;213:94–98. [PubMed] [Google Scholar]
- 10.Penninck DG, Brisson JO, Webster CRL. Sonographic assessment of gallbladder volume in normal cats. Vet Radiol Ultrasound. 2010;51:665–666. doi: 10.1111/j.1740-8261.2010.01708.x. [DOI] [PubMed] [Google Scholar]
- 11.Hittmair KM, Vielgrader HD, Loupal G. Ultrasonographic evaluation of gallbladder wall thickness in cats. Vet Radiol Ultrasound. 2001;42:149–155. doi: 10.1111/j.1740-8261.2001.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 12.Leveille R, Biller DS, Shiroma JT. Sonographic evaluation of the common bile duct in cats. J Vet Intern Med. 1996;10:296–299. doi: 10.1111/j.1939-1676.1996.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 13.Fossum TW. Small Animal Surgery. 2nd ed. St. Louis, Missouri: Mosby; 2002. [Google Scholar]
- 14.Boyden EA. The accessory gallbladder — An embryological and comparative study of aberrant biliary vesicles occurring in man and the domestic mammals. Am J Anat. 1926;38:177–231. [Google Scholar]
- 15.Bartlett LM. A divided intrahepatic gallbladder in a cat. Anat Rec. 1951;109:715–721. doi: 10.1002/ar.1091090408. [DOI] [PubMed] [Google Scholar]
- 16.Moentk J, Biller DS. Bilobed gallbladder in a cat: Ultrasonographic appearance. Vet Radiol Ultrasound. 1993;34:354–356. [Google Scholar]
- 17.Hirsch VM, Doige CE. Suppurative cholangitis in cats. J Am Vet Med Assoc. 1983;182:1223–1226. [PubMed] [Google Scholar]
- 18.Moores AL, Gregory SP. Duplex gall bladder associated with choledocholithiasis, cholecystitis, gall bladder rupture and septic peritonitis in a cat. J Small Anim Pract. 2007;48:404–409. doi: 10.1111/j.1748-5827.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiss DJ, Gagne JM, Armstrong PJ. Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis and nephritis in cats. J Am Vet Med Assoc. 1996;206:1114–1116. [PubMed] [Google Scholar]