Abstract
Management of brainstem mass lesions remains a controversial issue, especially when the lesion cannot be excised and when infiltration occurs; moreover, the benefits of a stereotactic procedure are still under debate. In most studies, treatment decisions are based solely on MRI features and do not include a histopathological diagnosis. In the current study, we compared MRI characteristics with histopathological findings of intrinsic brainstem lesions and identified the characteristics associated with the diagnosis of pathologies other than diffuse glioma. From February 1988 through August 2007, 96 brainstem biopsies were performed at the Roger Salengro Hospital in Lille, France, on adult patients with intrinsic brainstem lesions not amenable to excision. Of the 96 patients, 42 were women and 54 were men, with a mean age of 41 years (range, 18–75 years). Data analysis of the MRI findings revealed focal (P < .05) and contrast enhancing lesions (P < .05), and these lesions were significant factors associated with the diagnosis of pathologies other than diffuse glioma. Focal lesions were a significant factor associated with a diagnosis of nontumor lesions (P < .05). In conclusion, the diagnostic effect of stereotactic biopsy on intrinsic brainstem lesions was greater in patients with focal or enhancing lesions shown by MRI, in whom the diagnosis of diffuse glioma was less frequent.
Keywords: biopsy, brainstem tumors, MRI
Management of brainstem mass lesions remains a controversial issue, especially when the lesion cannot be excised and when infiltration occurs; moreover, the benefits of a stereotactic procedure are still under debate.1
In most studies, treatment decisions are based solely on MRI features and do not include a histopathological diagnosis. Most authors regard biopsy of intrinsic brainstem tumors as too dangerous and consider imaging methods to be sufficiently reliable.2,3 Thus, the effect of MRI findings on treatment decisions for brainstem tumors is very high, but the accuracy of MRI-based diagnosis of brainstem gliomas has not been conclusively verified by histopathological findings.3
In the current study, we compared MRI characteristics with histopathological findings of intrinsic brainstem lesions. We also discuss cases in which the biopsy had an important effect on treatment.
Patients and Methods
From February 1988 through August 2007, 96 brainstem biopsies were performed at the Roger Salengro Hospital in Lille, France, on adult patients with intrinsic brainstem lesions not amenable to excision. Of the 96 patients, 42 were women and 54 were men, with a mean age of 41 years (range, 18–75 years). Patients were followed up from 9 days to 147 months after biopsy (mean, 25.4 months).
In this study, the focal and diffuse tumors were differentiated by MRI according to the classification of Donaldson et al.4 In this system, focal tumors are well-marginated in MRI and occupy less than50% of the axial diameter of the brainstem, and diffuse tumors are poorly marginated, occupying more than50% of the axial diameter of the brainstem. In addition, in the current study, we grouped these tumors into enhancing and nonenhancing lesions.
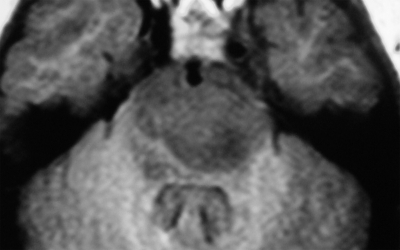
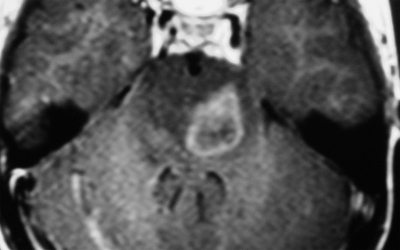
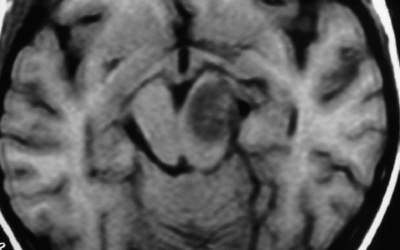
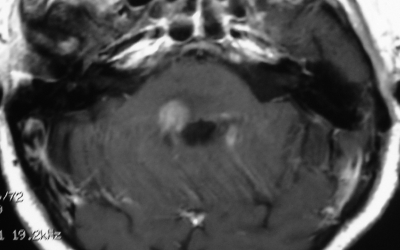
Therefore, brainstem lesions were differentiated by MRI findings into 4 groups: (1) diffuse nonenhancing brainstem lesion (MRI showed diffuse hypointense lesion on T1W, noncontrast enhancing lesion on T1W, and diffuse hyperintense lesion on T2W) (Fig. 1), (2) diffuse enhancing brainstem lesion (MRI showed diffuse hypointense lesion on T1W, contrast enhancing lesion on T1W, and diffuse hyperintense lesion on T2W) (Fig. 2), (3) focal nonenhancing brainstem lesions (MRI showed focal hypointense lesion on T1W, noncontrast enhancing lesion on T1W, and focal hyperintense lesion on T2W) (Fig. 3), and (4) focal enhancing brainstem lesions (MRI showed focal hypointense lesion on T1W, noncontrast enhancing lesion on T1W, and focal hyperintense lesion on T2W) (Fig. 4).
Fig. 1.
Axial Gd-enhanced Ti-weighted MR image showing a diffuse nonenhancing brainstem lesion.
Fig. 2.
Axial Gd-enhanced Ti-weighted MR image showing a diffuse enhancing brainstem lesion.
Fig. 3.
Axial Gd-enhanced Ti-weighted MR image showing a focal nonenhancing brainstem lesion.
Fig. 4.
Axial Gd-enhanced Ti-weighted MR image showing a focal enhancing brainstem lesion.
Surgical Technique
Following the induction of general anesthesia, 76 patients underwent a stereotactic biopsy procedure with a Talairach frame, and 20 underwent robotic brainstem biopsy (NeuroMate Systems). In all patients, targeting of the biopsy site within the lesion was achieved by MRI. Serial sampling was performed every 10 mm of the trajectory with use of a side-cutting Sedan needle.
The center of the lesion was targeted, and when enhancement was identified, this region was also targeted. The transfrontal approach was chosen in most cases, except in middle pontine lesions with infiltration of the middle cerebellar peduncule, for which a transcerebellar approach was preferable. Thus, a transfrontal approach was used in 79 patients and a transcerebellar approach in 17 patients. All biopsy specimens were formalin-fixed and analyzed after staining with hematoxylin and eosin, Masson trichrome, and immunostains.
Data Analysis and Statistical Analysis
Data analysis was performed using EpiInfo, version 6.02, and Medicalc, version 9.3.0.9. Univariate analysis of the following variables was performed with regard to the radiological findings and histological diagnosis: aspect in T1 diffuse versus focal and enhanced versus nonenhanced contrast. All lesions in T2 were hyperintense.
Results
The mean duration of symptoms was 2 months. Frequently reported symptoms consisted of waking disturbance in 46 patients, visual impairment in 43, dysphagia in 23, signs of intracranial hypertension in 22, facial paresis in 31, and hemiparesis in 48.
On the radiological findings, 32 patients had diffuse nonenhancing brainstem lesions, 31 patients had diffuse enhancing brainstem lesions, 10 patients had focal nonenhancing brainstem lesions, and 23 patients had focal enhancing brainstem lesions.
Histological Results and Complications
A precise histological diagnosis was established in 92 patients (95.8%), and the diagnosis was confirmed by their clinical course. A diffuse brainstem glioma diagnosis was determined in 63 patients.
Other neoplastic diseases were diagnosed in 19 patients: 7 lymphomas, 6 metastases, 4 pilocytic astrocytomas, 1 craniopharyngioma, and 1 ganglioglioma. Moreover, histological evaluation revealed nonneoplastic lesions in 10 patients: 5 cases of inflammatory disease, 2 ischemic lesions, 2 fungal abscesses, and 1 gliosis.
The overall morbidity rate associated with biopsy in the present study was 9% (9 patients), and in all these cases, the patients had only aggravation of their preexisting symptoms, showing no further symptoms: 2 patients had worsening of hemiparesis, progressing from grade 1 to grade 2; 3 patients developed worsening of facial paresis, progressing from grade 2 to grade 3 (House-Brackmann classification); 2 patients had aggravation of dysphagia; and 2 had aggravation of third nerve paresis.
One patient died of causes associated with the procedure. This patient, who had a pontine-enhanced lesion and aggravation of hemiparesis after biopsy, died 11 days after the procedure. Postoperative CT showed aggravation only of the associated edema. The diagnosis was metastasis.
Correlation of Histological and Radiological Findings
Histological evaluation revealed diffuse brainstem glioma in 25 (89.3%) of the patients with diffuse nonenhancing brainstem lesions. Of these, 18 cases were low-grade glioma and 7 were high-grade glioma; moreover, another neoplastic disease was identified in 1 patient (lymphoma), and there were 2 cases of inflammatory disease. The biopsy results were inconclusive in 4 cases (Table 1).
Table 1.
Histological diagnosis in patients with diffuse nonenhancing brainstem lesions
| Histology | No. | % |
|---|---|---|
| Low-grade glioma | 18 | 56.3 |
| High-grade glioma | 7 | 21.9 |
| Lymphoma | 1 | 3.1 |
| Inflammatory disease | 2 | 6.3 |
| Inconclusive | 4 | 12.5 |
| Total | 32 | 100 |
Diffuse brainstem gliomas were verified in 21 (68%) of the 31 patients showing diffuse enhancing brainstem lesions. Of these, 3 were low-grade glioma and 18 were high-grade glioma. Patients with pathologies other than diffuse glioma had a wide variety of other neoplastic diseases: 1 pilocytic astrocytoma, 4 lymphomas, 3 metastases, and 1 ganglioglioma. One case of inflammatory disease was also identified (Table 2).
Table 2.
Histological diagnosis in patients with diffuse enhancing brainstem lesions
| Histology | No. | % |
|---|---|---|
| Low-grade glioma | 3 | 9.7 |
| High-grade glioma | 18 | 58.1 |
| Lymphoma | 4 | 12.9 |
| Metastases | 3 | 9.7 |
| Pilocytic astrocytoma | 1 | 3.2 |
| Ganglioglioma | 1 | 3.2 |
| Inflammatory disease | 1 | 3.2 |
| Total | 31 | 100 |
In the cases involving focal nonenhancing brainstem lesions, a diagnosis of diffuse brainstem glioma was achieved in 9 patients; moreover, 1 ischemic lesion was verified (Table 3). In the 23 cases involving focal enhancing brainstem lesions, a diagnosis of diffuse brainstem glioma was verified in only 8 (34.7%), of which 1 was low-grade glioma and 7 were high-grade glioma. Pathologies different from diffuse glioma were diagnosed in 15 patients (65.3%) (Table 4). A wide variety of comorbidities was identified, ranging from other neoplastic diseases (3 pilocytic astrocytoma, 3 metastases, 2 lymphomas, and 1 craniopharyngioma) to nontumors, including gliosis (1 case), ischemic lesion (1 case), fungal abscess (2 cases), and inflammatory disease (2 cases).
Table 3.
Histological diagnosis in patients with focal nonenhancing brainstem lesions
| Histology | No. | % |
|---|---|---|
| Low-grade glioma | 9 | 90 |
| Ischemic lesion | 1 | 10 |
| Total | 10 | 100 |
Table 4.
Histological diagnosis in patients with focal enhancing brainstem lesions
| Histology | No. | % |
|---|---|---|
| Low-grade glioma | 1 | 4.3 |
| High-grade glioma | 7 | 30.4 |
| Pilocytic astrocytoma | 3 | 13.0 |
| Metastases | 3 | 13.0 |
| Lymphomas | 2 | 8.7 |
| Craniopharyngioma | 1 | 4.3 |
| Gliosis | 1 | 4.3 |
| Ischemic lesion | 1 | 4.3 |
| Fungal abscess | 2 | 8.7 |
| Inflammatory disease | 2 | 8.7 |
| Total | 23 | 100 |
Data analysis of the MRI findings revealed focal (P < .05) and contrast enhancing lesions (P < .05), and these lesions were significant factors associated with the diagnosis of pathologies other than diffuse glioma.
Focal lesions were a significant factor associated with a diagnosis of nontumor lesions (P < .05). However, MRI findings of contrast enhancing or nonenhancing lesions were not significant factors (P > .05).
Discussion
The use of image-guided stereotactic brain biopsy is regarded as a safe and reliable procedure for the management of supratentorial lesions. However, its application in lesions involving the brainstem remains limited.5 Recent progress in modern neuroimaging techniques, especially high-resolution MRI, permits greater precision in determining the location and extension of brainstem tumors and can indicate certain specific characteristics of their nature.6 In such cases, the challenge is to know whether the use of MRI alone is precise enough to provide an accurate diagnosis or at least to permit the classification of patients into specific treatment groups, and, consequently, whether a pathological diagnosis is still mandatory before initiating any therapy.7
Histological Results and Complications
Although several authors have discussed a wide variety of histological results in brainstem masses by biopsy, the indication for this procedure is still a controversial matter. One objection to performing a brainstem stereotactic biopsy procedure is that it may not be reliable because the tumor may be heterogeneous.8 In addition, heterogeneity often requires multiple sampling, which is potentially dangerous in the brainstem.9 Even in the most recent study by Kesari et al., involving a large number of cases, a histological diagnosis was obtained only in 53% of cases.10 Many authors claim a very restrictive indication for radiologically unclear lesions because of the presumed high-risk profile.2
In a number of published studies, a large number of complications occurred because of procedures involving stereotactic brainstem biopsies, reaching up to 10%.2 As a consequence, certain authors advocate a noninvasive approach.11 In the current study, one procedure-related death occurred in spite of the highly eloquent target localization. Other than this, 9 patients showed only slight deterioration in preoperative symptoms.
Others studies have found that stereotactic biopsy is a safe and reliable method with a high diagnostic yield.11 In the current study, the rate of diagnosis was 95.8%, in agreement with rates in the recent literature (range, 87% to100%).1,5,8,12–15
In addition, some authors have found that incorporation of PET into stereotactic planning allowed for better targeting of the biopsy sample and, thus, increased the diagnostic yield. Therefore, better targeting will decrease the number of biopsy trajectories and samples, thereby reducing the risk of brainstem injury.1,14,15
Massager et al.1 obtained a diagnostic accuracy of 100% in patients with brainstem tumors using MRI and 18F-2-fluoro-2-deoxy-D-glucose (FDG)-PET. In addition, Pirrote et al.14 reported, in a series of 20 patients with brainstem tumors, that FDG-PET and 11C-methionine (MET)-PET data contributed to the surgical planning and also improved biopsy target selection in all cases.16–18
According to Pirrote et al.,14 malignant tumors are characterized by an increased FDG uptake. This uptake is significantly correlated with the presence of anaplasia and is a more accurate reflection of tumor grade than MRI contrast enhancement.16 Furthermore, the accumulation of MET in tissues is influenced by cellular needs for protein synthesis precursors and is related to tissue proliferation and malignancy.17 Protein metabolism is much higher in the tumor than in brain tissue, and the sensitivity and specificity of MET-PET to detect tumor tissue are both approximately90%.18
Correlation of Histological and Radiological Findings
The current study analyzed the correlations between the histological and radiological findings in patients with brainstem tumor. Further analysis included the risk profile for stereotactic brainstem biopsies and identified the MRI characteristics associated with diagnoses other than diffuse brainstem glioma.
This study showed that the greatest effect of the biopsy on treatment occurred among patients with enhancing lesions; among those with enhancing diffuse or focal brainstem lesions, a diagnosis of diffuse glioma was achieved in only 67.8% (Fig. 2) and 34.7% (Fig. 4) of cases, respectively. Histological diagnosis verified a wide variety of comorbidities, including metastasis, lymphoma, pilocytic astrocytoma, and inflammatory disease. This confirmed the data analysis of the MRI findings, which showed that focal and contrast enhancing lesions were significant factors associated with the diagnosis of pathologies other than diffuse glioma. Moreover, analysis demonstrated that focal lesions were a significant factor associated with the diagnosis of nontumor lesions.
Therefore, with regard to the therapeutic consequences of radiotherapy and radiochemotherapy, patients with pathologies other than diffuse glioma might have gained some benefit from the biopsy procedure, especially patients with no histological evidence of tumor, in whom treatment based solely on radiological diagnosis could have severe consequences.3,19,20
The biopsy was also important in establishing the degree of malignancy in diffuse gliomas, which according to some authors, is an important factor in the prognosis of such patients. In a recent report, Rachinger et al.3 found that patients with diffuse low-grade glioma had a 1-year survival rate of 93%, whereas this rate was 42% among patients with diffuse high-grade glioma. These authors also noted that current data concerning prognosis and prognostic factors in brainstem gliomas in adults remain scarce. This may be attributable to the low incidence of these tumors and the fact that histopathological diagnosis is rarely confirmed.19,21,22
Nevertheless, with respect to patients with nonenhancing focal brainstem lesions, this study showed that the biopsy had no effect on treatment, because in such lesions, the diagnosis of diffuse low-grade gliomas accounted for 90% of the cases, and MRI was a sufficiently reliable diagnostic method. In addition, the remaining case was of ischemic injury, which can be diagnosed with imaging follow-up. Therefore, in these cases, biopsy should not be offered as a first choice for diagnosis.
In conclusion, the diagnostic effect of stereotactic biopsy on intrinsic brainstem lesions was greater in patients with focal enhancing lesions on MRI, in whom the diagnosis of diffuse glioma was less frequent. Stereotactic biopsy of brainstem tumors is a low-risk procedure with a high diagnostic value in experienced hands and, thus, should be regarded as standard in adult patients with enhancing brainstem lesions.
Conflict of interest statement. None declared.
References
- 1.Massager N, David P, Goldman S, et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. Neurosurg Focus. 2000;8:1–6. doi: 10.3171/jns.2000.93.6.0951. doi:10.3171/foc.2000.8.2.2. [DOI] [PubMed] [Google Scholar]
- 2.Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Curr Opin Neurol. 2001;14:711–715. doi: 10.1097/00019052-200112000-00006. doi:10.1097/00019052-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Rachinger W, Grau SJ, Holtmannspoetter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared to MRI only. J Neurol Neurosurg Psychiatr. 2009;80:1134–1139. doi: 10.1136/jnnp.2009.174250. doi:10.1136/jnnp.2009.174250. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. doi: 10.1200/JCO.2005.04.6599. doi:10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Lunsford LD. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg Neurol. 1995;43:558–562. doi: 10.1016/0090-3019(95)00009-7. doi:10.1016/0090-3019(95)00009-7. [DOI] [PubMed] [Google Scholar]
- 6.Levivier M, Massager N, Brotchi J. Management of mass lesions of the brainstem. Crit Rev Neurosurg. 1998;8:338–345. doi: 10.1007/s003290050099. doi:10.1007/s003290050099. [DOI] [PubMed] [Google Scholar]
- 7.Rajshekhar V, Chandy MJ. Computerized tomography-guided stereotactic surgery for brainstem masses: a risk-benefit analysis in 71 patients. J Neurosurg. 1995;82:976–981. doi: 10.3171/jns.1995.82.6.0976. doi:10.3171/jns.1995.82.6.0976. [DOI] [PubMed] [Google Scholar]
- 8.Aker FV, Hakan T, Karadereler S, Erkan M. Accuracy and diagnostic yield of stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimens. Neuropathology. 2005;25:207–213. doi: 10.1111/j.1440-1789.2005.00634.x. doi:10.1111/j.1440-1789.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 9.Ranjan A, Rajshekhar V, Joseph T, Chandy MJ, Chandi SM. Nondiagnostic CT-guided stereotactic biopsies in a series of 407 cases: influence of CT morphology and operator experience. J Neurosurg. 1993;79:839–844. doi: 10.3171/jns.1993.79.6.0839. doi:10.3171/jns.1993.79.6.0839. [DOI] [PubMed] [Google Scholar]
- 10.Kesari S, Kim RS, Markos V, Drappatz J, Wen PY, Prutt AA. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175–183. doi: 10.1007/s11060-008-9545-1. doi:10.1007/s11060-008-9545-1. [DOI] [PubMed] [Google Scholar]
- 11.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain-stem gliomas: a report from the Children's Cancer Group. Neurosurgery. 1993;33:1026–1030. doi: 10.1227/00006123-199312000-00010. doi:10.1227/00006123-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kreth FW, Muacevic A, Medele R, Bise K, Meyer T, Reulen HJ. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brains tumors - a prospective study. Acta Neurochir. 2001;143:539–543. doi: 10.1007/s007010170058. doi:10.1007/s007010170058. [DOI] [PubMed] [Google Scholar]
- 13.Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, Smith A. Brainstem stereotactic biopsy sampling in children. J Neurosurg. 2006;104(2 Suppl Pediatrics):108–114. doi: 10.3171/ped.2006.104.2.108. [DOI] [PubMed] [Google Scholar]
- 14.Pirotte BJ, Lubansu A, Massager N, Wikler D, Goldman S, Levivier M. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107(5 Suppl Pediatrics):392–399. doi: 10.3171/PED-07/11/392. [DOI] [PubMed] [Google Scholar]
- 15.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(Suppl Pediatrics):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 16.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 17.Pirrote B, Goldman S, Massager N, et al. Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy in gliomas. J Nucl Med. 2004;45:1293–1298. [PubMed] [Google Scholar]
- 18.Utriainen M, Metsähonkala L, Salmi TT, et al. Metabolic characterization of childhood brain tumors: comparison of 18F-fluorodeoxyglucose and 11C-methionine positron emission tomography. Cancer. 2002;95:1376–1386. doi: 10.1002/cncr.10798. doi:10.1002/cncr.10798. [DOI] [PubMed] [Google Scholar]
- 19.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. doi:10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 20.Yen CP, Sheehan J, Steiner M, Patterson G, Steiner L. Gamma knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8–17. doi: 10.3171/jns.2007.106.1.8. doi:10.3171/jns.2007.106.1.8. [DOI] [PubMed] [Google Scholar]
- 21.Jeuken JW, Van der Maazen RW, Wesseling P. Molecular diagnostics as a tool to personalize treatment in adult glioma patients. Technol Cancer Res Treat. 2006;5:215–229. doi: 10.1177/153303460600500305. [DOI] [PubMed] [Google Scholar]
- 22.Pierre-Kahn A, Hirsch JF, Vinchon M, et al. Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg. 1993;79:845–852. doi: 10.3171/jns.1993.79.6.0845. doi:10.3171/jns.1993.79.6.0845. [DOI] [PubMed] [Google Scholar]