Abstract
The diagnosis of primary central nervous system lymphoma (PCNSL) by radiographical examination is often difficult because of its similarity to other brain tumors. To test whether interleukin-10 (IL-10) and IL-6 can be used to distinguish PCNSL from other brain tumors that are radiographically similar, cerebrospinal fluid (CSF) levels of IL-10 and IL-6 were measured in 66 patients with intracranial tumors (PCNSLs: 26 cases; other brain tumors: 40 cases). In the patients with PCNSLs, the median CSF levels of IL-10 and IL-6 were 27 pg/mL and 5.4 pg/mL, respectively. The CSF IL-10 and IL-6 levels were significantly higher in PCNSLs than in the other brain tumors. To validate the diagnostic value of CSF IL-10 in PCNSL, we prospectively examined 24 patients with brain lesions that were suspected to be PCNSL. We observed that the CSF IL-10 levels were significantly higher in PCNSLs than in other brain tumors. At an IL-10 cutoff level of 9.5 pg/mL, the sensitivity and specificity were 71.0% and 100%, respectively. After therapy, the CSF IL-10 levels were decreased in all patients and were increased at relapse in most of these patients. Immunohistochemically, all PCNSLs, except for 1 unclassified PCNSL, expressed both IL-10 and IL-10 receptor-A. In the patients with high CSF IL-10, IL-10 expression levels in tumor were relatively higher, compared with low CSF IL-10; however, there was no significant difference between these groups. In addition, elevated CSF level of IL-10 was significantly associated with having a shorter progression-free survival (hazard ratio, 3.37; 95% confidence interval, 0.985–11.528; log-rank, P= .038). These results indicate that the CSF level of IL-10 may be a useful diagnostic and prognostic biomarker in patients with PCNSLs.
Keywords: biomarker, cerebrospinal fluid; IL-10; IL-6; primary CNS lymphoma
Primary central nervous system lymphomas (PCNSLs) represent 3%–5% of primary brain tumors. PCNSL is a rare type of non-Hodgkin lymphoma (NHL) and is predominantly of B cell origin.1 The incidence of PCNSLs has increased over the past 3 decades, especially in immunocompetent persons.1 In contrast to most primary malignant brain tumors, PCNSLs are sensitive to corticosteroids, chemotherapy, and radiotherapy. Durable, complete responses and long-term survival are possible with these treatments; however, outcome for patients with PCNSL is substantially worse than for patients with systemic NHL.2
PCNSL is currently diagnosed by performing a histological examination of a brain biopsy specimen. The preoperative diagnosis is often suggested by its radiographic appearance.3 Multiple lesions are observed in approximately30% of patients and frequently occur within the cerebral white matter near the corpus callosum, in the basal ganglia and thalamus, and in the cerebellum. MRI modality is fairly sensitive in detecting PCNSLs. Because of the high cellularity and decreased water content because of a high nucleus-to-cytoplasm ratio, there is T2 shortening combined with a relative lowering in signal intensity in these tumors.3 Diffusion-weighted imaging has also been helpful in distinguishing these masses from other lesions.3 However, PCNSL may be associated with a large spectrum of radiological presentations and can simulate other brain tumors, such as glioblastoma and metastatic brain tumor. Therefore, the preoperative diagnosis of PCNSL by radiographic appearance alone is often misleading.
Cytological and chemical examination of the cerebrospinal fluid (CSF) is also an important tool for the diagnosis of some brain tumors. High CSF concentrations of β-human chorionic gonadotropin or α-fetoprotein, plus concordant features on brain MRI, are used to diagnose CNS germ cell tumors and can eliminate the need for craniotomy.4 In addition, in patients with AIDS, detection of Epstein-Barr virus (EBV) DNA in the CSF using polymerase chain reaction (PCR), in addition to neuroimaging, may establish the presence of a CNS lymphoma. However, PCNSL in the immunocompetent population is not associated with EBV infection.5 Because early diagnosis and treatment of PCNSL attenuates disease progression and neurologic deterioration, useful biomarkers are required to facilitate diagnosis.
It is well established that cytokines are deregulated in a variety of hematological disorders.6 Some cytokines may act as tumor cell growth factors, inhibitors of apoptosis, attractors of immune cells, mediators affecting tumor-stromal cell interactions, facilitators of invasion or promoters of angiogenesis.6–8 IL-10 is a pleiotropic cytokine produced by type-2 helper cells (Th2), monocytes and macrophages, and normal and neoplastic B lymphocytes.9–11 IL-10 was first described as a growth and differentiation factor for B lymphocytes that induced activated B cells to secrete large amounts of immunoglobulin. IL-10 is also known to have broad anti-inflammatory properties because of its suppression of both macrophage and dendritic cell function.9,11,12 In addition, IL-10 increases Bcl-2 expression and protects malignant cells from apoptosis.13,14 Thus, IL-10 may play various roles in the development of lymphoma. Conversely, IL-6 is a potent lymphoid growth and differentiation cytokine that is produced by various cell types, including benign and malignant B and T lymphocytes, monocytes, macrophages, fibroblasts, and hepatocytes.6–8 Its pleiotropic activities are reflected in its participation in the physiological regulation of immune function and inflammatory responses, among others.
In some studies, serum IL-6 and IL-10 levels were identified to be high in patients with aggressive, systemic NHL.15,16 Serum IL-10 levels are also elevated in approximately half of patients with Hodgkin lymphoma and strongly correlated with prognosis independent of other factors.17 In addition, high IL-10 levels were reported to be in the vitreous of most patients with primary intraocular lymphoma. This is a helpful adjunct to further confirm diagnosis of primary intraocular lymphoma.18,19 In contrast, there are only a few reports stating that the occurrence of PCNSL is associated with an elevated CSF IL-10 level.20–22
The aim of this work was to evaluate the diagnostic value of IL-10 and IL-6 levels in the CSF samples from patients with PCNSL and other intracranial malignant tumors. We demonstrated that an elevated level of CSF IL-10 was highly associated with the occurrence of PCNSLs. Furthermore, the CSF IL-10 level reflected PCNSL disease progression. In addition, pretherapy high CSF IL-10 levels were associated with having a shorter progression-free survival (PFS) and overall survival (OS). These results suggest that the CSF level of IL-10 may be a helpful biomarker of PCNSLs for diagnosis and prediction of prognosis.
Materials and Methods
Case Control Study and Patients
To evaluate the diagnostic value of the CSF IL-10 and IL-6 levels in patients with PCNSL and other malignant brain tumors, 66 patients with preoperative brain tumors suspected of being PCNSLs on radiographic examination were retrospectively studied. Those patients were treated at the Department of Neurosurgery, University of Kobe (Japan), from January 2004 through November 2010. Preoperative diagnostic procedures included MRI and/or computed tomography (CT). In addition, for preoperative diagnosis, 2–5 mL of CSF was drawn from the patients by lumbar puncture after informed consent. This study included 26 PCNSLs and 40 other brain tumors. Of the 26 cases of PCNSL, 20 cases were diffuse large B cell lymphoma (DLBCL), 3 cases were PCNSL with intraocular lymphoma (IOL), 2 cases were T cell lymphoma (TCL), and 1 case was an unclassified PCNSL. Of the 40 cases of other brain tumor types, 16 cases were glioblastoma, 5 cases were anaplastic astrocytomas, 1 case was an anaplastic oligodendroglioma, 4 cases were low-grade gliomas, 1 case was a brain stem glioma, 2 cases were ependymomas, 4 cases were germ cell tumors, and 7 cases were metastatic tumors. To obtain a pathological diagnosis, brain biopsies or tumor removals were performed after written informed consent. Three patients with PCNSL with IOL received a diagnosis by vitrectomy. One patient with brain stem glioma did not undergo biopsy and received a diagnosis by MRI and treatment response.
Prospective Study for CSF IL-10
To verify the diagnostic value of IL-10 and IL-6 expression in the CSF from patients with PCNSL, we conducted a prospective study at the Department of Neurosurgery, University of Kobe, from December 2010 through August 2011, which was approved by the ethical review board of our institution. Patients with brain tumors or brain lesions that were preoperatively suspected of being PCNSLs after radiographic examination were prospectively studied. Patients who had symptoms of increasing intracranial pressure, such as severe headache, nausea, vomiting, and papilloedema, were excluded because lumbar puncture is considered to be high risk. In addition, the patients who had severe brain shift because of tumor and peritumoral edema were also excluded. Twenty-four patients were preoperatively suspected of having PCNSL by MRI at the time of admission (Supplementary Fig. S1). These 24 patients were enrolled in this prospective study. To examine the CSF samples, the patients underwent lumbar puncture after informed consent, and IL-10 and IL-6 CSF levels were preoperatively analyzed, in combination with routine biochemical examinations, including lactate dehydrogenase, β2-M, and sIL-2R. To obtain pathological diagnosis, brain biopsies or tumor removals were performed after written informed consent was provided. Twelve patients underwent biopsy, and 10 patients underwent tumor removal with craniotomy. Two patients with pontine glioma did not undergo biopsy and received a diagnosis by MRI and treatment response. Five patients received a diagnosis of PCNSLs, 8 patients with glioblastoma, 2 patients with oligodendroglioma, 1 patient with anaplastic ependymoma, 1 patient with diffuse astrocytoma, 2 patients with pontine glioma, 1 patient with chordoma, 1 patient with germinoma, 1 patient with metastatic tumor, and 2 patients with multiple sclerosis. This prospective study was approved by the ethical review board of our institution.
CSF Samples and Determining Cytokine Concentration
CSF samples (3–5 mL) were obtained from the patients by lumbar puncture after informed consent. All samples were coded. The CSF samples were immediately centrifuged (15 min at 2000 × g at 4°C) and stored at −70°C for protracted investigation without intermediate thawing. IL-10, IL-6, IL-2R, and β2-M levels in the CSF were examined during or after routine biochemical and pathological examinations. The CSF concentrations of IL-10 and sIL-2R were measured using a human enzyme-linked immunosorbent assay. IL-6 levels were measured using a human chemiluminescent enzyme immunoassay. β2-M was measured using a latex agglutination-turbidimetric immunoassay. The limits of the tests for the quantification of IL-10, IL-6, sIL-2R, and β2-M levels were 2 pg/mL, 0.3 pg/mL, 50 U/mL, and 200 μg/L, respectively.
Treatment of PCNSL and Follow-Up
Treatment of all patients with PCNSLs is displayed in Table 1. Of 31 patients with PCNSLs, 22 patients received high-dose methotrexate therapy. Methotrexate (4–6 g/m2) was given as an intravenous infusion over a 3-hour period on day 1. Leucovorin rescue began 24 h after the methotrexate infusion. One patient received rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone because of renal dysfunction. Eight patients did not receive chemotherapy (3 patients received steroid therapy alone, and 5 patients received radiotherapy alone). Twenty-six patients received radiotherapy (RT); of these, 25 patients underwent conventional RT (whole brain plus local irradiation) and 1 patient underwent stereotactic radiosurgery (γ-knife). In whole brain irradiation, both eyes were included in the RT field. Twenty-one patients received both chemotherapy and radiotherapy. After completion of the treatments, the patients underwent an MRI to evaluate treatment response. In addition, the patients underwent lumbar puncture, and CSF analyses were performed after informed consent. Additional follow-up scans and CSF analysis were performed if clinically necessary. Radiographic response was based on complete response (CR), which referred to the absence of tumor enhancement on post-treatment contrast MRI scan. Partial response (PR) was considered to be a reduction of at least 50% of the contrast-enhancing MRI volume, which is the sum of the products of all the maximum diameters of the measured lesion or lesions. Stable disease (SD) indicated objective regression of the measured contrast-enhancing MRI volume, less than required to meet the criteria for partial response or less than a25% increase in the measurable lesion. Progressive disease (PD) was considered to be an increase in the sum of the products of the maximum diameters by 25% or more. PFS was determined from the onset of treatment until relapse, disease progression, or the last follow-up evaluation. OS was determined from the onset of treatment until the last follow-up evaluation or death from any cause.
Table 1.
Patient clinical characteristics, CSF examination, and prognosis in all PCNSLs
| Case | Age/Sex | Pathology | Tumor No. single/multiple | CSF cytology Papanicolaou classification | IL-10 pre-treat (pg/mL) | IL-10 post-treat (pg/mL) | IL-10 rec. (pg/mL) | IL-6 pre-treat (pg/mL) | IL-6 post-treat (pg/mL) | Treatment | Respons to Treatment | PFS (mo.) | Rec. | OS (mo.) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77/M | DLBCL | Multiple | class II | 1280 | 950 | 235 | 51.3 | 202 | MTX/SRS | PR | 7 | + | 36 | Dead |
| 2 | 64/M | DLBCL | Multiple | class II | 1090 | <2 | – | 16.2 | 1.3 | MTX/RT | CR | 39 | − | 39 | Alive |
| 3 | 46/F | DLBCL | Multiple | class II | 647 | <2 | 522 | 7.8 | 1.6 | RT | CR | 12 | + | 19 | Dead |
| 4 | 73/F | DLBCL | Multiple | class I | 212 | <2 | – | 3.6 | 3.7 | MTX/RT | CR | 10 | − | 10 | Alive |
| 5 | 66/M | DLBCL | Single | – | 160 | <2 | – | 7.2 | 3.3 | MTX/RT | CR | 7 | + | 8 | Dead |
| 6 | 58/M | DLBCL | Multiple | class II | 69 | – | – | 10.1 | – | R-CHOP/RT | CR | 4 | + | 5 | Dead |
| 7 | 68/M | DLBCL | Multiple | class II | 62 | 6 | – | 5.1 | 5.9 | MTX/RT | CR | 8 | + | 9 | Dead |
| 8 | 67/M | DLBCL | Multiple | class II | 51 | <2 | 16 | 4.3 | 3 | MTX/RT | CR | 25 | + | 30 | Alive |
| 9 | 80/M | DLBCL | Multiple | – | 45 | 20 | – | 18.4 | 21.9 | Steroid | PD | 1 | + | 2 | Dead |
| 10 | 58/M | DLBCL | Single | – | 42 | <2 | – | 4.8 | 2.5 | MTX/RT | CR | 16 | − | 16 | Alive |
| 11 | 65/M | DLBCL | Multiple | class II | 42 | – | – | 23.8 | – | Steroid | PD | 1 | + | 2 | Dead |
| 12 | 67/F | DLBCL | Single | class II | 29 | 9 | 2400 | 3 | 5.3 | MTX/RT | PR | 6 | + | 7 | Dead |
| 13 | 68/M | DLBCL | Single | class II | 13 | <2 | – | 6.6 | 3.3 | MTX/RT | CR | 31 | − | 31 | Alive |
| 14 | 83/M | DLBCL | Multiple | class I | 10 | – | 3 | 264 | – | MTX/RT | CR | 39 | + | 53 | Alive |
| 15 | 61/M | DLBCL | Multiple | – | 10 | <2 | – | 2.8 | 2.4 | MTX/RT | CR | 10 | − | 10 | Alive |
| 16 | 76/F | DLBCL | Single | – | 8 | <2 | – | 4.2 | 2.6 | MTX/RT | CR | 27 | − | 27 | Alive |
| 17 | 55/M | DLBCL | Multiple | class I | 8 | <2 | – | 3.9 | 5.2 | MTX/RT | CR | 17 | − | 17 | Alive |
| 18 | 36/M | DLBCL | Multiple | class II | 7 | <2 | 8 | 4.9 | 6.4 | MTX/RT | PR | 4 | + | 16 | Dead |
| 19 | 67/F | DLBCL | Single | class II | <2 | <2 | <2 | 167 | 6.2 | MTX/RT | PR | 5 | + | 6 | Dead |
| 20 | 79/M | DLBCL | Multiple | class II | <2 | – | – | 5.2 | – | MTX | CR | 24 | − | 24 | Alive |
| 21 | 79/M | PCNSL with IOL | Single | class II | 1610 | <2 | 11 | 75.4 | 4 | MTX/RT | CR | 5 | + | 27 | Dead |
| 22 | 63/M | PCNSL with IOL | Single | class II | 27 | <2 | – | 6.5 | 7.4 | MTX | CR | 36 | − | 36 | Alive |
| 23 | 72/F | PCNSL with IOL | Single | class II | 12 | 9 | – | 1.2 | 1.5 | RT | CR | 40 | + | 44 | Dead |
| 24 | 67/M | TCL | Single | – | <2 | <2 | – | 5.4 | 12.1 | MTX/RT | CR | 9 | + | 10 | Dead |
| 25 | 47/M | TCL | Multiple | class II | <2 | <2 | – | 1.3 | 0.3 | RT | CR | 58 | − | 58 | Alive |
| 26 | 53/F | Unclassified | Single | class I | <2 | – | – | 3 | – | RT | CR | 53 | − | 53 | Alive |
| <Prospective study> | |||||||||||||||
| 1 | 60/M | DLBCL | Multiple | – | 5 | <2 | – | 4.4 | 286 | MTX/RT | CR | 7 | − | 7 | Alive |
| 2 | 71/M | DLBCL | Single | class I | 53 | <2 | – | 6.2 | 5.5 | MTX/RT | PR | 7 | − | 7 | Alive |
| 3 | 83/M | DLBCL | Single | – | 13 | <2 | – | 13.9 | 3.6 | Steroid | PD | 2 | + | 3 | Dead |
| 4 | 55/F | DLBCL | Multiple | class II | 42 | 26 | – | 6.8 | 7.4 | RT | CR | 5 | − | 5 | Alive |
| 5 | 62/M | DLBCL | Single | – | 118 | <2 | – | 6.8 | 4.6 | MTX/RT | PR | 3 | − | 3 | Alive |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; IOL, intraoular lymphoma; TCL, T-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, adriamycin, vincristine, prednisone; MTX, methotrexate; RT, radiation therapy; SRS, stereotactic radiosurgery; rec, recurrence; rec-, no recurrence; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Immunohistochemistry of IL-10, IL-10RA, and Bcl-2
Archived paraffin blocks of patients with PCNSL from the Department of Pathology of our hospital were used for immunostaining. Serial sections were deparaffinized, immersed in methanol with 0.3% hydrogen peroxide, and heated in 0.01 M citrate buffer (pH, 6.0) for 15 min by autoclaving (121°C, 2 atm). The sections were incubated with IL-10 (ab34843; Abcam), IL-10RA (Millipore), or Bcl-2 (C-2; Santa Cruz Biotechnology) primary antibodies at 4°C overnight. The sections were allowed to react with peroxidase-conjugated anti-rabbit IgG polyclonal antibody for IL-10RA and anti-mouse IgG polyclonal antibody for Bcl-2 (Histofine Simple Stain MAX-PO; Nichirei) for 60 min. Reaction products were visualized by immersing the sections in 0.03% diaminobenzidine solution containing 2 mM hydrogen peroxide for 5 min. Nuclei were lightly stained with Mayer's hematoxylin. Immunohistochemical reactivity was evaluated and classified into 4 groups: (-) negative in lymphoma cells, (1+) weakly positive in lymphoma cells, (2+) moderately positive in lymphoma cells, and (3+) strongly positive in lymphoma cells. Examples of this classification are displayed in Supplementary Fig. S5.
Statistical Analysis
The values of IL-10 and sIL-2R below the detection limits of the assays were set at 2 pg/mL and 50 U/mL, respectively. Differences between the groups were analyzed using the Mann-Whitney U test. The correlation between the groups was assessed using Spearman's rank test. Survival (PFS and OS) was estimated using the Kaplan-Meier method, and significance was determined by the log-rank test. Univariate analysis was performed with Cox's proportional hazard model. P < .05 was considered to be statistically significant. Statistical analysis was performed using the SPSS, version 12.0, software package (SPSS).
Results
Case-Control Study of Pretreatment CSF Levels of IL-10, IL-6, β2-M, and sIL-2R
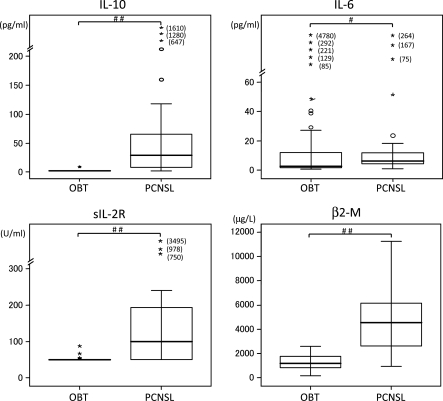
IL-10, IL-6, β2-M, and sIL-2R concentrations of CSF were measured in 66 patients with brain tumors. Twenty-six patients had PCNSLs (20 cases of DLBCL, 3 cases of PCNSL with IOL, 2 cases of T-cell lymphoma, and 1 case of unclassified PCNSL), and 40 patients had other brain tumor types (16 cases of glioblastoma, 5 cases of anaplastic astrocytoma, 1 case of anaplastic oligodendroglioma, 4 cases of low-grade glioma, 1 case of brain stem glioma, 2 cases of ependymoma, 4 cases of germ cell tumors, and 7 cases of metastatic tumor). Five patients with PCNSLs and all 40 patients with other brain tumor types had IL-10 CSF levels below the detection limit (<2 pg/mL) (Table 1). Seven patients with PCNSLs and 30 patients with other brain tumor types also had CSF levels of IL-2R below the detection limit (<50 U/mL) (Table 1). Median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were 27 pg/mL (range, <2 to 1610 pg/mL), 5.4 pg/mL (range, 1.2–264 pg/mL), 4084 μg/L (range, 970–11239 μg/L), and 100 U/mL (range, <50 to 978 U/mL), respectively (Table 1). On the other hand, in the patients with other brain tumor types, the median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were <2.0 pg/mL, 2.7 pg/mL (range, 0.8–4780 pg/mL), 1200 μg/L (range, 172–2600 μg/L), and <50 U/mL (range, <50 to 87 U/mL), respectively (Table 1). Median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were significantly higher in the patients with PCNSL than in those with other brain tumors (P < .001, P= .017, P< .001, and P< .001, respectively; calculated using the Mann-Whitney U test) (Fig. 1).
Fig. 1.
Pretherapy CSF levels of IL-10, IL-6, β2-M, and sIL-2R in PCNSL and other brain tumor types. The difference between PCNSL and other brain tumor types was statistically significant (##P< .001, #P< .05, Mann–Whitney U test). PCNSL, primary CNS lymphoma; OBT, other brain tumors.
Prospective Study of Pretreatment CSF Levels of IL-10, IL-6, β2-M, and sIL-2R
Twenty-four patients were enrolled in this prospective study (Supplementary material, Table S1). All patients had brain lesions that were suspected of being PCNSL on the basis of MRI findings (Supplementary Fig. S1). All patients, except for 2 patients with brain stem tumors, underwent surgery for diagnosis (Supplementary material, Table S1). The 2 patients with brain stem lesions received a diagnosis by a radiological examination (eg, MR spectroscopy) and treatment response. All patient characteristics are listed in Supplementary material, Table S1. Six patients had a high concentration of IL-10 in their CSF, and 18 patients had a concentration of IL-10 below the detection limit. Five patients received a diagnosis of PCNSLs, 8 patients with glioblastoma, 2 patients with oligodendroglioma, 2 patients with pontine glioma, 1 patient with diffuse astrocytoma, 1 patient with anaplastic ependymoma, 1 patient with germinoma, 1 patient with chordoma, 1 patient with metastatic tumor, and 2 patients with multiple sclerosis. Five of 6 patients with high concentrations of IL-10 received a diagnosis of PCNSL; the other patient received a diagnosis of anaplastic ependymoma, and concentration of the CSF IL-10 was 9 pg/mL. However, there were no PCNSLs in patients with CSF IL-10 levels below the detection limit. In patients with PCNSLs, median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were 42 pg/mL (range, 5–118 pg/mL), 6.8 pg/mL (range, 4.4–13.9 pg/mL), 4983 μg/L (range, 2303–6166 μg/L), and 88 U/mL (range, <50 to 3495 U/mL), respectively. In the patients with other brain tumor types, the median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were <2.0 pg/mL (range, <2 to 9 pg/mL), 2.7 pg/mL (range, 0.8–221 pg/mL), 1164 μg/L (range, 410–2075 μg/L), and <50 U/mL, respectively. The median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were significantly higher in patients with PCNSLs than in those with other brain tumors (P< .001, P= .017, P< .001, and P< .001, respectively; calculated using the Mann-Whitney U test).
Twelve patients had biopsy performed, and 10 patients underwent tumor removal with craniotomy. Preoperative IL-10 measurement in the CSF was useful for clinical decision-making, especially for determining appropriate operation methods. For example, in patient 11, who had a right temporal lobe tumor, the CSF IL-10 level was under the detection limit (<2 pg/mL). This tumor was resectable, and therefore, tumor removal with a craniotomy was performed. In contrast, in patient 15, who had a left temporal lobe tumor, the tumor was resectable but the CSF IL-10 was high (118 pg/mL). A biopsy was planned, and chemo-radiotherapy was started immediately after surgery. One patient, who had a left parietal anaplastic ependymoma (patient 13), had a slightly high IL-10 concentration (9 pg/mL) in CSF. A craniotomy with general anesthesia was planned for this patient because of the low levels of sIL-2R and β2-M. In addition, a total tumor resection was performed after the intraoperative diagnosis of ependymoma. Taken together, the preoperative measurement of CSF IL-10 was very useful for diagnosis and treatment decision-making.
Measuring the CSF Levels of IL-10 Is a Useful Diagnostic Biomarker for PCNSLs
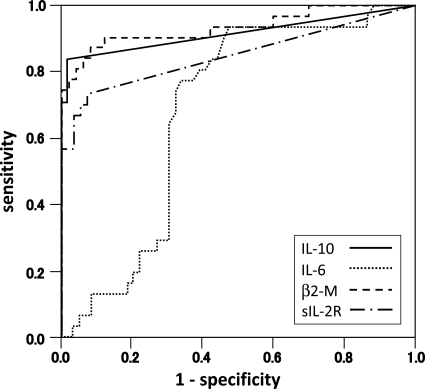
Figure 2 displays the receiver-operator characteristic (ROC) curves of the CSF levels of IL-10, IL-6, β2-M, and sIL-2R in all patients. The areas under the curves and confidence intervals are 0.916 (0.837–0.994), 0.681 (0.568–0.793), 0.934 (0.871–0.998), and 0.848 (0.747–0.950) for IL-10, IL-6, β2-M, and sIL-2R, respectively. ROC curves showed that the discrimination power of IL-10 or β2-M levels were better than that of sIL-2R or IL-6 levels for the presence of a PCNSL (Fig. 2). At an IL-10 cutoff level of 9.5 pg/mL, the sensitivity and specificity were 71.0% and 100%, respectively. Similarly, a cutoff of 2056 μg/L in β2-M yielded a specificity of 90.3% and a sensitivity of 88.0%. Finally, a cutoff of 77 U/mL in sIL-2R yielded a specificity of 56.7% and a sensitivity of 81.0%.
Fig. 2.
Receiver-operator characteristic (ROC) curves of the CSF IL-10, IL-6, β2-M, and sIL-2R levels (IL-10: sensitivity 0.71, specificity 1.00 at 9.5 pg/mL; IL-6: sensitivity 0.77, specificity 0.63 at 4.0 pg/mL; β2-M: sensitivity 0.90, specificity 0.88 at 2056 μg/L; sIL-2R: sensitivity 0.57, specificity 0.81 at 77 U/mL).
Relationship Between the CSF Levels of IL-10, Tumor Number, and Histology
The number of tumors (single/multiple) was not associated with the CSF levels of IL-10 (Table 1). We examined the association between dissemination and the CSF levels of IL-10 in 22 patients with PCNSL; however, there were no patients with CSF dissemination (Table 1). In 25 patients with DLBCL, the median levels of IL-10, IL-6, β2-M, and sIL-2R were 42 pg/mL, 6.6 pg/mL, 4700 μg/L, and 121 U/mL, respectively (Supplementary material, Table S2). In adddition, in 3 patients with PCNSL with IOL, the median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were 27 pg/mL, 6.5 pg/mL, 3110 μg/L, and <50 U/mL, respectively. In 3 patients with other PCNSLs, the median CSF levels of IL-10, IL-6, β2-M, and sIL-2R were <2.0 pg/mL, 3.0 pg/mL, 1297 μg/L, and 55.4 U/mL, respectively. The CSF level of IL-10 was significantly higher in the patients with DLBCLs and PCNSL with IOL than in the patients with other PCNSLs (P< .01); however, differences in IL-6, sIL-2R, and β2-M were not observed (Supplementary Fig. S2). These results indicated that the IL-10 CSF levels in patients with DLBCL are higher than in patients with other PCNSLs.
Posttreatment CSF Concentrations of IL-10 and IL-6
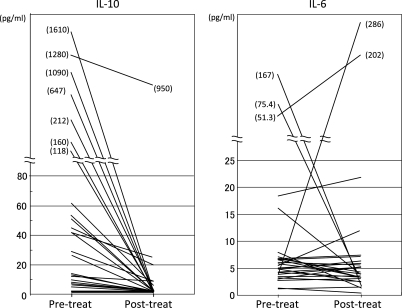
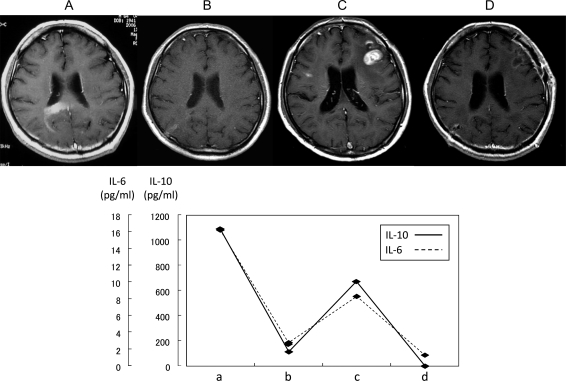
In 26 of 31 patients with PCNSLs, the CSF levels of IL-10 and IL-6 were measured after completion of therapy (Fig. 3, Table 1). The posttherapy CSF concentration of IL-10 was lower than at the pretherapy measurement in all patients analyzed. In 20 patients (80%), the CSF levels of IL-10 were below the detection limit (<2 pg/mL). The median CSF value of IL-10 was <2 pg/mL. Only 1 patient (case 1) had a high posttherapy level of IL-10 (950 pg/mL); however, this patient's pretherapy IL-10 level was higher than at posttherapy. The CSF levels of IL-6 were increased in 12 patients and decreased in 14 patients at posttherapy; the median CSF levels of posttreatment IL-6 were 4.3 pg/mL. These results suggested that the posttreatment measurements of IL-10 agreed with the tumor status in each of the patients with PCNSL who had high CSF levels of IL-10 before treatment. One example (case 2) is illustrated in Fig. 4. This patient had a PCNSL in the right occipital lobe and corpus callosum, with an IL-10 concentration of 1090 pg/mL (Fig. 4A). When the tumor rapidly regressed spontaneously without any treatment, such as steroid or antitumor drugs, the CSF concentration of IL-10 decreased to 113 pg/mL (Fig. 4B). Subsequently, tumors developed at the bilateral frontal lobes, and the CSF concentrations of IL-10 were elevated again to 675 pg/mL (Fig. 4C). After chemo-radiotherapy, the tumors disappeared (complete response) and the CSF IL-10 levels decreased to <2 pg/mL (Fig. 4D).
Fig. 3.
Changes of the CSF levels of IL-10 and IL-6 from pretreatment to posttreatment in the patients with PCNSLs.
Fig. 4.
The relationship between the state of the tumor and the CSF concentrations of IL-10 and IL-6. (Case 2) (A) Pre-treatment. Brain MRI revealed enhanced lesions in the right occipital lobe and the corpus callosum. At this time, IL-10 and IL-6 concentration were 1090 pg/mL and 16.2 pg/mL, respectively. (B) Spontaneous regression. Three weeks later, (A) the tumor rapidly regressed without any treatment. CSF concentrations of IL-10 and IL-6 fell to 113 pg/mL and 2.8 pg/mL, respectively. (C) Tumor re-progression. After spontaneous regression, the tumors developed again in the bilateral frontal lobes. CSF concentrations of IL-10 and IL-6 were elevated at 675 pg/mL and 8.3 pg/mL, respectively. (D) Post-treatment. After chemo-radiotherapy, the tumors disappeared (complete response) and CSF IL-10 and IL-6 levels fell to <2 pg/mL and 1.3 pg/mL, respectively.
CSF Levels of IL-10 at Recurrence
Tumor recurrence occurred in a total of 16 patients. In 8 of these patients, IL-10 measurements were performed at the time of recurrence. In 5 patients, the IL-10 CSF levels were elevated (Table 1). A high level of IL-10 (2400 pg/mL) was revealed in 1 patient (case 12) who had many lymphoma cells in the CSF, and diagnosis of CSF dissemination was made. (Supplementary Fig. S3). One patient (case 19) had IL-10 levels <2 pg/mL at recurrence, which was likely because both pre- and posttreatment IL-10 levels were below the detection limit (<2 pg/mL). Serial CSF measurements of IL-10 agreed with tumor status on the basis of objective criteria (neuroimaging) in each of the patients with PCNSL who had high levels of IL-10 before treatment. Two examples are depicted in Supplementary Fig. S3 and S4.
IL-10 and IL-10RA Expression in PCNSLs
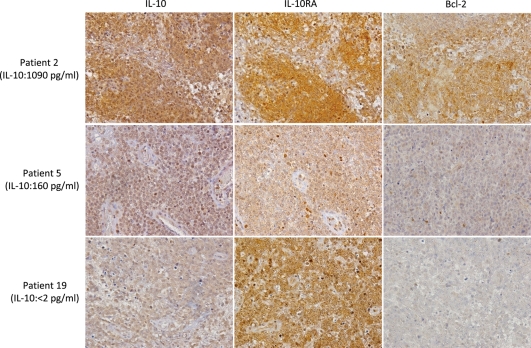
To examine whether the PCNSL tumor cells express IL-10 protein, we performed immunohistochemical examination of 28 PCNSL specimens, except for the 3 PCNSLs with IOL. In 27 of these specimens, IL-10 was expressed in tumor cells. Most of the tumor cells stained positive for IL-10. Immunohistochemical reactivity was evaluated and classified into 4 groups: negative (−), weak (1+), moderate (2+), and strong (3+) (Supplementary Fig. S5). Negative, weak, moderate, and strong expression was observed in 1 case (3%), 7 cases (25%), 10 cases (36%), and 10 cases (36%), respectively. Of 6 patients with high levels of IL-10 (>100 pg/mL), 4 patients showed strong expression of IL-10 in lymphoma cells and 2 patients showed moderate expression of IL-10. However, of 5 patients with an IL-10 value below the detection limits, the strong, moderate, weak, and negative expression of IL-10 in lymphoma cells was observed in 1 case, 1 case, 2 cases, and 1 case, respectively. Next, PCNSL specimens were classified into 2 groups based on the CSF levels of IL-10: >20 pg/mL was defined as high CSF IL-10, and <20 pg/mL was defined as low CSF IL-10. In the group with high CSF IL-10 (n = 15), negative, weak, moderate, and strong expression was observed in 0 cases (0%), 1 case (7%), 5 cases (33%), and 9 cases (60%), respectively (Table 2). On the other hand, in the group of low CSF IL-10 (n = 13), negative, weak, moderate, and strong expression was observed in 1 case (8%), 6 cases (46%), 5 cases (38%), and 1 case (8%), respectively (Table 2). There was no significant difference between the high CSF IL-10 group and the low CSF IL-10 group in terms of IL-10 immunoreactivities in tumor cells. However, the patients expressing high levels of IL-10 in the CSF had a tendency to express high IL-10 levels in the tumor cells (Fig. 5).
Table 2.
Relationship between CSF IL-10 and immunostaining of IL-10, IL-10RA and Bcl-2 in lymphoma cells
| CSF IL-10 | CSF IL-10 | ||
|---|---|---|---|
| Low (<20 pg/mL) | High (>20 pg/mL) | ||
| n = 13 | n = 15 | ||
| IL-10 expression | – | 1 | 0 |
| 1+ | 6 | 1 | |
| 2+ | 5 | 5 | |
| 3+ | 1 | 9 | |
| IL-10RA expression | – | 1 | 0 |
| 1+ | 3 | 1 | |
| 2+ | 2 | 7 | |
| 3+ | 7 | 7 | |
| Bcl-2 expression | – | 6 | 0 |
| 1+ | 2 | 6 | |
| 2+ | 3 | 2 | |
| 3+ | 2 | 7 |
Fig. 5.
Immunohistochemistry of IL-10, IL-10RA, and Bcl-2 in 3 patients with PCNSL. Upper panels: Patient 2 displays a very high CSF concentration of IL-10. Expression levels of IL-10, IL-10R, and Bcl-2 were all strong (3+). Middle panels: Patient 5 displays high CSF concentration of IL-10. Expression levels of IL-10, IL-10R, and Bcl-2 were moderate (2+), moderate (2+), weak (1+), respectively. Lower panels: Patient 19 displays a CSF concentration of IL-10 that is below the detection limit. Expression levels of IL-10, IL-10R, and Bcl-2 were moderate (2+), strong (3+), and weak (1+), respectively. (Original magnification: 400×).
To compare the expression levels of IL-10 in PCNSLs with those in other brain tumors, we investigated immunohistochemstry of IL-10 with use of 14 glioma specimens (9 glioblastomas, 1 anaplastic astrocytoma, 1 diffuse astrocytoma, 1 anaplastic oligodendroglioma, 1 oligodendroglioma, and 1 ependymoma). Negative, weak, moderate, and strong expression was observed in 2 cases, 6 cases, 6 cases, and 0 cases, respectively. Compared with PCNSLs, the expression levels of IL-10 in glioma cells were relatively low (Supplementary Fig. S6).
Next, we examined IL-10RA expression in PCNSL cells. Most of the tumor cells were positive for IL-10RA and IL-10. Negative, weak, moderate, and strong expression was observed in 1 case (4%), 4 cases (3%), 9 cases (29%), and 14 cases (45%), respectively (Table 2). However, there was no association between CSF IL-10 levels and IL-10RA expression in tumor cells (Table 2).
Association of IL-10 Levels with Bcl-2 Expression
Previous studies determined that the activation of IL-10R by IL-10 results in an increase in activated STAT3 and subsequent Bcl-2 expression.13,14,23 Therefore, to examine the correlation between CSF IL-10 levels and the protein expression of Bcl-2 in PCNSLs, immunohistochemical analyses were performed. The PCNSL samples were classified into 2 groups based on the CSF levels of IL-10 and the levels of IL-10 and IL-10RA. In 15 cases in the group with high CSF IL-10, the negative, weak, moderate, and strong expressions of Bcl-2 were observed in 0 cases (8%), 6 cases (50%), 2 cases (50%), and 7 cases (42%), respectively (Table 2). On the other hand, in 13 cases with low CSF IL-10, the negative, weak, moderate, and strong expressions of Bcl-2 were seen in 6 cases (46%), 2 cases (46%), 3 cases (38%), and 2 cases (16%), respectively. There was no statistically significant difference between these groups. However, Bcl-2 expression was moderately higher in the group with high CSF IL-10 than in the group with low CSF IL-10. Next, we analyzed the correlation between the IL-10 and Bcl-2 immunoreactivities in tumor cells using Spearman's rank test. Significant correlation was determined (P= .0077). In addition, there were significant correlations between IL-10RA expression and Bcl-2 expression in PCNSL cells (P= .038). These results indicate that IL-10 and IL-10RA expression levels in tumor cells may influence Bcl-2 expression.
Association of CSF IL-10 Levels with Prognosis
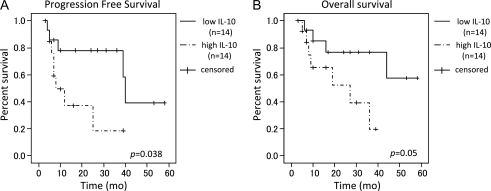
During initial treatment, 21 patients underwent chemotherapy plus radiotherapy, 5 patients underwent radiotherapy alone, 2 patients underwent chemotherapy alone, and 3 patients underwent steroid therapy alone. After treatment, 22 patients had a CR, 6 patients had a PR, and 3 patients had PD. In 28 patients treated with radiotherapy and/or chemotherapy, the pretreatment CSF levels of IL-10 and IL-6 were not associated with treatment response. In addition, CSF levels of IL-10 and IL-6 after therapy were not associated with the treatment response. The mean follow-up period was 21.9 months (range, 3–58 months). Of 28 patients treated with radiotherapy and/or chemotherapy, 17 patients survived and 11 patients died. Median survival time was 44 months, and the median time to progression was 39 months. With use of a univariate analysis with Cox's proportional hazards model and the log-rank test, elevated CSF levels of IL-10 were significantly associated with having a shorter PFS (hazard ratio [HR], 3.37; 95% confidence interval [CI], 0.985–11.528; log-rank, P= .038) (Fig. 6A). In addition, no statistically significant association was identified between the CSF IL-10 levels and OS; however, trends were observed for the elevated CSF IL-10 level and having shorter OS (HR, 3.58; 95% CI, 0.918–13.94; log-rank, P= .050) (Fig. 6B). No statistically significant correlation was identified between OS or PFS and the CSF levels of IL-6, β2-M, or IL-2R. In addition, IL-10, IL-10R, and Bcl-2 expression levels in tumor cells were not statistically associated with OS or PFS.
Fig. 6.
(A) Comparison of Kaplan–Meier PFS curves according to CSF IL-10 levels (low IL-10: <28 pg/mL (n = 14), high IL-10: ≥28 pg/mL (n = 14)) (log-rank test). (B) Comparison of Kaplan-Meier OS curves according to CSF IL-10 levels (low IL-10: <28 pg/mL (n = 14), high IL-10: ≥28 pg/mL (n = 14)) (log-rank test).
Discussion
In this study, we determined that measuring IL-10 levels in the CSF is a useful tool for the diagnosis of PCNSL. A high CSF IL-10 level was observed in most of the patients with PCNSLs, especially in DLBCL and PCNSL with IOL. Of surprise, all 59 other brain tumor types analyzed, except for 1 anaplastic ependymoma, had a CSF concentration of IL-10 below the detection limit (<2 pg/mL). On the basis of the ROC curve, sensitivity was 71.0% and specificity was 100% at an IL-10 level of 9.5 pg/mL (Fig. 2). Both sensitivity and specificity of CSF IL-10 were better than those of CSF IL-6 or sIL-2R and are similar to those of CSF β2-M. Three patients had very high concentrations of IL-10 (>1000 pg/mL) before treatment. In contrast, 5 cases of PCNSLs had IL-10 levels below the detection limit (<2 pg/mL) before treatment. Of these, 2 cases were T cell lymphoma, and 1 case was an unclassified PCNSL. In this study, CSF IL-10 levels were associated with lymphoma histology; however, further studies are required because the number of patients with T cell lymphoma or other types of lymphoma was very small.
Preoperative diagnosis is very important for neurosurgeons, because the surgical procedure is different for different intracranial tumor types. For example, total resection is frequently required for metastatic brain tumors, and gross total resection is the standard care for malignant gliomas. However, a biopsy or partial removal is sufficient in PCNSLs, because chemo-radiotherapy is quite effective. Therefore, if the preoperative diagnosis is PCNSL, stereotactic biopsy by burr hole surgery would be selected. In contrast, if the preoperative findings strongly suggested another brain tumor, such as malignant glioma, tumor removal with large craniotomy would be performed. Radiographically, PCNSLs can simulate other brain tumors, such as malignant gliomas, germ cell tumors, and brain metastases.3 MR spectroscopy and perfusion MRI seem to be helpful tools when showing some suggestive abnormalities; however, none of these signs is specific.3 In our prospective study, preoperative IL-10 measurement of CSF was practically very useful in clinical decision-making. For example, when the IL-10 levels were low and the tumor was resectable, tumor removal with craniotomy was performed. In contrast, when the tumor was large and resectable but the CSF IL-10 was high, stereotactic biopsy or open biopsy with local anesthesia was selected and postoperative chemo-radiotherapy could be started immediately. The results of our study indicate that a high IL-10 CSF level, in addition to neuroimaging of the brain tumor, may establish the presence of PCNSL and may help neurosurgeons make a prompt decision regarding brain biopsy.
Several proteins in the CSF are reported to be helpful for the diagnosis of PCNSL. Jeffery et al. found that the CSF β2-M level is a useful adjunct to the cytological diagnosis of CNS lymphoma.24 In addition, Kersten et al. reported that soluble CD27 levels in the CSF are elevated in patients with meningeal localization of lymphoid malignancies involving PCNSLs.25 In addition, the CSF level of sIL-2R is reported to be a valuable marker of CNS involvement in patients with NHL or acute lymphocytic leukemia.26,27 Recently, Roy et al. proposed that an elevated CSF level of antithrombin III was associated with the presence of CNS lymphoma.28
The high level of IL-10 in the CSF of patients with PCNSL was first reported in 1997;22 however, there are only 2 reports on this topic. Whitcup et al. found that the elevated CSF levels of IL-10 were associated with the presentation of malignant cells in CSF in patients with PCNSLs.22 They also showed that an IL-10/IL-6 ratio >1 is associated with CNS lymphoma. Subsequently, Salmaggi et al. analyzed the CSF levels of IL-10 in various brain tumors, including PCNSLs.21 The CSF IL-10 level in PCNSL (mean, 14.8 ± 23 pg/mL) was significantly higher than in other CNS tumors (mean, 0.23 ± 0.26 pg/mL), which is consistent with our results. However, the number of patients analyzed in their study was very small (11 cases of PCNSLs and 11 cases of other CNS tumors), compared with our study (31 cases of PCNSLs and 59 cases of other CNS tumors).
Several investigators have measured the CSF level of IL-10 in patients with other diseases. Gallo et al. investigated CSF IL-10 levels in 120 patients with various inflammatory and noninflammatory diseases of the CNS and observed high levels of IL-10 in patients with acute viral (aseptic) meningitis, HIV-related encephalitis, HIV-related cryptococcal meningitis, and encephalomeningeal sarcoidosis.29 Luca et al. also observed high levels of IL-10 in various AIDS-related CNS diseases, such as AIDS-related CNS lymphoma, cryptococcal meningitis, and Toxoplasma gondii encephalitis.30 Furthermore, the level of IL-10 in the CSF was markedly high in patients with eosinophilic meningitis associated with angiostrongyliasis.31 In contrast, in patients with neuro-Behcet,32 multiple sclerosis,32 tuberculous meningitis,33 Alzheimer's disease,34 and migraine headache,35 the CSF level of IL-10 was not elevated. Recently, Krzyszkowski et al. reported that the CSF level of IL-10 was decreased in patients with high-grade astrocytoma, compared with that in age- and sex-matched control patients.36 Their results are very interesting, because in our study, IL-10 CSF levels in the patients with high-grade astrocytoma were below the detection limit (<2 pg/mL).
Here, the CSF level of IL-10 was not elevated in the patients with CNS T cell lymphoma. Although IL-10 is one of the cytokines implicated in the pathogenesis of DLBCL and acts as an autocrine or paracrine growth factor for B cell lymphoma cells, some evidence suggests that IL-10 may be associated with progression in T cell NHLs.37 Increased production of IL-10 mRNA was demonstrated in peripheral T cell lymphomas and nasal natural-killer/T cell lymphomas.38,39 In addition, there have been some reports of high IL-10 level in the serum of T cell lymphoma.40–42 In our study, tumor cells in 2 patients with CNS T cell lymphoma were positive for IL-10; however, both patients had low CSF levels of IL-10. Because there were only 2 patients with T cell lymphoma in this study, additional studies should be performed to examine the CSF level of IL-10 in patients with CNS T cell lymphomas.
Several reports have shown that an elevated IL-10 plasma level is correlated with poor prognosis in systemic DLBCL.15,16,43,44 On the basis of these results, Nacinović-Duletić et al. proposed that the IL-10 serum level before treatment of patients with systemic DLBCL may give some insight into the prognosis and, thus, facilitate the therapeutic decision-making process for individual patients.16 In addition to DLBCL, high IL-10 serum levels were reported to be an unfavorable prognostic factor for patients with Hodgkin lymphoma17 and adult T cell leukemia/lymphoma.40 However, no studies have linked the CSF level or serum level of IL-10 to prognosis of PCNSL. In this study, a significant association was found between PFS and the CSF level of IL-10. In addition, trends were observed for patients having elevated CSF IL-10 levels to shorter OS. To our knowledge, ours is the first report to associate a higher CSF level of IL-10 with an unfavorable prognosis in patients with PCNSL.
High levels of Bcl-2 are known to be a poor prognostic factor in patients with lymphoma. Bcl-2 is detectable in 56%–85% of PCNSL cases.45,46 In this study, 76% of PCNSLs were positive for Bcl-2 by immunohistochemical analysis; however, there was no association between Bcl-2 expression and OS or PFS. IL-10/IL-10R interaction leads to survival of lymphocytes via tyrosine phosphorylation of JAK1 and TYK2.47 In addition, several reports have described the association of IL-10 with Bcl-2 through the transcriptional factor STAT3.13,14,23 In our study, patients with high levels of CSF IL-10 had a tendency to exhibit high expression of Bcl-2 in lymphoma cells. In addition, expression levels of IL-10 in tumor cells were significantly correlated with Bcl-2 expression levels. In contrast, Bcl-2 expression was very low in most of the gliomas, which also exhibit low levels of IL-10 in tumor cells and in the CSF (data not shown). Although various factors regulate Bcl-2 expression48 and although the exact role of IL-10 in the pathogenetic mechanisms of proliferation and apoptosis of PCNSL cells has not yet been fully established, our results indicate that the CSF level of IL-10 might be associated with Bcl-2 expression in PCNSLs.
In conclusion, the IL-10 levels in the CSF were identified as being a useful marker in the diagnosis of PCNSLs, especially of DLBCL. PCNSL progression and therapeutic response were reflected by the increase and decrease in the CSF levels of IL-10 in many of the patients. In addition, this study suggests a significant association between the elevated CSF level of IL-10 and an unfavorable prognosis; however, the number of patients analyzed in this study was small. Therefore, larger numbers of patients are required to determine whether an elevated CSF level of IL-10 is truly associated with poor prognosis and outcome.
Supplementary Material
Funding
This work was supported in part by Grants-in-Aid for Scientific Research to Takashi Sasayama (22591610) and to Katsu Mizukawa (22791344) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Supplementary Material
Acknowledgments
We thank Mariko Ueda for technical assistance with the immunohistochemical analysis.
Conflict of interest statement. None declared.
References
- 1.Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. doi:10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- 2.Sierra del Rio M, Rousseau A, Soussain C, et al. Primary CNS lymphoma in immunocompetent patients. Oncologist. 2009;14:526–539. doi: 10.1634/theoncologist.2008-0236. doi:10.1634/theoncologist.2008-0236. [DOI] [PubMed] [Google Scholar]
- 3.Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. doi:10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein M, Berger M. Neuro-Oncology: The Essentials. New York, NY: Thieme Medical Publishers; 2000. [Google Scholar]
- 5.Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17:554–560. doi: 10.1200/JCO.1999.17.2.554. [DOI] [PubMed] [Google Scholar]
- 6.Salles G, Coiffier B. Inherited cytokine response and risk of lymphoma. Lancet Oncol. 2006;7:3–4. doi: 10.1016/S1470-2045(05)70513-1. doi:10.1016/S1470-2045(05)70513-1. [DOI] [PubMed] [Google Scholar]
- 7.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92:1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. doi:10.1002/1097-0142(20010915)92:6+<1684::AID-CNCR1497>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Yee C, Biondi A, Wang XH, et al. A possible autocrine role for interleukin-6 in two lymphoma cell lines. Blood. 1989;74:798–804. [PubMed] [Google Scholar]
- 9.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. doi:10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 10.Moore KW, de Waal Malefyt R, Coffman RL. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. doi:10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 11.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. doi:10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Garra A, Barrat FJ, Castro AG. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. doi:10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 13.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's lymphoma to apoptosis. Clin Cancer Res. 2001;7:709–723. [PubMed] [Google Scholar]
- 14.Vega MI, Huerta-Yepaz S, Garban H, et al. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23:3530–3540. doi: 10.1038/sj.onc.1207336. doi:10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- 15.el-Far M, Fouda M, Yahya R. Serum IL-10 and IL-6 levels at diagnosis as independent predictors of outcome in non-Hodgkin's lymphoma. J Physiol Biochem. 2004;60:253–258. doi: 10.1007/BF03167070. doi:10.1007/BF03167070. [DOI] [PubMed] [Google Scholar]
- 16.Nacinović-Duletić A, Stifter S, Dvornik S. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30:230–239. doi: 10.1111/j.1751-553X.2007.00951.x. doi:10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 17.Vassilakopoulos TP, Nadali G, Angelopoulou MK, et al. Serum interleukin-10 levels are an independent prognostic factor for patients with Hodgkin's lymphoma. Haematologica. 2001;86:274–281. [PubMed] [Google Scholar]
- 18.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259. doi: 10.1167/iovs.06-0031. doi:10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13:411–418. doi: 10.1097/00055735-200212000-00012. doi:10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Cassoux N, Merle-Beral H, Lehoang P. Interleukin-10 and intraocular-central nervous system lymphoma. Ophthalmology. 2001;108:426–427. doi: 10.1016/s0161-6420(00)00401-2. doi:10.1016/S0161-6420(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 21.Salmaggi A, Eoli M, Corsini E, et al. Cerebrospinal fluid interleukin-10 levels in primary central nervous system lymphoma: a possible marker of response to treatment? Ann Neurol. 2000;47:137–138. [PubMed] [Google Scholar]
- 22.Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 23.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 2001;61:5137–5144. [PubMed] [Google Scholar]
- 24.Jeffery GM, Frampton CM, Legge HM. Cerebrospinal fluid β2-microglobulin levels in meningeal involvement by malignancy. Pathology. 1990;22:20–23. doi: 10.3109/00313029009061421. doi:10.3109/00313029009061421. [DOI] [PubMed] [Google Scholar]
- 25.Kersten MJ, Evers LM, Dellemijn PL, et al. Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood. 1996;87:1985–1989. [PubMed] [Google Scholar]
- 26.Chang CS, Liu HW, Lin SF. Soluble interleukin-2 receptor levels in cerebrospinal fluid of patients with acute lymphocytic leukemia or with non-Hodgkin's lymphoma. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1989;22:132–137. [PubMed] [Google Scholar]
- 27.Lee W, Kim SJ, Lee S, et al. Significance of cerebrospinal fluid sIL-2R level as a marker of CNS involvement in acute lymphoblastic leukemia. Ann Clin Lab Sci. 2005;35:407–412. [PubMed] [Google Scholar]
- 28.Roy S, Josephson SA, Fridlyand J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol. 2008;26:96–105. doi: 10.1200/JCO.2007.12.1053. doi:10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo P, Sivieri S, Rinaldi L, et al. Intrathecal synthesis of interleukin-10 (IL-10) in viral and inflammatory diseases of the central nervous system. J Neurol Sci. 1994;126:49–53. doi: 10.1016/0022-510x(94)90093-0. doi:10.1016/0022-510X(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 30.De Luca A, Antinori A, Cingolani A, et al. Evaluation of cerebrospinal fluid EBV-DNA and IL-10 as markers for in vivo diagnosis of AIDS-related primary central nervous system lymphoma. Br J Haematol. 1995;90:844–849. doi: 10.1111/j.1365-2141.1995.tb05205.x. doi:10.1111/j.1365-2141.1995.tb05205.x. [DOI] [PubMed] [Google Scholar]
- 31.Intapan PM, Kittimongkolma S, Niwattayakul K, et al. Cerebrospinal fluid cytokine responses in human eosinophilic meningitis associated with angiostrongyliasis. J Neurol Sci. 2008;267:17–21. doi: 10.1016/j.jns.2007.09.023. doi:10.1016/j.jns.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Saruhan-Direskeneli G, Yentür SP, Akman-Demir G, et al. Cytokines and chemokines in neuro-Behçet's disease compared to multiple sclerosis and other neurological diseases. J Neuroimmunol. 2003;145:127–134. doi: 10.1016/j.jneuroim.2003.08.040. doi:10.1016/j.jneuroim.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Kashyap RS, Deshpande PS, Ramteke SR, et al. Changes in cerebrospinal fluid cytokine expression in tuberculous meningitis patients with treatment. Neuroimmunomodulation. 2010;17:333–339. doi: 10.1159/000292023. doi:10.1159/000292023. [DOI] [PubMed] [Google Scholar]
- 34.Rota E, Bellone G, Rocca P, et al. Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer's disease patients. Neurol Sci. 2006;27:33–39. doi: 10.1007/s10072-006-0562-6. doi:10.1007/s10072-006-0562-6. [DOI] [PubMed] [Google Scholar]
- 35.Bø SH, Davidsen EM, Gulbrandsen P, et al. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia. 2009;29:365–372. doi: 10.1111/j.1468-2982.2008.01727.x. doi:10.1111/j.1468-2982.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 36.Krzyszkowski T, Dziedzic T, Czepko R. Decreased levels of interleukin-10 and transforming growth factor-beta 2 in cerebrospinal fluid of patients with high grade astrocytoma. Neurol Res. 2008;30:294–296. doi: 10.1179/016164107X235149. doi:10.1179/016164107X235149. [DOI] [PubMed] [Google Scholar]
- 37.Lee JJ, Kim DH, Lee NY, et al. Interleukin-10 gene polymorphism influences the prognosis of T-cell non-Hodgkin lymphomas. Br J Haematol. 2007;137:329–336. doi: 10.1111/j.1365-2141.2007.06570.x. doi:10.1111/j.1365-2141.2007.06570.x. [DOI] [PubMed] [Google Scholar]
- 38.Ho JW, Liang RH, Srivastava G. Preferential type 1-1 cytokine gene expressions in peripheral T-cell lymphomas. Hematol Oncol. 1999;17:117–129. doi: 10.1002/(sici)1099-1069(199909)17:3<117::aid-hon640>3.0.co;2-1. doi:10.1002/(SICI)1099-1069(199909)17:3<117::AID-HON640>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Shen L, Chiang AK, Liu WP, et al. Expression of HLA class I, beta(2)-microglobulin, TAP1 and IL-10 in Epstein-Barr virus-associated nasal NK/T-cell lymphoma: implications for tumor immune escape mechanism. Int J Cancer. 2001;92:692–696. doi: 10.1002/1097-0215(20010601)92:5<692::aid-ijc1237>3.0.co;2-z. doi:10.1002/1097-0215(20010601)92:5<692::AID-IJC1237>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Inagaki A, Ishida T, Ishii T, et al. Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer. 2006;118:3054–3061. doi: 10.1002/ijc.21688. doi:10.1002/ijc.21688. [DOI] [PubMed] [Google Scholar]
- 41.Bhat PV, Jakobiec FA, Papaliodis G. Primary T-cell lymphoma of the retina and cerebellum: immunophenotypic and gene rearrangement confirmation. Am J Ophthalmol. 2009;148:350–360. doi: 10.1016/j.ajo.2009.04.005. doi:10.1016/j.ajo.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Lighter J, Tse DB, Kaul A. Hyper interleukin-10 in an HIV-positive child with t-cell lymphoma and candidal sepsis. J Int Assoc Physicians AIDS Care (Chic Ill) 2008;7:228–231. doi: 10.1177/1545109708325467. doi:10.1177/1545109708325467. [DOI] [PubMed] [Google Scholar]
- 43.Lech-Maranda E, Bienvenu J, Broussais-Guillaumot F, et al. Plasma TNF-alpha and IL-10 level-based prognostic model predicts outcome of patients with diffuse large B-Cell lymphoma in different risk groups defined by the International Prognostic Index. Arch Immunol Ther Exp (Warsz) 2010;58:131–141. doi: 10.1007/s00005-010-0066-1. doi:10.1007/s00005-010-0066-1. [DOI] [PubMed] [Google Scholar]
- 44.Ozdemir F, Aydin F, Yilmaz M, et al. The effects of IL-2, IL-6 and IL-10 levels on prognosis in patients with aggressive Non-Hodgkin's Lymphoma (NHL) J Exp Clin Cancer Res. 2004;23:485–488. [PubMed] [Google Scholar]
- 45.Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190–196. doi: 10.1182/blood-2005-03-1024. doi:10.1182/blood-2005-03-1024. [DOI] [PubMed] [Google Scholar]
- 46.Raoux D, Duband S, Forest F, et al. Primary central nervous system lymphoma: Immunohistochemical profile and prognostic significance. Neuropathology. 2010;30:232–240. doi: 10.1111/j.1440-1789.2009.01074.x. doi:10.1111/j.1440-1789.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 47.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155(3):1079–1090. [PubMed] [Google Scholar]
- 48.Park YH, Sohn SK, Kim JG, et al. Interaction between BCL2 and interleukin-10 gene polymorphisms alter outcomes of diffuse large B-cell lymphoma following rituximab plus CHOP chemotherapy. Clin Cancer Res. 2009;15:2107–2115. doi: 10.1158/1078-0432.CCR-08-1588. doi:10.1158/1078-0432.CCR-08-1588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.