Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are rapidly progressive Schwann cell neoplasms. The erbB family of membrane tyrosine kinases has been implicated in MPNST mitogenesis and invasion and, thus, is a potential therapeutic target. However, tyrosine kinase inhibitors (TKIs) used alone have limited tumoricidal activity. Manipulating the autophagy lysosomal pathway in cells treated with cytostatic agents can promote apoptotic cell death in some cases. The goal of this study was to establish a mechanistic basis for formulating drug combinations to effectively trigger death in MPNST cells. We assessed the effects of the pan erbB inhibitor PD168393 on MPNST cell survival, caspase activation, and autophagy. PD168393 induced a cytostatic but not a cytotoxic response in MPNST cells that was accompanied by suppression of Akt and mTOR activation and increased autophagic activity. The effects of autophagy modulation on MPNST survival were then assessed following the induction of chloroquine (CQ)–induced lysosomal stress. In CQ-treated cells, suppression of autophagy was accompanied by increased caspase activation. In contrast, increased autophagy induction by inhibition of mTOR did not trigger cytotoxicity, possibly because of Akt activation. We thus hypothesized that dual targeting of mTOR and Akt by PD168393 would significantly increase cytotoxicity in cells exposed to lysosomal stress. We found that PD168393 and CQ in combination significantly increased cytotoxicity. We conclude that combinatorial therapies with erbB inhibitors and agents inducing lysosomal dysfunction may be an effective means of treating MPNSTs.
Keywords: apoptosis, autophagy; chloroquine; erbB inhibitor; malignant peripheral nerve sheath tumor
Autophagy is a ubiquitously occurring physiological process by which long-lived proteins and organelles are engulfed in membrane-bound vacuoles (autophagosomes) and delivered to lysosomes for degradation. Autophagy is traditionally considered to be a cellular response to nutrient stress and is negatively regulated by mTOR. Therefore, deactivation of the mTOR signaling cascade stimulates autophagic vacuole (AV) formation.1 It is now well established that there is a complex cross-talk between autophagy and apoptosis that can determine cell fate. Depending on the cellular context and stress stimulus, autophagy may be pro-survival and antagonize apoptosis or may cooperate with the apoptotic death program to potentiate death. Recently, therapy-induced autophagy in cancer cells has drawn significant attention. This underscores the need to supplement studies of drugs manipulating cell death pathways with a careful analysis of their impact on autophagy. Moreover, this interplay between apoptosis and autophagy affords an opportunity to tailor drug combinations to efficiently induce tumor cell death.
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive sarcomas that arise from Schwann cell–like elements in plexiform and intraneural neurofibromas. Approximately half of MPNSTs occur in patients with neurofibromatosis type 1 (NF1), an autosomal dominant cancer predisposition syndrome. NF1 is the most common genetic disease affecting the human nervous system, occurring in 1 of 3500 individuals.2,3 The NF1 gene encodes a tumor suppressor protein called neurofibromin.4 Patients with NF1 develop several types of neoplasms, with neurofibromas being the most common. However, MPNSTs remain the primary cause of NF1-related mortality, primarily because few therapeutic options are available to these patients beyond surgery; current chemotherapeutic and radiation regimens have no effect on the survival of patients with MPNSTs.5 The poor prognosis associated with MPNSTs underscores the need to develop new therapeutic approaches, including combinatorial therapies.
Compared with Schwann cells, MPNSTs display significant alterations in their expression of members of the epidermal growth factor receptor (EGFR/erbB) family of receptor tyrosine kinases.6 Furthermore, the erbB receptors expressed by MPNST cells are constitutively activated (phosphorylated).7 The EGFR family, which has been implicated in the pathogenesis of numerous tumor types, is thus an attractive therapeutic target in MPNSTs. Although specific targeting of EGFR with drugs, such as gefitinib, only marginally decreases proliferation in human MPNSTs,8 pan-erbB inhibitors (e.g., PD168393 and Cl-1033) effectively shut down mitogenesis in these cells.7,8 However, tyrosine kinase inhibitors (TKIs), such as PD168393, primarily exert a cytostatic effect and are relatively poor inducers of cell death.
In addition to their cytostatic action, TKIs can activate autophagy by inhibiting the PI3K-mTOR-Akt axis.9 This raises the question of whether TKIs in combination with agents blocking the autophagy lysosomal pathway might effectively induce MPNST cell death. The use of lysosomotropic agents as adjuvant therapeutic agents provides an excellent example of this strategy. Lysosomotropic agents accumulate in acidic compartments, such as lysosomes, altering the functioning of lysosomal enzymes and preventing degradation of AVs, thereby leading to AV accumulation.10 The prototypical lysosomotropic agent chloroquine (CQ) blocks autophagy completion and induces death in glioma cells.11 CQ-induced lysosomal dysfunction also circumvents resistance to TKIs, such as imatinib, in a Myc-induced model of lymphoma and sarcatinib in prostate cancer xenografts.9,12 However, it is not known whether a similar strategy will be effective against MPNSTs.
The goal of this study was to determine the effects of pan erbB inhibition and lysosomal dysfunction on apoptosis and autophagy in MPNST cells and to test the hypothesis that modulating autophagy will trigger increased cytotoxicity in these cells. We first assessed the effect of the pan erbB inhibitor PD168393 on autophagy and observed that, similar to other TKIs, PD168393 increases autophagy in human MPNST cell lines. As expected, PD168393 inhibits MPNST cell proliferation but does not induce caspase activation and cell death. We then assessed the ability of CQ to trigger death in MPNST cells and found that CQ induces death that has apoptotic features and is attenuated by broad caspase inhibition. Next, we examined the effects of modulating autophagy in MPNST cells exposed to lysosomal stress. In contrast to previous observations in glioma cells,11 suppression of autophagy induction in CQ-treated MPNST cells does not provide significant protection, possibly because of a compensatory upregulation in caspase activation. On the other hand, increasing autophagy induction via mTOR inhibition does not result in increased cytotoxicity, possibly because of concurrent Akt activation. Because of the ability of PD168393 to suppress Akt activation and induce autophagy, we tested the hypothesis that PD168393 may trigger increased apoptosis when administered in combination with CQ. We found that addition of PD168393 to CQ-treated MPNST cells leads to enhanced apoptosis and cytotoxicity. We thus conclude that combinatorial therapy with pan erbB inhibitors and lysosomal dysfunction-inducing agents, such as CQ, may represent an attractive new approach to MPNST treatment.
Materials and Methods
Chemicals
CQ, 3-methyladenine (3-MA), rapamycin, and staurosporine (STS) were purchased from Sigma. BOC-aspartyl(Ome)-fluoromethyl ketone (BAF) was purchased from MP Biomedicals, and bafilomycin A1 (BafA1) was from A.G. Scientific. PD168393 was from Enzo Life Sciences.
Cell Cultures
We previously described the sources of the human MPNST lines used in this study (ST88-14, T265, S462, and 90-8).7,13 The identity of these cell lines was routinely verified in accordance with the specifications outlined in the ATCC Technical Bulletin 8. In brief, morphology and doubling times of all cell lines were routinely assessed. Identity of the cell lines was verified by STR analysis. Cells were also regularly tested for Mycoplasma infection. Cells were cultured in DMEM (Invitrogen) containing 1% penicillin/streptomycin (Invitrogen), 1% L-glutamine (Sigma), and 10% fetal bovine serum (Hyclone) and were incubated at 37°C in humidified 5% CO2, 95% air atmosphere. Cells were plated onto uncoated 48-well plates at a density of 15 000 cells/well. Cultures were then incubated for 48 h before being used in experiments. During treatments, cell culture medium was switched to DMEM without fetal bovine serum. However, assessment of effects of PD168393 on mTOR and Akt activity was performed on cells in serum containing medium.
Cell Viability and In Vitro Caspase Cleavage Assays
Calcein-AM conversion was used to measure cell viability. Caspase activation was assessed by the in vitro caspase-3 cleavage assay using the chemical substrate DEVD-7-amino-4-methylcoumarin (BIOMOL). We previously described both of these methods.11
Cell Proliferation Assays
Incorporation of 3H-thymidine was used to quantify DNA synthesis and was performed in accordance with our previously described methodology.7
Immunocytochemistry
Primary antibodies and their sources and working concentrations were as follows: LC3 (Abgent; 1:2000), cathespin D (Santa Cruz Biotechnology; 1:500), and Lamp1 (1D4B; John Hopkins University School of Medicine; 1:1000). The sources and working concentrations of the secondary antibodies used in this study were horseradish peroxidase (HRP)–conjugated anti-rabbit Super Picture (Invitrogen; 1:100) for LC3, HRP-conjugated donkey anti-goat polyclonal antibody (Jackson Immunoresearch; 1:2000) for cathepsin D, and anti-mouse ImmPRESS (Vector Laboratories; 1:100) for Lamp1. Immunoreactivity was detected using a tyramide signal amplification system (Perkin-Elmer Life Science Products). Bisbenzimide (2 µg/mL; Hoechst 33258; Sigma) was used for nuclear counterstaining. Samples were examined using a Zeiss Axioskop fluorescent microscope equipped with an AxioCam digital camera. Images were captured and analyzed using Axio Vision Rel. 4.8 software (Carl Zeiss MicroImaging).
Western Blot
Whole cell lysates were prepared by washing cells with phosphate-buffered saline, scraping them with cell scrapers, and resuspending pelleted cells in lysis buffer containing 20 mM Tris-HCl (pH, 7.4), 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 10% glycerol, protease inhibitor cocktail (Sigma), and phosphatase inhibitor cocktails 1 and 3 (Sigma). Primary antibodies were used against the following proteins: β-tubulin and cathepsin D (Santa Cruz Biotechnology); cleaved caspase-3 (Asp175), Akt, phospho-Akt (Ser473), mTOR, phospho-mTOR (Ser2448), GAPDH, poly-ADP ribose polymerase (PARP), and Atg7 (Cell Signaling); and LC3 (Abgent). Secondary antibody was HRP-conjugated goat anti-rabbit antibody (Biorad). Signals were detected using ECL Western blotting analysis system (GE Healthcare) or Supersignal chemiluminescence (Pierce).
RNAi
Lentiviral shRNA constructs (Atg7) were purchased from Open Biosystems. Lentiviruses were packaged as previously described.14 ST88-14 cells were plated in 6-well dishes and subjected to infection in the presence of Polybrene overnight. After 48 h, cells were passaged and plated in the presence of 1.5 µg/mL puromycin. Individual clones were selected and transferred to 24-well plates. After subsequent expansion in 60 mm dishes, cells were allowed to grow to confluence before collecting lysates for assessment of protein levels.
Statistics
All data points represent mean ± standard deviation (6 wells for all experiments). All experiments were repeated at least 3 times unless stated otherwise. Representative data are shown. Statistical significance was determined by analysis of variance and post-hoc analysis with use of Bonferroni's test, in which P < .05 was considered to be statistically significant.
Results
Inhibition of erbB Signaling Pathway Blocks Mitogenesis
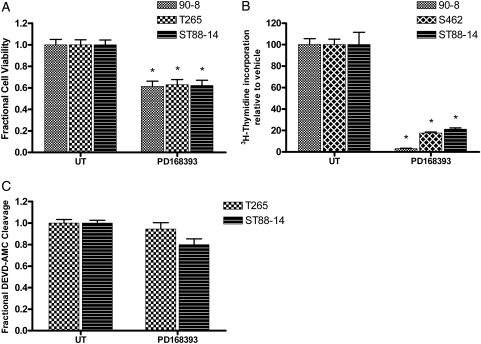
PD168393 is a 6-acrylamido-4-anilinoquinazoline compound that irreversibly inhibits erbB receptors.15 The effect of pan erbB inhibition on viability was examined in human MPNST cells after PD168393 treatment. This assessment showed a decrease in the number of live cells relative to untreated controls (Fig. 1A). The decrease in MPNST cell number after PD168393 treatment could reflect decreased proliferation and/or increased cell death. To distinguish between these possibilities, we first examined the effect of PD168393 on DNA synthesis. PD168393 inhibited mitogenesis in MPNST cells, as evidenced by decreased tritiated thymidine incorporation in treated cells (Fig. 1B). To assess whether PD168393 induced apoptosis, we assessed the cleavage of DEVD-7-amino-4-methylcoumarin, a fluorogenic caspase-3 substrate, in PD168393-treated MPNST cells. Activation of executioner caspases, such as caspase-3, is a major hallmark of apoptotic cell death. PD168393-treated cells demonstrated no change in levels of caspase-3–like activity relative to untreated cells (Fig. 1C). Taken together, these data indicate that PD168393-induced block in erbB signaling is largely cytostatic and has no effect on MPNST cell apoptosis.
Fig. 1.
PD168393 blocks mitogenesis in malignant peripheral nerve sheath tumor (MPNST) cells. Exposure to PD168393 (2 µM; 24–48 h) induced a decrease in (A) the number of viable cells and (B) incorporation of tritiated thymidine. * P < .05 relative to untreated. (C) No change was detected in levels of caspase-3–like activity, relative to untreated cells.
Inhibition of erbB Signaling Pathway Triggers Autophagy
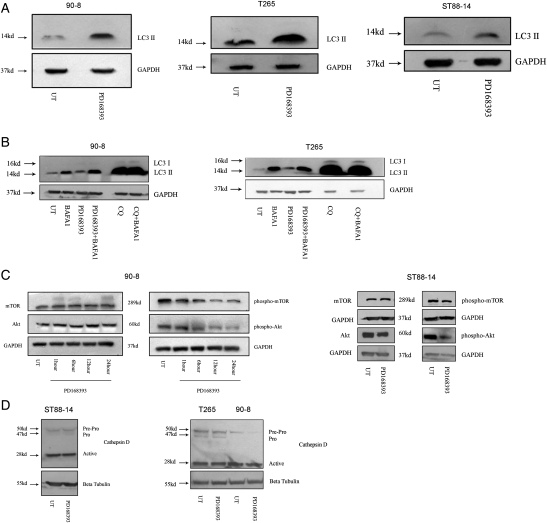
The erbB receptor family feeds into a number of downstream signaling cascades, with the PI3K-mTOR-Akt axis being among the most prominent. Classically, TKIs are thought to stimulate autophagy by virtue of their effect on the PI3K-Akt-mTOR axis.9 To examine the ability of PD168393 to induce autophagy, MPNST cells were treated with PD168393 and lysed. Whole cell lysates were immunoblotted and probed for levels of LC3-II, a widely accepted surrogate marker for AVs. LC3-II is an AV-associated form of the LC3/Atg8 protein, which is formed by cleavage and subsequent lipidation of LC3-I (soluble form of LC3).16 As expected, PD168393-treated cells displayed an increase in levels of LC3-II relative to untreated cells (Fig. 2A). LC3-I could not be visualized in the MPNST cells at the same exposure, in accordance with what has been reported previously.17 However, increased LC3-II levels may result from either increased AV formation or decreased AV degradation. To distinguish between these possibilities, autophagic flux was measured by collecting lysates from cells exposed to bafilomycin A1 during the last 4 hours of their respective treatments. Bafilomycin A1 (inhibitor of vacuolar ATPase) blocks AV clearance, and thus, any increase in AVs may be attributed to increased induction.18 PD168393-treated cells demonstrated an increase in autophagic flux (Fig. 2B). Because increased AV formation is often associated with a block in mTOR and Akt activation,1 we examined the levels of phosphorylated mTOR and Akt in lysates of PD168393-treated cells. We found that levels of phosphorylated forms of mTOR and Akt decreased in a time-dependent manner, suggesting that the increase in autophagy induction in PD168393-treated cells resulted from decreased negative regulation of mTOR and Akt (Fig. 2C). To determine whether the PD168393-induced accumulation of AVs was associated with inhibition of lysosomal function, lysates from PD168393-treated cells were probed with an antibody against cathepsin D (the principal lysosomal aspartate protease mediating long-lived protein degradation). This antibody recognizes all 3 forms of cathepsin D (the 50 kDa inactive pre-pro molecule, the 47 kDa pro form, and the 28 kDa active form). No change was seen in levels of the active form in PD168393-treated cells (Fig. 2D), indicating that the increase in AVs was not attributable to impaired lysosomal function.
Fig. 2.
PD168393 induces autophagy in malignant peripheral nerve sheath tumor (MPNST) cells. Whole cell lysates from PD168393-treated cells (2 µM; 24 h) demonstrated an increase in (A) levels of LC3II and (B) autophagic flux and (C) a decrease in levels of phospho-mTOR (Ser2448) and phospho-Akt (Ser473). (D) No change was seen in the levels of the active form of cathepsin D.
CQ-Induced Lysosomal Dysfunction Blocks Autophagy Completion in MPNST Cells
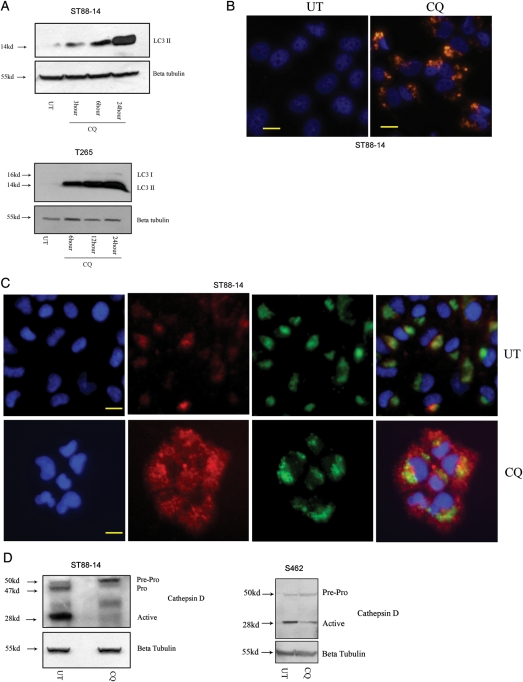
CQ has been shown to block autophagy completion and enhance accumulation of AVs in several cell types, including neurons and glioma cells.11,19 To validate the effects of CQ on autophagy in human MPNST cells, whole cell lysates from CQ-treated cells were immunoblotted and probed for LC3-II. We found that CQ induced a time-dependent increase in levels of LC3-II in all cell lines (Fig. 3A). To confirm these results immunocytochemically, the subcellular distribution of LC3 immunoreactivity in CQ-treated cells was examined. Untreated cells demonstrated relatively weak cytoplasmic LC3 immunoreactivity typical of LC3-I, whereas CQ-treated cells showed clumped LC3 immunoreactivity, indicative of LC3-II association with AVs (Fig. 3B). CQ-treated cells displayed no change in autophagic flux (Fig. 2B). To determine whether the CQ-induced accumulation of AVs was associated with inhibition of lysosomal function, CQ-treated cells were stained with antibodies recognizing cathepsin D and Lamp 1, a marker of lysosomal membranes. In untreated cells, cathepsin D immunoreactivity appeared to be clumped and colocalized with Lamp1, consistent with its presence in lysosomes. In contrast, cells treated with CQ displayed a diffuse cytoplasmic cathepsin D immunoreactivity with decreased colocalization with Lamp1 immunoreactivity (Fig. 3C). This nonlysosomal-associated cathepsin D distribution is consistent with disruption of lysosome membrane integrity and/or abnormal subcellular trafficking and processing of cathepsin D.20 To determine whether CQ had any effect on the processing of cathepsin D, whole cell lysates were prepared from CQ-treated cells and probed with the cathepsin D antibody. We found that CQ treatment decreased the levels of the active form of cathepsin D (Fig. 3D), suggesting a direct or indirect effect on its processing. Taken together, changes in subcellular localization and processing of cathepsin D are indicative of dysfunctional lysosomal activity.20 Thus, these results support the conclusion that CQ-induced lysosomal dysfunction blocks autophagy at a late stage in MPNST cells.
Fig. 3.
Chloroquine (CQ)–induced lysosomal stress blocks autophagy completion in malignant peripheral nerve sheath tumor (MPNST) cells. (A) CQ -treated cells (50 µM) demonstrated a time-dependent increase in levels of LC3II, as assessed by immunoblot anlaysis. (B) Cells exposed to CQ (25 µM; 24 h) demonstrated significantly higher levels of autophagic vacuole (AV)–associated LC3 immunoreactivity (red), relative to untreated (UT) cells. (C) UT cells showed discrete Lamp1-associated (green) cathepsin D immunoreactivity (red), whereas CQ–treated cells (50 µM; 24 h) exhibited diffuse cytoplasmic cathepsin D immunoreactivity. Cell nuclei were counterstained with bisbenzimide (blue). Scale bar equals 20 microns. (D) CQ (50 µM; 24 h) induced a decrease in levels of active cathepsin D, as assessed by immunoblot analysis.
CQ-Induced Lysosomal Dysfunction Triggers Apoptotic Death in MPNST Cells
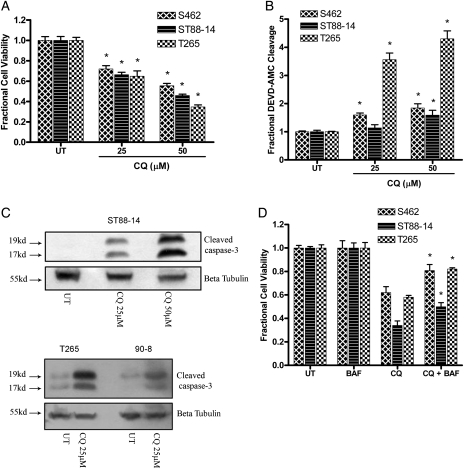
Lysosomal dysfunction has been shown to be a trigger for cell death in several experimental settings.14 To determine whether CQ-induced lysosomal dysfunction is cytotoxic in MPNST cells, the effect of CQ treatment on the viability of MPNST cells was assessed. CQ induced a concentration-dependent decrease in cell viability in MPNST cells (Fig. 4A). We have previously shown that CQ-treated glioma cells demonstrate apoptotic features.11 To determine whether CQ similarly triggers MPNST cell apoptosis, we assessed the cleavage of DEVD-7-amino-4-methylcoumarin and observed a concentration-dependent increase in caspase-3–like activity in CQ-treated MPNST cells (Fig 4B). In addition, immunoblot analyses performed on whole cell lysates collected from CQ-treated cells revealed an increase in levels of cleaved caspase-3 (Fig. 4C). In contrast to our previous studies in cultured glioma cells and telencephalic neurons,11,19 treatment of the human MPNST cell lines with the broad caspase inhibitor BAF significantly attenuated CQ-induced death (Fig. 4D). This indicates that CQ has cytotoxic actions in human MPNST cells and that its mechanism of cell death is, at least in part, caspase-dependent apoptosis.
Fig. 4.
Chloroquine (CQ) triggers apoptotic death in malignant peripheral nerve sheath tumor (MPNST) cells. CQ (24–48 h) induced a concentration-dependent (A) decrease in number of viable cells and (B) an increase in levels of caspase-3–like activity. * P < .05 relative to untreated. (C) Levels of cleaved caspase-3 were found to be elevated concentration-dependently following CQ treatment (24 h). (D) Broad caspase inhibition by BAF (50 µM, 1 h pretreatment) attenuated CQ-induced death (24–48 h). * P < .05 relative to cells treated with CQ alone.
Inhibition of Autophagy Induction Coupled with Lysosomal Dysfunction Leads to a Further Increase in Caspase Activation
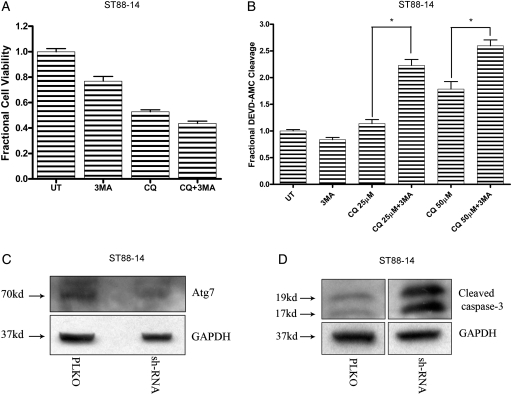
As described above, dying MPNST cells displayed a dramatic accumulation of AVs after CQ treatment. Therefore, we assessed the effects of modulating AV formation in cells exposed to CQ-induced lysosomal stress. First, the effects of suppressing autophagy in CQ-treated MPNST cells were assessed using 3-MA, a class III-PI3K inhibitor that blocks pre-autophagosome formation. It has been reported previously that blocking autophagy induction in cells that have impaired ability to clear AVs may provide protection from lysosomal dysfunction–induced death.11,14 However, we found that a 1-h pretreatment with 3-MA did not protect MPNST cells from CQ-induced death (Fig. 5A).
Fig. 5.
Inhibition of autophagy induction in chloroquine (CQ)–treated cells leads to increased caspase activation. Treatment with 3-MA (3 mM; 1 h pretreatment) (A) did not significantly affect CQ-induced death (50 µM; 48 h) but (B) induced an increase in levels of caspase-3 like activity. * P < .05 relative to cells treated with CQ alone. Lentiviral-mediated knockdown of Atg7 in CQ-treated cells (50 µM; 24 h) resulted in (C) decreased levels of Atg7 and (D) an increase in levels of cleaved caspase-3.
Autophagy and apoptosis can occur simultaneously in a dying cell, and suppression of one pathway can lead to a compensatory upregulation of the other. To test the possibility that autophagy inhibition can trigger apoptosis, caspase-3–like activity was assessed in MPNST cells pretreated with 3-MA prior to CQ treatment. Increased levels of caspase-3–like activity were observed in cells treated with both 3-MA and CQ, compared with cells treated with CQ or 3-MA alone (Fig. 5B). Similar results were obtained when autophagy induction was suppressed in MPNST cells by lentiviral-mediated knockdown of Atg7, a critical regulator of autophagy initiation (Fig. 5C and D). These findings indicate that blocking autophagy induction leads to a compensatory upregulation of caspase activation in MPNST cells exposed to lysosomal stress.
Autophagy Induction by mTOR Inhibition Alone Does Not Induce Increased Apoptosis in Combination with Lysosomal Dysfunction
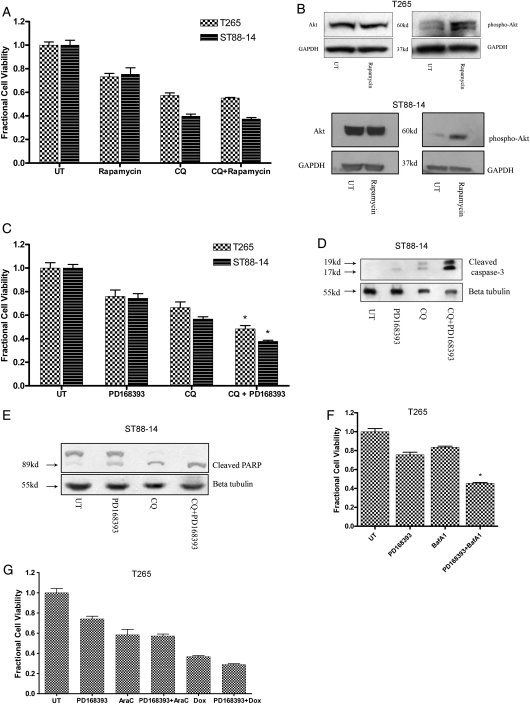
We hypothesized that, because continued induction of autophagy in combination with blocked AV degradation triggers death,21 pretreatment with the mTOR inhibitor rapamycin would increase the sensitivity of MPNST cells to CQ-induced cell death. To test this hypothesis, cells were pretreated for 1 h with rapamycin, followed by addition of CQ, and viability was assessed. Dual treatment with rapamycin and CQ did not lead to increased cytotoxicity, compared with cells receiving CQ alone (Fig. 6A). Rapamycin-induced suppression of mTOR activity has been reported to trigger a feedback mechanism that results in upregulation in the activity of the Akt survival pathway.22 This was confirmed by immunoblot analyses indicating that, in addition to autophagy, rapamycin-treated cells also activate Akt (Fig. 6B).
Fig. 6.
Coupling lysosomal dysfunction with pan erbB inhibition leads to increased cytotoxicity. (A) Treatment with rapamycin (3 µM; 1 h pretreatment) did not affect chloroquine (CQ)–induced death (50 µM; 24–48 h) but (B) induced an increase in Akt activation. (C) Pretreatment with PD168393 (2 µM; 1 h pretreatment) triggered increased cytotoxicity in CQ-treated cells (36–72 h). * P < .05 relative to cells treated singly with CQ or PD168393. Immunoblot analyses demonstrated (D) an increase in levels of cleaved caspase-3 and (E) cleaved PARP in cells receiving dual treatment with CQ and PD168393 relative to cells treated with either agent alone (24 h). (F) Cells receiving dual treatment with BafA1 (100 nM) and PD168393 demonstrated increased cell death relative to cells receiving either agent alone (24 h). *P < .05 relative to cells treated singly with CQ or BafA1. (G) Cells receiving dual treatment with cytosine arabinoside (50 µM) or doxorubicin (0.5 µg/mL) and PD168393 did not demonstrate any difference in viability relative to cells receiving single treatment (24 h).
Coupling Lysosomal Dysfunction with pan erbB Inhibition Leads to Increased Cytotoxicity
We hypothesized that, because PD168393 blocks both mTOR and Akt activation and triggers an increase in autophagy induction, pretreatment with PD168393 would increase the sensitivity of MPNST cells to CQ-induced cell death. To test this hypothesis, MPNST cells were subjected to 1 h of pretreatment with PD168393, followed by addition of CQ, and the number of viable cells assessed. A significant decrease in the number of viable cells was observed in cultures receiving both drugs, compared with cells receiving either of the 2 drugs alone (Fig. 6C). To assess whether this was attributable to increased apoptosis, cell lysates were subjected to immunoblot analyses and probed for levels of cleaved caspase-3. Cells receiving dual treatment displayed increased levels of cleaved caspase-3, compared with cells receiving either drug alone (Fig. 6D). To further test the effects of dual treatment on caspase-3 activity, levels of cleaved PARP (a known physiological substrate for active caspase-323,24) were assessed. Immunoblot analyses of PD168393-treated cells with use of an antibody recognizing both unmodified and cleaved forms of PARP demonstrated an increase in the levels of cleaved PARP in cells receiving dual treatment, compared with cells receiving either treatment alone (Fig. 6E). This finding indicates that, although pan erbB inhibition by PD16839 is not especially cytotoxic, it can trigger death in cells with ongoing lysosomal dysfunction. However, in addition to inducing lysosomal stress, CQ is a genotoxic stress-inducing agent.25 To confirm that increased CQ cytotoxicity in combination with pan erbB inhibition is attributable to autophagy blockade by CQ, analogous experiments were repeated using bafilomycin A1 in place of CQ. Increased cell death was observed in cells after combined treatment with PD168393 and bafilomycin A1 (Fig. 6F). In contrast, combining PD168393 with the genotoxic agents cytosine arabinoside or doxorubicin did not affect the viability of cells relative to either agent alone (Fig. 6G). This demonstrates that the increased cytotoxicity resulting from combined CQ and PD168393 therapy can be attributed to a lysosomal dysfunction–induced block in autophagy completion.
Discussion
In the present study, we investigated the hypothesis that manipulation of the autophagy lysosomal pathway could be used to enhance drug-induced MPNST cell death. We assessed the effect of pan erbB inhibition on autophagy and observed an increase in AV accumulation. In addition, we established the mechanisms of action of lysosomal dysfunction–induced death in MPNST cells using CQ, a prototypical lysosomotropic agent. By virtue of its alkalinity, CQ accumulates in and alters the pH of acidic compartments, such as lysosomes (lysosomotropism).18 This inhibits lysosomal enzyme function, resulting in lysosomal dysfunction. We found that CQ produced a cytotoxic response that was attenuated by broad caspase inhibition. The role of autophagy was studied in this in vitro model of lysosomal dysfunction by pharmacologic and genetic manipulations of the autophagy pathway. Inhibition of autophagy induction did not significantly affect viability of CQ-treated cells, nor did stimulation of autophagic activity. However, cells receiving dual treatment with PD168393 and CQ displayed increased caspase activation and cell death, leading us to conclude that pan erbB inhibition is a potent enhancer of lysosomal stress-induced MPNST apoptotic cell death.
Traditionally considered to be a homeostatic response to nutrient stress, autophagy is now recognized as a major cellular pathway regulating the response of cells to a wide variety of stressors. There is increasing interest in designing drug combinations that take advantage of autophagy modulation, especially those that use agents disrupting lysosomal trafficking and/or function. However, autophagy may have contrasting roles that are stimulus and cell type dependent. Autophagy can be upregulated as a stress response to drug exposure, suppressing apoptosis; alternatively, autophagy can potentiate death either by itself or by promoting apoptosis. The effects of TKIs on autophagy also vary. Inhibitors of the Src and Bcr-Abl kinases stimulate a high level of autophagy that, when blocked at a late stage, sensitizes cancer cells to apoptosis.9,12 On the other hand, dasatinib-induced autophagy in glioma cells potentiates cell death by itself, and in this scenario, a further increase in autophagy induction by temozolomide significantly increases cell death.26 This underscores the need to individually characterize the mechanisms of action of each drug for a given tumor type before formulating drug combinations to target tumor cells.
Because of the potential importance of pan erbB inhibitors in MPNST treatment, we assessed the effects of the pan erbB inhibitor PD168393 on cell survival, caspase activation, and autophagy. We found that PD168393 retards MPNST cell proliferation but is not particularly cytotoxic. This is consistent with previously published reports on the cytostatic effects of pan erbB inhibitors, such as canertinib in MPNST cell lines.8 We observed a decrease in mTOR activity accompanied by an increase in autophagy induction in PD168393-treated MPNST cells. Because mTOR negatively regulates autophagy, drugs that target the mTOR pathway often induce an upsurge in autophagy. Increased autophagy induction affords the possibility of formulating drug combinations with agents affecting lysosomal function. Successful completion of the autophagy lysosomal pathway requires fusion of the AVs with lysosomes and degradation of the intralumenal content. Inhibition of lysosomal function and resultant AV accumulation triggers death in tumor cells, suggesting the possibility that inducing autophagy in cells exposed to lysosomal stress will trigger cytotoxicity. Accordingly, TKIs have been used in combination with lysosomal dysfunction–inducing agents to trigger apoptosis in tumor cells.9 However, to our knowledge, this strategy has not previously been tested in MPNSTs.
Several studies have proposed the use of lysosomal stress inducers as adjuvants in chemotherapy.27 Indeed, CQ has been shown to potentiate the cytotoxic effects of histone deacetylase inhibitors in MPNST cells.28 However, lysosomal dysfunction–induced death has not been previously characterized in MPNSTs. Here, we treated MPNST cells with the prototypical lysosomotropic agent CQ to induce lysosomal stress and examined the roles that apoptosis and autophagy play in CQ-induced MPNST cell death. CQ induced a concentration-dependent increase in levels of cleaved caspase- 3, a marker of apoptosis. However, in contrast to our studies in glioma cells in which CQ-induced death was caspase independent,11 broad caspase inhibition significantly attenuated CQ-induced cytotoxicity in MPNST cells, indicating that there may be cell-type specific differences in mechanisms of CQ-induced death.
We assessed the effect of suppressing autophagy in MPNST cells undergoing lysosomal stress. Contrary to what we had expected, pharmacological inhibition of AV formation had no significant effect on MPNST cell viability. Autophagy suppression has been shown to provide protection from CQ-induced death, possibly because it leads to decreased AV accumulation in cells with compromised clearance capability.11,14 However, after further investigation, we observed a concomitant increase in levels of caspase activity in MPNST cells treated with both CQ and 3-MA, raising the possibility that a compensatory upsurge in apoptosis may mask any protection afforded by blocking autophagic activity in CQ-treated cells. Numerous studies demonstrating the cooperative relationship between apoptosis and autophagy exist in the literature, indicating the potential need to suppress both programs to achieve a significant increase in cell survival.29
In some experimental models, autophagy-inducing agents coupled with late-stage autophagy inhibitors trigger cell death.30 We therefore examined the effect of increasing autophagy induction in CQ-treated cells by using rapamycin to block the activity of mTOR. We found no increase in cytotoxicity in cells treated with CQ and rapamycin. However, an assessment of rapamycin-treated cells revealed that, although there was a decrease in mTOR activity, there was also a concomitant increase in Akt activation, which is a pro-survival signal. Feedback activation of Akt has been reported in rapamycin-treated cells,22 suggesting that simultaneous inhibition of pro-survival Akt signaling may be needed to circumvent resistance to strategies using late-stage autophagy blockade in combination with mTOR inhibition. The use of dual inhibitors of PI3K/Akt and mTOR has been proposed in this regard.31 We found that PD168393 dually inhibited mTOR and Akt signaling, suggesting that pretreatment of cells with PD168393 would sensitize MPNST cells to CQ-induced lysosomal stress and potentiate cell death. Our results indicate that there is significantly increased apoptosis and cytotoxicity in cells receiving dual treatment, validating our hypothesis. The use of bafilomycin A1, which also induces lysosomal dysfunction, although through a different mechanism than CQ, lends further support to our conclusion that pan erbB inhibition in cells exposed to lysosomal stress is a potent trigger for cell death in MPNST cells.
The present study provides a rationale for developing additional drug combinations using pan erbB agents dually targeting mTOR and Akt in combination with lysosomotropic agents to effectively target MPNST cells. There has been a study reporting the efficacy of PI-103, a dual inhibitor of mTOR/PI3K-Akt in shutting down mitogenesis in MPNST cells.31 Our findings warrant the testing of agents, such as PI-103, in combination with lysomotropic agents to increase their therapeutic effectiveness.
Funding
This work was supported by the National Institutes of Health grants NS41962, CA134773 and P50CA097247 (K.A.R.) and CA122804, NS048353 and the Department of Defense grant W81XWH-09-1-0086 (S.L.C.).
Acknowledgements
We thank Barbara Klocke, Mary Ballestas, and the UAB Neuroscience Core facilities (NS47466 and NS57098) for technical assistance.
Conflict of interest statement. None declared.
References
- 1.Noda T, Ohsumi Y. Tdor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. doi:10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- 2.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. doi:10.1001/jama.1997.03550010065042. [PubMed] [Google Scholar]
- 3.Gutmann DH. The neurofibromatoses: when less is more. Hum Mol Genet. 2001;10:747–755. doi: 10.1093/hmg/10.7.747. doi:10.1093/hmg/10.7.747. [DOI] [PubMed] [Google Scholar]
- 4.Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci USA. 1991;88:9658–9662. doi: 10.1073/pnas.88.21.9658. doi:10.1073/pnas.88.21.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. [PubMed] [Google Scholar]
- 6.Holtkamp N, Malzer E, Zietsch J, et al. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro Oncol. 2008;10:946–957. doi: 10.1215/15228517-2008-053. doi:10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stonecypher MS, Byer SJ, Grizzle WE, et al. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24:5589–5605. doi: 10.1038/sj.onc.1208730. doi:10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- 8.Dilworth JT, Wojtkowiak JW, Mathieu P, et al. Suppression of proliferation of two independent NF1 malignant peripheral nerve sheath tumor cell lines by the pan-ErbB inhibitor CI-1033. Cancer Biol Ther. 2008;7:1938–1946. doi: 10.4161/cbt.7.12.6942. doi:10.4161/cbt.7.12.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellodi C, Lidonnici MR, Hamilton A, et al. Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119:1109–1123. doi: 10.1172/JCI35660. doi:10.1172/JCI35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seglen PO, Gordon PB. Effects of lysosomotropic monoamines, diamines, amino alcohols, and other amino compounds on protein degradation and protein synthesis in isolated rat hepatocytes. Mol Pharmacol. 1980;18:468–475. [PubMed] [Google Scholar]
- 11.Geng Y, Kohli L, Klocke BJ, et al. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro-Oncology. 2010;12:473–481. doi: 10.1093/neuonc/nop048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Chang PC, Yang JC, et al. Autophagy Blockade Sensitizes Prostate Cancer Cells towards Src Family Kinase Inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. doi:10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byer SJ, Eckert JM, Brossier NM, et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor-independent manner. Neuro Oncol. 2011;13:28–41. doi: 10.1093/neuonc/noq146. doi:10.1093/neuonc/noq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walls KC, Ghosh AP, Franklin AV, et al. Lysosome dysfunction triggers Atg7-dependent neural apoptosis. J Biol Chem. 2010;285:10497–10507. doi: 10.1074/jbc.M110.103747. doi:10.1074/jbc.M110.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry DW, Bridges AJ, Denny WA, et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci USA. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. doi:10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. doi:10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. doi:10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidi AU, McDonough JS, Klocke BJ, et al. Chloroquine-induced neuronal cell death is p53 and Bcl-2 family-dependent but caspase-independent. J Neuropathol Exp Neurol. 2001;60:937–945. doi: 10.1093/jnen/60.10.937. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nature Reviews Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. doi:10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 21.Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Molecular and Cellular Biology. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. doi:10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. doi:10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 23.Tewari M, Quan LT, O'Rourke K, et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. doi:10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. doi:10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee T, Muhkopadhyay A, Khan KA, et al. Comparative mutagenic and genotoxic effects of three antimalarial drugs, chloroquine, primaquine and amodiaquine. Mutagenesis. 1998;13:619–624. doi: 10.1093/mutage/13.6.619. doi:10.1093/mutage/13.6.619. [DOI] [PubMed] [Google Scholar]
- 26.Milano V, Piao Y, LaFortune T, et al. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. doi:10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 27.Briceno E, Reyes S, Sotelo J. Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus. 2003;14:e3. doi: 10.3171/foc.2003.14.2.4. [DOI] [PubMed] [Google Scholar]
- 28.Lopez G, Torres K, Lev D. Autophagy blockade enhances HDAC inhibitors’ pro-apoptotic effects: Potential implications for the treatment of a therapeutic-resistant malignancy. Autophagy. 2011;7:440–441. doi: 10.4161/auto.7.4.14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maclean KH, Dorsey FC, Cleveland JL, et al. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118:79–88. doi: 10.1172/JCI33700. doi:10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shingu T, Fujiwara K, Bogler O, et al. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer. 2009;124:1060–1071. doi: 10.1002/ijc.24030. doi:10.1002/ijc.24030. [DOI] [PubMed] [Google Scholar]
- 31.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. doi:10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]