Abstract
Stem cells have generated great interest in the past decade as potential tools for cell-based treatment of human high-grade gliomas. Thus far, 3 types of stem cells have been tested as vehicles for various therapeutic agents: embryonic, neural, and mesenchymal. The types of therapeutic approaches and/or agents examined in the context of stem cell–based delivery include cytokines, enzyme/prodrug suicide combinations, viral particles, matrix metalloproteinases, and antibodies. Each strategy has specific advantages and disadvantages. Irrespective of the source and/or type of stem cell, there are several areas of concern for their translation to the clinical setting, such as migration in the adult human brain, potential teratogenesis, immune rejection, and regulatory and ethical issues. Nonetheless, a clinical trial is under way using neural stem cell–based delivery of an enzyme/prodrug suicide combination for recurrent high-grade glioma. A proposed future direction could encompass the use of stem cells as vehicles for delivery of agents targeting glioma stem cells, which have been implicated in the resistance of high-grade glioma to treatment. Overall, stem cells are providing an unprecedented opportunity for cell-based approaches in the treatment of high-grade gliomas, which have a persistently dismal prognosis and mandate a continued search for therapeutic options.
Keywords: cancer stem cells, cell-based therapy, high-grade glioma, stem cells
The use of stem cells (SC) as therapeutic vehicles for brain tumors has garnered much attention over the past decade. This is attributable to the fundamental ability of SC to migrate, or home, to brain tumors1 irrespective of the blood brain barrier (BBB) and to be manipulated into expressing various therapeutic molecules.2 These characteristics, together with their inherent immunosuppressive properties3–5 and the difficulties encountered in the use of viruses in gene therapy clinical trials 6 spurred the exploration of SC as vehicles for cell-based therapy of human high-grade gliomas (hHGG), the most common and devastating type of primary malignant brain tumor.
Thus far, hHGG continue to carry an extremely poor prognosis. Patients with glioblastoma, the most common type of hHGG 7,8 have an overall survival of less than 10% at 5 years after standard-of-care treatment with surgery, ionizing radiation, and temozolomide.9 Recent evidence has revealed the presence of cancer SC in gliomas, also known as glioma stem cells (GSC), and suggested that they may be the culprits behind the resistance of hHGG to therapy.10
Initial strategies to improve delivery of genes or other therapeutic agents for hHGG used neural stem cells (NSC) as vehicles 2 but as knowledge of SC expanded, mesenchymal stem cells (MSC)11 and embryonic stem cells (ESC)12 were also tested. Important to the development of SC as vehicles were observations in preclinical models that SC have immunomodulatory functions enabling immune evasion and suppression of the immune system, particularly of T cells 3–5 the main effectors of cellular rejection. In NSC, this effect has been postulated to be indirect via peripheral mechanisms 3 whereas MSC and ESC appear to have more direct effects.4,5 In addition, MSC have been reported to induce T cell apoptosis4 and ESC to have diminished T cell activation from low major histocompatibility molecule expression, although susceptible to epigenetic modification.5
Preclinical testing of SC-based therapies is typically performed in immunodeficient mouse models in which tumors are created by the injection of hHGG cells either intracranially or into the flank.13 Intracranial injection of hHGG cells (i.e., orthotopic xenograft model) has the advantage of providing a native environment. However, it has significant limitations 13 including low histopathologic similarity of the resultant tumors to clinical ones and the inability to recapitulate tumor-specific immune responses with implications for SC migration. These limitations heighten concern over the translation of results to the clinic, particularly with respect to SC migration as highlighted in the discussion. Nevertheless, this type of model is a mainstay of preclinical testing based on a number of practical factors, such as cost, availability, and ease of handling.13,14
To date, SC have been manipulated to deliver the following: cytokines, enzyme/prodrug suicide combinations, viral particles, matrix metalloproteinases, and antibodies. Table 1 provides a summary of the agents delivered by SC, as discussed below. Of note, the therapeutic agents are classified according to the final target being delivered, because viruses are often used to transfect SC. Viral particles refer to oncolytic viruses, where by definition, the virus is the effector mechanism.
Table 1.
Summary of stem cells (SC) as vehicles for the treatment of human high-grade glioma (hHGG).
| SC | Therapeutic strategy | Agent | (Refs) |
|---|---|---|---|
| Embryonic | cytokine | TRAIL | (18,21,23) |
| mda-7/IL-24 | (24) | ||
| Neural | cytokine | IL-4 | (26) |
| IL-12 | (27) | ||
| IL-23 | (28) | ||
| TRAIL ± BMZ | (29,30) | ||
| S-TRAIL ± MIR/TMZ | (31,32,34,35) | ||
| enzyme/prodrug | tk/GCV | (37–40) | |
| CD/5FC ± IFNβ | (41–44) | ||
| viral particles | mutant HSV-1 | (45) | |
| CRAd-survivin | (46,47,49) | ||
| matrix metalloproteinase | PEX | (51) | |
| Mesenchymal | cytokine | IL-2 | (53) |
| IFNβ | (55) | ||
| IFNα | (56) | ||
| IL-18 | (57) | ||
| TRAIL ± PI3KI | (58–61,64) | ||
| IL-12 | (62,63) | ||
| enzyme/prodrug | tk/GCV | (66,67) | |
| viral particles | CRAd-CXCR4 | (68) | |
| CRAd-Rb | (69) | ||
| CRAd-survivin | (71) | ||
| antibody | EGFRvIII | (72,73) |
ESC
ESC are found in the inner cell mass of a blastocyst formed after the union of sperm and egg.15 A major advantage of ESC over other types of SC is their capacity to be permanently and genetically modified using homologous recombination.12 The enthusiasm of using ESC is tempered by the regulatory, political, and ethical issues behind their procurement.16 Recent work on inducible pluripotent stem cells (iPSC), for which patient-specific cells may be easily obtained from peripheral blood and reprogrammed into pluripotent SC similar to ESC, may overcome these limitations.17 However, no studies to date have tested iPSC as vehicles for cell-based therapy against hHGG. Experimental use of ESC for hHGG has focused on cytokine delivery.
Cytokines
With use of a novel and unique approach, ESC were genetically modified to express a doxycyline-inducible transgene and differentiated into astrocytes with more than 95% purity per flow cyometric analysis of glial fibrillary acidic protein.18 The astrocyte lineage was chosen as the differentiation end point for several reasons. Astrocytes are highly secretory cells with intracellular machinery in place for the secretion of multiple growth factors and metabolic intermediates critical for neuronal function.19 Astrocytes do not interfere with neural circuitry and migrate along white matter tracts.18,20 The transgene of choice was tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), based on its ability to selectively induce apoptosis in tumor cells.20 ESC-derived astrocyte-mediated delivery of TRAIL significantly induced apoptosis of hHGG in vitro21,22 and in vivo.23 Because the effects of TRAIL were not uniform across hHGG lines 23 ESC-astrocytes were engineered with doxycycline-inducible melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24), a cytokine that induces apoptosis in a variety of cancer cells while sparing normal ones via viral-mediated delivery.24,25 When cocultured with hHGG cells, mda7/IL24-expressing ESC-derived astrocytes increase hHGG apoptosis, autophagy, and radiosensitivity and overcome resistance to TMZ.24 Further work is under way to confirm these results in vivo.
NSC
In addition to tumor tropism, infiltrative potential across the BBB and manipulation of cell survival times in vivo, a major advantage of NSC is that it is the only SC native to the brain.2 Disadvantages include the possibility of de-differentiation. NSC have been used in 4 therapeutic strategies. In order of frequency, these consist of cytokines, enzyme/prodrug suicide combinations, viral particles, and matrix metalloproteinases.
Cytokines
The most frequently delivered therapeutic agents using NSC are cytokines. One of the first studies to report the use of NSC as cell-based vehicles for gene therapy delivered interleukin (IL)–4 to experimental hHGG.26 Investigators found increased survival rates with administration of IL-4 expressing NSC into established experimental hHGG.26 The second study tested IL-12 with similar effects and corroborated the technical feasibility of cytokine delivery via NSC.27 NSC-mediated delivery of IL-23, belonging to the same family as IL-12, also resulted in increased survival times in mice via increased cytotoxic T cell and natural killer cell responses.28 Finally, intracranial injection of TRAIL-expressing NSC also resulted in tumor apoptosis and reduction of tumor area.29 Efficacy of TRAIL-expressing NSC was enhanced by the concomitant administration of the proteosome inhibitor bortezomib.30 An additional technique that increased the effectiveness of TRAIL-based NSC delivery was creation of a soluble form of TRAIL (S-TRAIL) by fusion of the extracellular domain of TRAIL with that of Flt3L, a ligand for the Flt3 tyrosine kinase receptor.31,32 This is in keeping with experiments in rodent models showing that adenoviral-mediated delivery of Flt3L alone inhibits glioma growth and improves survival.33 S-TRAIL–expressing NSC have also been combined with other strategies, such as microRNAs34 and TMZ 35 with positive results. Novel molecular imaging techniques allowed noninvasive confirmation of NSC tumor infiltration.32 These experimental results, combined with the increasing repertoire of TRAIL-based clinical trials for other cancers 36 warrant continued development of SC-based TRAIL delivery.
Enzyme/Prodrug
Two enzyme/prodrug systems for suicide gene therapy have been transfected into NSC for hHGG treatment: thymidine kinase/ganciclovir (tk/GCV) and cytosine deaminase/5-fluorocytosine (CD/5FC). In these systems, after administration of the prodrug, the enzyme converts the prodrug into its active form (phosphorylated ganciclovir and 5-fluorouracil, respectively), which interferes with DNA synthesis and induces chain termination apoptosis in proliferating tumor cells, causing them to commit “suicide”. Although the herpes simplex virus (HSV) tk/GCV system is the most well-studied in viral-mediated gene therapy 6 a comparably small number of studies have examined its efficacy in NSC. Evaluation in rodent intracranial models confirmed NSC migratory abilities after transfection and demonstrated reduction in tumor growth attributable to bystander effects.37–39 Bystander effects correlated with expression of gap junctions and were dependent on cell-to-cell contact.40 Although seen by some as a drawback because of the potential for gap junction modification in hHGG, others argue it is a major reason for lower neurotoxicity, compared with CD/5FC, in which delivery is based on diffusion.40,41 Studies examining the therapeutic efficacy of NSC-mediated delivery of CD/5FC also found reductions in tumor volumes and extended survival in experimental rodent models.41–43 This was accompanied by significant neurotoxicity as manifested by peritumoral necrosis, edema, demyelination, and hydrocephalus.42 NSC double transduction of CD/5FC and interferon (IFN)–β shows promising results 44 but the potential for neurotoxicity inherent in this strategy remains a serious concern.
Viral Particles
Data on the delivery of viral particles via NSC are limited, with relatively few reports in the literature.45–47 The first was a proof-of-concept study demonstrating the feasibility of loading NSC with a conditionally replicating mutant HSV-1 (mutant HSV-1) and manipulating both viral replication and particle release with the use of mimosine (an agent that can arrest viral replication without compromising NSC) to achieve distribution throughout tumor parenchyma.45 The second study loaded NSC with a conditionally replicating adenovirus under control of a survivin promoter (CRAd-survivin) overexpressed in hHGG cells to restrict viral replication to these cells; in addition to demonstrating viral particle delivery in vitro, authors demonstrated that virally loaded NSC retained their migratory capacity and could reduce tumor volume in a subcutaneous hHGG model.46 A subsequent study in an orthotopic xenograft model using this system extended these results and demonstrated a significant increase in median survival and upregulation of chemoattractant receptors.47 Several hurdles are inherent in the use of NSC for the delivery of viral particles, not the least of which is to achieve an adequate balance between viral loading and lysis of the carrier cell. Areas of investigation that could enhance the development of NSC adequate for preclinical and/or clinical studies include improving the transduction and extending the life-span of virus-loaded carrier cells.48 A recent direct comparison between virally loaded CRAd-survivin NSC and MSC has shown a significantly higher amount of viral particle release from NSC despite similar capacities for viral replication and longer median survival time.49
Matrix Metalloproteinase
A naturally occurring fragment of the enzyme matrix metalloproteinase-2, PEX is expressed in samples of hHGG and can inhibit angiogenesis, suppress tumor cell proliferation, and induce apoptosis.50 When tested in vivo, intraperitoneal injections of PEX resulted in nearly complete tumor eradication.50 These dramatic results motivated examination of PEX delivery via NSC.51 Experiments revealed significant decrease in hHGG cell viability in vitro and tumor volume in vivo.51 Histological analysis of tumors revealed a significant decrease in tumor vascularization and proliferative index.51 Of interest, there was no change in apoptotic index, which was attributed to delivery of a lower PEX concentration and was consistent with prior work showing that a higher concentration was necessary to induce apoptosis, whereas a lower one was sufficient to inhibit angiogenesis.50,51 Overall, these studies provide the first documentation of a SC-based delivery system of an anti-angiogenic therapeutic agent.
MSC
MSC are multipotent SC deriving from ESC that reside in the bone marrow and can differentiate into cells of mesenchymal lineage, such as osteoblasts, adipocytes, chondrocytes, and myocytes.11 The traditional source of MSC is the bone marrow, but there are other sources, including adipose tissue, umbilical cord blood, and placenta.11 The major advantage of MSC is their relative ease of availability, compared with other types of SC 52 while retaining the fundamental characteristics of SC, such as tumor tropism and infiltrative potential across the BBB. Comparison of MSC and NSC migration has revealed no significant differences.35,49 MSC have been used to deliver a number of therapeutic agents and/or systems, including cytokines, enzyme/prodrug combinations, viral particles, and antibodies (ranging from most to least frequently used).
Cytokines
MSC have been used to deliver several types of cytokines for the treatment of hHGG, including ILs, IFNs, and TRAIL. After confirming that the function of MSC was not affected by IL-2 expression, investigators first showed that IL-2–expressing MSC migrated toward hHGG both in vitro and in vivo and significantly prolonged rodent survival, in association with decreased tumor volume and increased lymphocyte infiltration.53 Of interest, decreased expression of IL-2 and IFNγ constitute part of the endogenous defective anti-glioma immune response in a spontaneous hHGG model 54 providing further basis for those findings and suggesting IFNγ as a possible cytokine of interest. The data with IL-2–expressing MSC provided the basis for use of MSC as cell-based vehicles in treatment of hHGG. The technical feasibility of this approach was confirmed soon afterward by a study delivering IFNβ-expressing MSC in both ipsilateral and contralateral rodent carotid arteries; cells homed to the established hHGG, inhibited proliferation, and prolonged rodent survival.55 Since then, the repertoire of cytokines successfully tested has expanded to include IFNα 56 IL-18 57 TRAIL 58–61 and IL-12.62,63 The addition of a systemically administered PI3K/mTOR inhibitor to MSC-delivered TRAIL further reduced tumor growth in an experimental model, compared with either treatment alone.64 The effects of TRAIL-expressing MSC were notably present against hHGG associated with high levels of Akt expression.58 These studies raised several issues, such as kinetics of SC-based delivery, injection site, and effect of MSC on tumor growth. For example, one study described persistence of TRAIL-expressing MSC for 14 days; however, expression began to decrease after 7 days, correlating with diminished therapeutic effect and raising the possibility of multiple injections.58 Data in rodents demonstrate induction of apoptosis and prolongation of survival times even with systemic intravascular and/or intravenous injections 61 suggesting that the potential for repeat injections may be a less-daunting obstacle in the clinical setting. MSC alone may have effects on tumors, with some studies noting positive effects on recipient survival55,57 and others not 58,61 possibly because of differences in the soluble factor microenvironment.65
Enzyme/Prodrug
The only enzyme/prodrug suicide gene therapy combination tested in MSC to date is HSV tk/GCV.66,67 In addition to confirmation of in vivo migratory characteristics, investigators confirmed gap junction formation between MSC and hHGG cells66 and found tumor growth suppression.67 Bystander effects were observed following intratumoral administration of MSC and intraperitoneal injection of GCV, with a significant fraction of mice surviving long-term.66 A novel and noteworthy aspect of this study was the use of PET and MRI, both clinically well established imaging modalities, to track the injected cells and determine therapeutic efficacy.66 Although the translation potential of this therapeutic strategy to the clinic may be arguable, there is no question as to the value of developing molecular imaging techniques to track cells in vivo for therapies based on cell-mediated gene delivery.
Viral Particles
With respect to MSC-mediated delivery of viral particles, proof of principle was first provided using a conditionally replicating adenovirus under control of the CXCR4 promoter (CRAd-CXCR4) in MSC; when injected distant to the tumor in an intracranial model, MSC succesfully migrated to the tumor site in the setting of increasing intracellular viral replication and released viral particles that infected hHGG cells.68 The next study, using a conditionally replicating adenovirus under control of a promoter regulated by retinoblastoma pathways (CRAd-Rb), provided information on the effect on tumor volume and, for the first time, used an intravascular mode of delivery for the virally loaded MSC.69 Cells migrated to the intracranial tumor site, inhibited hHGG growth, and increased median rodent survival.69 Investigation of immune mechanisms underlying the function of CRAd-survivin MSC using the semi-permissive cotton rat model70 demonstrated attenuation of the antiviral response via T cell suppression, with subsequent enhancement of adenoviral dissemination and persistence, compared with adenoviral injection alone.71
Antibodies
Expression of surface antibodies on the surface of MSC serves as an alternative to the delivery of soluble factors. Thus far, this concept has been demonstrated with expression of a single-chain antibody against EGFRvIII on the surface of human MSC.72,73 This choice of target was based on several lines of evidence, including a frequency of EGFRvIII expression of 20%–30% in glioblastoma samples and promising preclinical data.74 MSC expressing the EGFRvIII antibody accumulated within EGFRvIII-positive experimental HGG to a significantly higher degree, compared with the nonmodified MSC, and were associated with significantly smaller tumor volumes.73 Furthermore, when investigated in an intracranial model, injection of MSC expressing EGFRvIII significantly prolonged recipient survival and was associated with a significant decrease in phosphorylated Akt expression.73
Discussion
Thus far, ESC, NSC, and MSC have been used to deliver multiple therapeutic agents for the treatment of experimental hHGG, each with specific advantages and limitations as reviewed. These agents may be classified in 5 categories: cytokines, enzyme/prodrug combinations, viral particles, matrix metalloproteinases, and antibodies. Preclinical results have shown promising effects on hHGG cell survival and tumor growth with all 3 types of SC. However, irrespective of the SC source, there are several areas of current concern in translating results to the clinical setting.
The first area involves migration. Rodents display a higher degree of NSC migration 75–79 and it is unclear whether it is secondary to the SC or recipient brain. Although preclinical data demonstrate that human SC injected into immunocompromised rodents migrate toward hHGG, it is not certain that the same extent of migration will occur after engraftment in human brains, with serious implications for therapeutic efficacy. Another major issue is teratogenesis. The potential for teratogenesis from transplanted SC has been highlighted in a recent report documenting the case of a 13-year-old patient who developed a donor-derived brain tumor after receiving a NSC transplant for ataxia telangiectasia.80 Teratogenesis remains a concern and requires continued optimization of cell sorting, purification, and ablation strategies.81 Most recently, spontaneous tumor formation in MSC long-term cultures has become the subject of controversy.82–84 Although likely arising from the cross-contamination of cell lines, further work is necessary to develop strategies for the elimination of this risk. Immune-based concerns may be 2-fold. On one hand, mechanisms leading to SC immune privilege may be subject to modification, rendering them susceptible to immune rejection and hampering their therapeutic efficacy. On the other hand, the immunosuppressive functions of SC could theoretically lead to increased risk of infection, which could also be heightened from oral immunosuppressant adjunct therapy. There are multiple regulatory issues of concern, including the establishment of institutional oversight and optimized protocols for the safe and reliable production of SC vehicles.85,86 This would also involve development of robust screening strategies to avoid infectious contamination of human pathogens.85,86 Lastly, there continue to be significant ethical and social concerns on the use of SC, particularly revolving around ESC.87 This has involved not only issues with their procurement but has also focused on the question of the moral and legal status of the embryo requiring protection.87 Although some argue that iPS may overcome these concerns 16 others note that there is no ethical bypass with iPS on the basis of the moral complicity involved in the development of iPS technology.88
Although many aspects of SC-based therapy need to be optimized for clinical use, several clinical trials are under way that use NSC and MSC for cell replacement therapy in various non-glioma central nervous system pathologies. NSC are being used for the treatment of neuronal ceroid lipofuscinoses89,90 and amyotrophic lateral sclerosis.91 Results of a phase I study including 6 patients with neuronal ceroid lipofuscinosis who underwent intracerebral and intraventricular transplantation of NSC with postoperative immunosuppression have been reported.89 The procedure was well-tolerated with evidence of donor cell engraftment and survival at 11 months after transplantation.89 Longer follow-up is awaited to evaluate potential therapeutic effects. Use of MSC has been reported more widely to date in pilot clinical trials involving stroke 92 multiple sclerosis 93 and amyotrophic lateral sclerosis93 in adults and metachromatic leukodystrophy and Hurler syndrome94 in the pediatric population. Study size was small across all trials, ranging from 5 to 12 patients, but no significant adverse events were reported deriving from the transplantation procedure and/or infection, a primary end point for all studies. With respect to therapeutic efficacy, results were not conclusive. Preliminary data in stroke suggest potential improvement in median daily stroke scores and lesion volumes, but the study was not blinded and was limited by several other factors, including delayed cell delivery and disease natural history.92 In patients with metachromatic leukodystrophy, the authors reported significant improvement in nerve conduction velocities and mild improvement in bone mineral density but no clinically apparent changes in the patients' condition.94 Neither of these studies documented the biodistribution of the infused MSC, with implications for therapeutic efficacy and cell number requirement. The tolerability of the procedure and lack of serious adverse events, however, have supported the continued investigation of SC for treatment of central nervous system pathologies. In December 2007, the US Food and Drug Administration approved the first NSC-based clinical trial for recurrent hHGG that began enrollment in August 2010, with a projected completion date of August 2012.95,96 In this pilot study, the CD/5FC enzyme/prodrug system is being used. Patients receive intracerebral implantation of NSC carrying CD at the time of tumor resection, and then are given oral 5-fluorocytosine every 6 hours on postoperative days 4–10.96 The primary objective is determination of safety and feasibility of the technique, and secondary objectives include assessment of pharmacokinetics and immunogenicity.
Proposed Future Direction
A conceptually appealing yet unexplored idea would be to use SC vehicles in the fight against GSC that have been implicated in the resistance of hHGG to treatment. In this therapeutic approach, SC-based delivery of therapeutic agents would be combined with systemic GSC-directed agents. A recent study used a systemic PI3K/mTOR inhibitor (a GSC-directed agent with effects on GSC proliferation and invasion) with MSC-delivered TRAIL in an experimental model;64 investigators administered the inhibitor prior to the injection of TRAIL-expressing MSC and found decreased tumor growth.64 This study, although the first to use a PI3K/mTOR inhibitor together with SC-based TRAIL delivery, notably did not specifically investigate the effects of combined therapy on GSC.
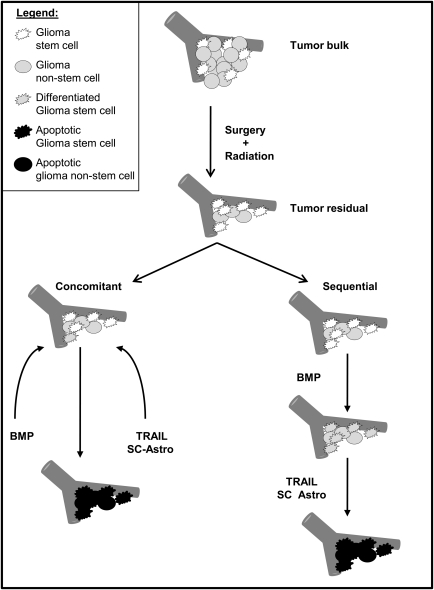
There are many ways in which SC could be used to target GSC and/or in which targeting strategies against GSC could be combined with SC-based delivery of therapeutic agents. One novel combination strategy for the potential elimination of GSC is shown in Fig. 1. It consists of inducing GSC differentiation via an agent, such as bone morphogenic protein (BMP)97 and then either concomitantly or subsequently delivering a pro-apoptotic agent, such as TRAIL, via an SC-based delivery system. A problem in using the concomitant approach is that the SC could be affected by the prodifferentiation agent and could potentially lose the capacity to both migrate and deliver the pro-apoptotic agent. To circumvent this obstacle, one could fully differentiate SC prior to engraftment, as has been shown feasible with ESC-derived astrocytes.12,18,20–24 Alternatively, in the sequential approach, in which the prodifferentiation agent is administered before SC-based delivery of the proapoptotic agent, the SC-vehicle would remain unaffected, and undifferentiated SC could be used. SC could also be engineered to deliver a GSC-specific agent, although this would require that the SC-vehicle either remain unaffected by the agent that it is delivering or become affected at a point when it is no longer a necessary component of the therapy.
Fig. 1.
Schematic presentation of potential concomitant and sequential therapeutic combinations involving a prodifferentiation agent (e.g., BMP) and SC-based delivery of a pro-apoptotic agent (e.g., TRAIL). In the concomitant approach, BMP would be administered to differentiate the GSC, which would then be eliminated by SC-delivered TRAIL. With this approach, it would be optimal for the SC vehicle to be fully differentiated, such as has been shown with ESC-derived astrocytes, to avoid the prodifferentiation effects of BMP (see text). In the sequential approach (in which BMP is administered before the delivery of SC-based TRAIL), the SC-vehicle may be undifferentiated SC (such as NSC or MSC) or alternatively be fully differentiated into a terminal cell, such as an astrocyte. The overall concept depicted in this schematic is to combine the use of SC-based delivery with GSC-directed therapeutics to more effectively eliminate GSC. Abbreviations: SC-Astro, SC-derived astrocytes; SC Astro, SC that can be either differentiated intro astrocytes or remain undifferentiated.
Regardless of the specific type of agent, cell, and timing schematic, the concept of combining SC-based delivery with GSC-specific agents for potential elimination of GSC merits further consideration. Because of the demands inherent in performing experiments to the highest technical standards, close collaboration between groups may be necessary to efficiently link the 2 areas that investigate SC as vehicles and GSC as targets.
Conclusions
In summary, the use of SC as cell-based vehicles for the delivery of agents targeting hHGG has accrued promising preclinical data using a variety of agents reviewed here that warrant continued investigation of this methodology. There are several areas of concern for translation to the clinical setting, including migration, teratogenicity, immune rejection, and regulatory and ethical issues. Nonetheless, a Food and Drug Administration–approved clinical trial is under way that uses NSC-based therapy for recurrent hHGG. SC-based therapy could be combined with GSC-targeting strategies for the potential elimination of GSC, implicated as the culprits behind the treatment resistance of hHGG. Overall, SC are providing an unprecedented opportunity for cell-based approaches to the treatment of hHGG that continues to carry a dismal patient prognosis.
Conflict of interest statement. None declared.
Funding
This work was supported in part by NIH/NCI (RO1 CA129489-01A1 to I.M.G.).
References
- 1.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. doi:10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. doi:10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 3.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. doi:10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 4.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. doi:10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 5.English K, Wood KJ. Immunogenicity of embryonic stem cell-derived progenitors after transplantation. Curr Opin Organ Transplant. 2011;16:90–95. doi: 10.1097/MOT.0b013e3283424faa. doi:10.1097/MOT.0b013e3283424faa. [DOI] [PubMed] [Google Scholar]
- 6.Germano IM, Binello E. Gene therapy as an adjuvant treatment for malignant gliomas: from bench to bedside. J Neurooncol. 2009;93:79–87. doi: 10.1007/s11060-009-9869-5. doi:10.1007/s11060-009-9869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Central Brain Tumor Registry of the United States. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States 2004–2006. [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 10.Germano IM, Swiss V, Casaccia P. Primary brain tumors, neural stem cells, and brain tumor cancer cells: where is the link? Neuropharmacol. 2010;58:903–910. doi: 10.1016/j.neuropharm.2009.12.019. doi:10.1016/j.neuropharm.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picinich SC, Mishra PJ, Glod J, et al. The therapeutic potential of mesenchymal stem cells. Expert Opin Biol. 2007;7:965–973. doi: 10.1517/14712598.7.7.965. doi:10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- 12.Germano IM, Uzzaman M, Keller G. Gene delivery by embryonic stem cells for malignant glioma therapy: hype or hope? Cancer Biol Ther. 2008;7:1341–1347. doi: 10.4161/cbt.7.9.6711. doi:10.4161/cbt.7.9.6711. [DOI] [PubMed] [Google Scholar]
- 13.deVries NA, Beijnen JH, van Tellingen O. High-grade glioma mouse models and their applicability for pre-clinical testing. Cancer Treat Rev. 2009;31:714–723. doi: 10.1016/j.ctrv.2009.08.011. doi:10.1016/j.ctrv.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. doi:10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 15.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. doi:10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 16.Das S, Bonaguidi M, Muro K, et al. Generation of embryonic stem cells: limitations of and alternatives to inner cell harvest. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/3-4/E3. [DOI] [PubMed] [Google Scholar]
- 17.Staerk J, Dawlaty MM, Gao Q, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. doi:10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benveniste RJ, Keller G, Germano IM. Embryonic stem cell-derived astrocytes expressing drug-inducible transgenes: differentiation and transplantation into the mouse brain. J Neurosurg. 2005;103:115–123. doi: 10.3171/jns.2005.103.1.0115. doi:10.3171/jns.2005.103.1.0115. [DOI] [PubMed] [Google Scholar]
- 19.Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. doi:10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uzzaman M, Benveniste RJ, Keller G, et al. Embryonic stem cell-derived astrocytes: a novel gene therapy vector for brain tumors. Neurosurg Focus. 2005;19:E6. doi: 10.3171/foc.2005.19.3.7. [DOI] [PubMed] [Google Scholar]
- 21.Germano IM, Uzzaman M, Benveniste RJ, et al. Apoptosis in human glioblastoma cells produced using embryonic stem cell-derived astrcoytes expressing tumor necrosis factor-related apoptosis-inducing ligand. J Neurosurg. 2006;105:88–95. doi: 10.3171/jns.2006.105.1.88. doi:10.3171/jns.2006.105.1.88. [DOI] [PubMed] [Google Scholar]
- 22.Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on temozolomide-resistant glioma cells. J Neurosurg. 2007;106:646–651. doi: 10.3171/jns.2007.106.4.646. doi:10.3171/jns.2007.106.4.646. [DOI] [PubMed] [Google Scholar]
- 23.Uzzaman M, Keller G, Germano IM. In vivo gene delivery by embryonic stem cell-derived astrocytes for malignant gliomas. Neurooncol. 2009;11:102–108. doi: 10.1215/15228517-2008-056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Germano IM, Emdad L, Qadeer ZA, et al. Embryonic stem cell (ESC)-mediated transgene delivery induces growth suppression, apoptosis and radiosensitization, and overcomes temozolomide resistance in malignant gliomas. Cancer Gene Ther. 2010;17:664–674. doi: 10.1038/cgt.2010.31. doi:10.1038/cgt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emdad L, Lebedeva IV, Su ZZ, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009;8:391–400. doi: 10.4161/cbt.8.5.7581. doi:10.4161/cbt.8.4.8156. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nature Med. 2000;6:447–450. doi: 10.1038/74710. doi:10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 27.Ehtesham M, Kabos P, Kabosova A, et al. The use of interleukin-12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 28.Yuan X, Hu J, Belladonna ML, et al. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer Res. 2006;66:2630–2638. doi: 10.1158/0008-5472.CAN-05-1682. doi:10.1158/0008-5472.CAN-05-1682. [DOI] [PubMed] [Google Scholar]
- 29.Ehtesham M, Kabos P, Gutierrez MAR, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 30.Balyasnikova I, Ferguson SD, Han Y, Liu F, Lesniak MS. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Lett. 2011;310:148–159. doi: 10.1016/j.canlet.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah K, Tung CH, Yang K, et al. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64:3236–3242. doi: 10.1158/0008-5472.can-03-3516. doi:10.1158/0008-5472.CAN-03-3516. [DOI] [PubMed] [Google Scholar]
- 32.Shah K, Bureau E, Kim DE, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57:34–41. doi: 10.1002/ana.20306. doi:10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 33.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. doi:10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corsten MF, Miranda R, Kasmieh R, et al. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell-delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. doi:10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 35.Hingten S, Ren X, Terwilliger E, et al. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and temozolomide. Mol Cancer Ther. 2008;7:3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. doi:10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. doi:10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 37.Li S, Tokuyama T, Yamamoto J, et al. Bystander effect-mediated gene therapy of gliomas using genetically engineered neural stem cells. Cancer Gene Ther. 2005;12:600–607. doi: 10.1038/sj.cgt.7700826. doi:10.1038/sj.cgt.7700826. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Tokuyama T, Yamamoto J, et al. Potent bystander effect in suicide gene therapy using neural stem cells transduced with herpes simplex virus thymidine kinase gene. Oncol. 2005;69:503–508. doi: 10.1159/000091032. doi:10.1159/000091032. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Gao Y, Tokuyama T, et al. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Lett. 2007;251:220–227. doi: 10.1016/j.canlet.2006.11.024. doi:10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Uhl M, Weiler M, Wick W, et al. Migratory neural stem cells for improved thymidine kinase-based gene therapy of malignant gliomas. Biochem Biophys Res Comm. 2005;328:125–129. doi: 10.1016/j.bbrc.2004.12.164. doi:10.1016/j.bbrc.2004.12.164. [DOI] [PubMed] [Google Scholar]
- 41.Barresi V, Belluardo N, Sipione S, et al. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10:396–402. doi: 10.1038/sj.cgt.7700580. doi:10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa T, Tamiya T, Adachi Y, et al. In vivo efficacy and toxicity of 5-fluorocytosine/cytosine deaminase gene therapy for malignant gliomas mediated by adenovirus. Cancer Gene Ther. 2000;7:74–82. doi: 10.1038/sj.cgt.7700086. doi:10.1038/sj.cgt.7700086. [DOI] [PubMed] [Google Scholar]
- 43.Lee DH, Ahm Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15:4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. doi:10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 44.Ito S, Natsume A, Shimato S, et al. Human neural stem cells transduced with IFN-β and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2010;17:299–306. doi: 10.1038/cgt.2009.80. doi:10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- 45.Herrlinger U, Woiciechowski C, Sena-Esteves M, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1:347–357. doi: 10.1006/mthe.2000.0046. doi:10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- 46.Tyler M, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–278. doi: 10.1038/gt.2008.165. doi:10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed AU, Thaci B, Alexiades N, et al. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol Ther. 2011;19:1714–1726. doi: 10.1038/mt.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21:119–126. doi: 10.1016/j.cytogfr.2010.02.004. doi:10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed AU, Tyler MA, Thaci B, et al. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm. 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bello L, Lucini V, Carrabba G, et al. Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion of a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001;61:8730–8736. [PubMed] [Google Scholar]
- 51.Kim SK, Cargioli TG, Machluf M, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. doi:10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 52.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. doi:10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2009;11:1155–1164. doi: 10.1038/sj.gt.3302276. doi:10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 54.Thang NNT, Derouazi M, Philippin G, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70:4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. doi:10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 55.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 56.Sato H, Kuwashima N, Sakaida T, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005;12:757–768. doi: 10.1038/sj.cgt.7700827. doi:10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 57.Xu G, Jiang XD, Xu Y, et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int. 2009;33:466–474. doi: 10.1016/j.cellbi.2008.07.023. doi:10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 58.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. doi:10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 59.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. doi:10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menon LG, Kelly K, Yang HW, et al. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. doi:10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 61.Yang B, Wu X, Mao Y, et al. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurg. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. doi:10.1227/01.NEU.0000350227.61132.A7. [DOI] [PubMed] [Google Scholar]
- 62.Ryu CH, Park SH, Park SA, et al. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 63.Hong X, Miller C, Savant-Bhonsale S, Kalkanis SN. Anti-tumor treatment using interleukin-12-secreting marrow stromal cells in invasive glioma model. Neurosurg. 2009;64:1139–1147. doi: 10.1227/01.NEU.0000345646.85472.EA. doi:10.1227/01.NEU.0000345646.85472.EA. [DOI] [PubMed] [Google Scholar]
- 64.Bagci-Onder T, Wakimoto H, Anderegg M, et al. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. doi:10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 65.Kang SG, Jeun SS, Lim JY, et al. Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv Sys. 2008;24:293–302. doi: 10.1007/s00381-007-0515-2. doi:10.1007/s00381-007-0515-2. [DOI] [PubMed] [Google Scholar]
- 66.Miletic H, Fischer Y, Litwak S, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. doi:10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 67.Uchibori R, Okada T, Ito T, et al. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11:373–381. doi: 10.1002/jgm.1313. doi:10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 68.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. doi:10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 69.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Δ24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. doi:10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toth K, Spencer JF, Tollefson AE, et al. Cotton rat tumor model for the evaluation of oncolytic adenoviruses. Hum Gene Ther. 2005;16:139–146. doi: 10.1089/hum.2005.16.139. doi:10.1089/hum.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed AU, Rolle CE, Tyler MA, et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. doi:10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balyasnikova IV, Franco-Gou R, Mathis JM, et al. Genetic modification of mesenchymal stem cells to express a single-chain antibody against EGFRvIII on the cell surface. J Tissue Eng Regen Med. 2010;4:247–258. doi: 10.1002/term.228. doi:10.1002/term.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balyasnikova IV, Ferguson SD, Sengupta S, et al. Mesenchymal stem cells modified with a single-chain antibody against EGFRvIII successfully inhibit the growth of human xenograft malignant glioma. PLoS ONE. 2010;5:e9750. doi: 10.1371/journal.pone.0009750. doi:10.1371/journal.pone.0009750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gan HK, Kaye AH, Luwor R. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16:748–754. doi: 10.1016/j.jocn.2008.12.005. doi:10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. doi:10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin J, Galvan V. Endogenous neural stem cells in the adult brain. J Neuroimmune Pharmacol. 2007;2:236–242. doi: 10.1007/s11481-007-9076-0. doi:10.1007/s11481-007-9076-0. [DOI] [PubMed] [Google Scholar]
- 77.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. doi:10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 78.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. doi:10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 79.Sanai N, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. doi:10.1126/science.1145011. [DOI] [PubMed] [Google Scholar]
- 80.Amariglio N, Hirschberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an Ataxia Telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. doi:10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 82.Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. doi:10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 83.Røsland GR, Svendsen A, Torsvik A, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. doi:10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 84.Torsvik A, Røsland GV, Svendsen A, et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track – letter. Cancer Res. 2010;70:6393–6396. doi: 10.1158/0008-5472.CAN-10-1305. doi:10.1158/0008-5472.CAN-10-1305. [DOI] [PubMed] [Google Scholar]
- 85.Master Z, McLeod M, Mendez I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson's disease. J Med Ethics. 2007;33:169–173. doi: 10.1136/jme.2005.013169. doi:10.1136/jme.2005.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Experiment Gerontol. 2008;43:1018–1023. doi: 10.1016/j.exger.2008.07.004. doi:10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Zarzecny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research and the current role of the moral status of the embryo. Stem Cell Rev and Rep. 2009;5:96–101. doi: 10.1007/s12015-009-9062-4. doi:10.1007/s12015-009-9062-4. [DOI] [PubMed] [Google Scholar]
- 88.Brown M. No ethical bypass of moral status in stem cell research. Bioethics. 2011 doi: 10.1111/j.1467-8519.2011.01891.x. doi:10.1111/j.1467-8519.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 89.Selden NR, Guillame DJ, Huhn SL, Koch TK, Al-Uzri A, Steiner RD. CNS transplantation of purified human neural stem cells in neuronal ceroid lipofuscinoses: phase I trial. American Association of Neurological Surgeons, Annual Meeting; May 1–5, 2010; Philadelphia. Abstract #600. [Google Scholar]
- 90. http://www.clinicaltrials.gov/ct2/show/NCT00337636?term=NCT00337636&rank=1. Accessed October 16, 2011. [Google Scholar]
- 91. http://www.clinicaltrials.gov/ct2/show/NCT01348451?term=NCT01348451&rank=1. Accessed October 16, 2011. [Google Scholar]
- 92.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. doi:10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freedman MS, Bar-Or A, Atkins HL, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Multiple Sclerosis. 2010;16:503–510. doi: 10.1177/1352458509359727. doi:10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 94.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone marrow transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. doi:10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 95.Najbauer J, Danks MK, Kim SU, et al. Neural stem cell mediated tumor-selective gene delivery: towards high grade glioma clinical trials. Mol Ther. 2008;16(Supplement 1):S136. [Google Scholar]
- 96. http://www.clinicaltrials.gov/ct2/show/NCT01172964?term=NCT01172964&rank=1. Accessed October 16, 2011. [Google Scholar]
- 97.Piccirillo SGM, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. doi:10.1038/nature05349. [DOI] [PubMed] [Google Scholar]