Abstract
Purpose
This study was performed to determine if there is an age-related specificity in the response of muscle protein metabolism to severe burn injury during acute hospitalization. This is a retrospective analysis of previously published data. Methods: Nineteen adult and 58 pediatric burn-injured patients (age 43.3 ± 14.3 vs. 7.2 ± 5.3 years, adult vs. children) participated in stable isotope [ring-2H5]phenylalanine (Phe) infusion studies. Femoral arterial and venous blood samples and muscle biopsy samples were collected throughout the study. Data are presented as means ± standard deviation (SD). A p value less than 0.05 was considered statistically significant.
Results
Muscle net protein balance (NB) was higher in children (adult vs. children, -43 ± 61 vs. 8 ± 68 nmol Phe/min/100 ml leg volume, p < 0.05). Muscle protein fractional synthesis rate (FSR) was higher in children (adult vs. children, 0.11 ± 0.05 vs. 0.16 ± 0.10 %/h, p < 0.05). Leg muscle protein breakdown was not different between the groups (adult vs. children, 179 ± 115 vs. 184 ± 124 nmol Phe/ min/100 ml leg volume, p < 0.05; synthesis rate was 134 ± 96 and 192 ± 128 nmol Phe/min/100 ml leg volume in adults and children, respectively (p = 0.07). Age significantly correlated with muscle protein NB (p = 0.01) and FSR (p = 0.02); but not with breakdown (p = 0.67) and synthesis (p = 0.07) rates measured by using a three-pool model.
Conclusion
In burn injury, the muscle protein breakdown may be affected to the same extent in adults and children, whereas synthesis may have age-related specificities, resulting in a better but still low NB in children.
Keywords: Burn, Muscle protein metabolism, Age
Introduction
Severe burn injury results in a hypermetabolic and catabolic state with loss of lean body mass in both adults and children. The muscle protein net balance (NB), a result of the balance between protein synthesis and breakdown, determines the rate of protein loss or gain. The distinction of whether synthesis or breakdown is altered and responsible for muscle loss in burns will be beneficial to better target therapeutic strategies developed to ameliorate the muscle loss.
Stable isotope tracer techniques are widely used to measure skeletal muscle protein kinetics in vivo. Skeletal muscle protein kinetics in healthy adults have been evaluated extensively. Although in many cases the studies were performed using different protocols or under different nutritional conditions, e.g., fasted, amino acid ingestion or infusion, they have allowed a good estimation of values of muscle protein kinetics in healthy adults. Our research group has conducted several studies to measure muscle protein kinetics in adult [1–5] and pediatric [1, 6–9] burn patients during acute hospitalization. Many of these studies were done under the same nutritional conditions (enteral feeding with Vivonex T.E.N.) and using the same tracer protocol. These similarities between studies provide a unique opportunity to compare muscle protein kinetics in a large number of burn-injured children and adults.
Many factors contribute to muscle catabolism and outcome of burn injury. Hart et al. [1] reported that age is one of the determinants of muscle loss in burn injury. They showed that leg muscle protein net deposition was lower in adults compared to children; however, they did not distinguish whether the difference was due to differences in the protein synthesis or breakdown rates. Although it is well established that a severe burn injury results in a significant loss of lean body mass in both adults and children, a more positive net protein balance in children logically can be expected. Children are growing, therefore their protein kinetics would be expected to be more anabolic, rather than catabolic. Clarifying the nature of any age-dependent difference in muscle protein net deposition could help in developing age-related strategies for amelioration of the loss of lean body mass in burn injury.
In this study we retrospectively analyzed data from our previously reported tracer infusion studies [1–9] to evaluate the differences between adults and children in muscle protein synthesis and breakdown rates, as well as net muscle protein balance, in response to burn injury.
Patients and methods
We retrospectively analyzed data from adult and pediatric patients treated at the Blocker Adult Burn Unit, University of Texas Medical Branch (UTMB) and Shriners Hospital for Children, Galveston, TX, respectively. All patients had provided a signed informed consent form to the UTMB Institutional Review Board approved protocol and the data were previously presented elsewhere [1–9]. The inclusion criteria for data analyses were admittance to the hospitals during the acute phase after burn injury; receiving only a standard of care treatment (i.e., no experimental drug was given); participation in a stable isotope infusion protocol to evaluate leg muscle protein kinetics (as described below) within 32 days following the burn injury; availability of data on muscle protein fractional synthesis rate (FSR) and amino acid kinetics using the arteriovenous balance method.
Stable isotope infusion protocol
The stable isotope infusion protocol was described previously [1–9]. Briefly, all patients participated in a 5-h study with primed constant infusion of [ring-2H5]phenylalanine. Arteriovenous blood samples were drawn at 20-min intervals between hours 4 and 5. At hours 2 and 5 muscle biopsy samples were obtained from the vastus lateralis. Leg blood flow was measured using indocyanine green (ICG) as described [1–10], and normalized for leg volume. The subject's leg circumference at prescribed points relative to anatomic landmarks, and the distances between these points were used to mathematically model the leg volume [10].
Each patient was studied during enteral feeding with Vivonex T.E.N. (Sandoz Nutrition, Minneapolis, MN, USA). The composition of Vivonex is 82% carbohydrate, 15% protein (as amino acids), and 3% fat. Daily caloric intake was titrated to 1,500 kcal/m2 of total body surface area plus an additional 1,500 kcal/m2 of burned area surface.
Analysis of samples
Sample processing has been previously described [1–10]. Plasma and muscle intracellular and bound phenylalanine enrichments (tracer-to-tracee ratio) were determined on their tert-butyldimethylsilyl (t-BDMS) derivatives using gas chromatography-mass spectrometry (GC-MS HP 5989; Hewlett-Packard, Palo Alto, CA, USA) with electron impact ionization [10]. The internal standard technique was used to measure the plasma phenylalanine concentration [10].
The serum concentration of ICG was determined by means of spectrophotometry at λ = 805 nm.
Calculations
The protocol was designed to simultaneously determine skeletal muscle amino acid kinetics from the enrichments and concentrations of phenylalanine in the femoral artery and vein and from the enrichment of tissue-free phenylalanine, using a three-pool model, as previously described and validated [4, 5, 10, 11]. The three-pool model parameters were calculated as follows: delivery to the leg, Fin = CA × BF; release from the leg, F out = CV × BF; net balance across the leg, NB = (CA − CV) × BF; transport into muscle, FM,A = {[(EM − EV)/(EA − EM)] × CV + CA} × BF; transport from muscle, FV,M = {[(EM − EV)/(EA − EM)] × CV + CV} × BF; arteriovenous shunting, FV,A = Fin − FM,A; muscle protein breakdown, FM,O = FM,A × (EA/EM − 1); muscle protein synthesis, FO,M = (CA × EA − CV × EV) × BF/EM. CA and CV are plasma phenylalanine concentrations in the femoral artery and vein, respectively; EA, EV, and EM are phenylalanine enrichments (tracer/tracee ratio) in the femoral arterial, venous plasma, and muscle, respectively; BF is the leg blood flow. Data are presented as nanomoles of phenylalanine per minute per 100 ml leg volume.
The muscle protein FSR was calculated from the determination of the rate of tracer incorporation into the protein and the enrichment of the intracellular pool as the precursor [10]: FSR = [(Ep2 − Ep1)/(EM × t)] × 60 × 100, where Ep1 and Ep2 are the enrichments of the protein-bound [ring-2H5]phenylalanine at 2 and 5 h; EM is the average intracellular [ring-2H5]phenylalanine enrichment over the time of incorporation; and t is the time in minutes between the biopsies. The factors 60 and 100 are required to express FSR in percent per hour (%/h).
Data presentation and statistical analysis
Data are presented as means ± SD. The Student's t test was used to evaluate the differences between the groups; linear regression and correlation analyses were performed to determine the relationship between the age and the parameters of leg muscle protein turnover. Differences were considered statistically significant at p < 0.05.
Results
Patients
Data from 19 adult and 58 pediatric patients were eligible for analysis according to the inclusion criteria. The demographic parameters are presented in Table 1. There was no difference between the groups in gender distribution, total body surface area burned (TBSA), and average days after burn injury when the infusion study was conducted (Table 1). All the patients received the standard of care prior to the infusion study.
Table 1.
Patient demographics
| Parameters | Adults | Children | P |
|---|---|---|---|
| N | 19 | 58 | – |
| Age | 42.2 ± 14.7 | 7.2 ± 5.3 | <0.001 |
| Gender (F/M) | 5/14 | 22/36 | 0.417 |
| TBSA (%) | 59.9 ± 20.5 | 58.1 ± 18.4 | 0.715 |
| Days postburn (days) | 10.8 ± 7.8 | 11.9 ± 6.7 | 0.520 |
| Leg blood flow (ml/min/100 ml leg volume) | 10.1 ± 6.3 | 13.8 ± 7.2 | 0.048 |
Data are presented as mean ± SD
TBSA total body surface area burned; Days postburn postburn day when the tracer study was conducted
Leg muscle amino acid kinetics and muscle protein metabolism
The leg blood flow was significantly higher in children, p = 0.048 (Table 1). Phenylalanine kinetics are presented in Table 2. There were no differences in the phenylalanine transport parameters (Table 2).
Table 2.
Leg muscle phenylalanine kinetics
| Parameters | Adult | Children | P |
|---|---|---|---|
| Delivery to the leg | 1,007 ± 720 | 1,379 ± 942 | 0.12 |
| Release from the leg | 1,051 ± 763 | 1,370 ± 948 | 0.18 |
| Transport into the muscle | 196 ± 168 | 308 ± 275 | 0.10 |
| Transport from the muscle | 239 ± 197 | 299 ± 283 | 0.39 |
| A-V shunting | 811 ± 595 | 1,071 ± 776 | 0.18 |
| Muscle protein breakdown rate | 179 ± 115 | 184 ± 124 | 0.82 |
| Muscle protein synthesis rate | 134 ± 96 | 192 ± 128 | 0.07 |
| Net balance | −43 ± 61 | 8 ± 68 | <0.05 |
| Muscle protein FSR (%/h) | 0.11 ± 0.05 | 0.16 ± 0.10 | 0.02 |
Data are presented as mean ± SD. Units for measurements calculated using the three-pool model are presented in nanomoles of phenylalanine per minute per 100 ml of leg volume (nmol Phe/min/100 ml leg volume); FSR data are presented in percent per hour (%/h)
A-V arteriovenous, FSR fractional synthesis rate
The leg muscle protein breakdown measured using the three-pool model was only 4% different between the groups (p = 0.82), protein synthesis was higher in the children by 31% (Table 2), although the difference did not reach statistical significance (p = 0.07). However, as a result of the difference in the protein synthesis rates, the net protein deposition (the balance between protein synthesis and breakdown) was significantly higher in children (p < 0.05) (Table 2). The muscle protein FSR, measured by the direct incorporation method, was significantly higher in children (Table 2 p < 0.05).
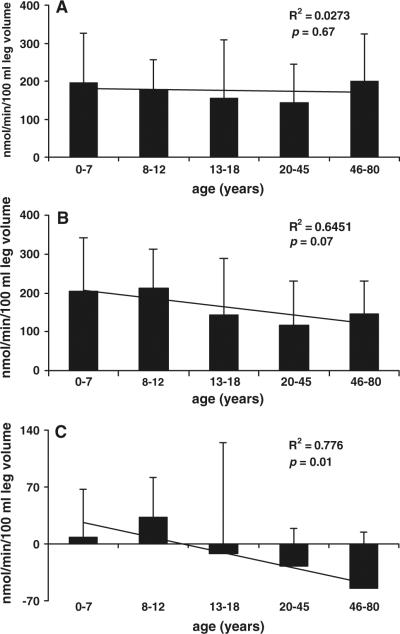
Correlation and linear regression analyses demonstrated that age significantly and negatively correlated with leg muscle protein net balance (r2 = 0.78, p = 0.01) (Fig. 1c) and FSR (r2 = 0.76, p = 0.01) (Fig. 2). Correlation analysis demonstrated that age accounted for 64% of the variability (r2 = 0.64) in the synthesis rate of leg muscle protein (measured by using the three-pool model); however, by the linear regression analysis the correlation did not reach statistical significance (p = 0.07) (Fig. 1b). Age did not correlate with the breakdown rate of leg muscle protein (r2 = 0.02, p = 0.67) (Fig. 1a).
Figure 1.
The breakdown (a) and synthesis (b) rates and the net balance (c) of leg muscle protein calculated by using the three-pool model. The data are presented as means (nmol Phe/min/100 ml leg volume) per age groups: age 0–7 (n = 35), age 8–12 (n = 10), age 13–18 (n = 6), age 20–45 (n = 8), age 46–80 (n = 11), and standard deviations of means
Figure 2.
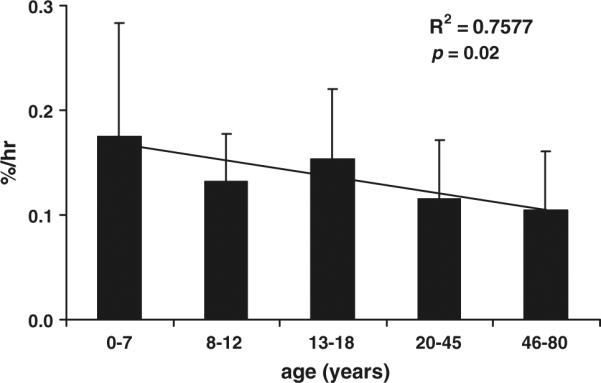
The fractional synthesis rate of leg muscle protein measured by using the direct incorporation method. The data are presented in means (percent per hour, %/h) per age groups: age 0–7 (n = 35), age 8–12 (n = 10), age 13–18 (n = 6), age 20–45 (n = 8), age 46–80 (n = 11), and standard deviations of means
Discussion
The current results demonstrate that the catabolic response of muscle protein turnover to severe burn injury may be greater in adults as compared to children. The loss of muscle mass may affect survival and certainly limits recovery from the injury. These results are consistent with the earlier report from this institution by Hart et al. [1], who showed that adult burn patients have lower muscle protein net balance as compared to children, but these authors did not assess the role of alterations in protein synthesis and breakdown in contributing to the catabolic response.
The main result of this study is that during the acute phase after burn injury there are similarities and differences in leg muscle protein metabolism between adults and children. The leg muscle protein breakdown was almost the same in both groups. This suggests that the effect of burn injury on the mechanism(s) responsible for muscle protein catabolism is (are) not age-dependent. In contrast, the protein synthesis was higher in children than in adults, and this difference resulted in a significantly better net balance in children. In addition, there was a significant correlation between age and net protein balance in leg muscle.
We measured muscle protein synthesis by two different methods, namely A-V balance and the direct incorporation method (FSR). In both cases the protein synthesis was higher by about 30% in the pediatric patient group, although the difference was significant only in the case of muscle protein FSR. Sample size analysis demonstrated that in the case of protein synthesis rate measured by the A-V balance method 78 patients per group would have been required to reach statistical significance with a power of 0.8 and α = 0.05. This large sample size can be explained by the fact that the A-V balance method calculations are based on many variables including leg blood flow, and measures made on arterial and venous blood and muscle biopsy samples. In contrast, the direct incorporation method used to calculate protein FSR requires only measurement of tracer enrichment in muscle biopsy samples [10]. Thus, although only the FSR indicates a statistically significant difference, it is reasonable to conclude that muscle protein synthesis was higher in the children than adults.
Evaluation of the causes of the similarities and differences in protein kinetics in children and adults is beyond the scope of this study. However, some speculation is possible. The rates of blood flow were different between the groups, with values in children being significantly higher. The calculation of muscle protein synthesis by the three-pool model requires blood flow measurement; therefore one may suggest that the higher leg blood flow in children artificially resulted in higher protein synthesis. However, we believe this is not the case for two reasons: (1) other amino acid kinetic parameters that require blood flow measurement, such as phenylalanine transport from artery to the leg muscle, were not significantly different between the groups; and (2) muscle protein FSR calculated by the direct incorporation method (which does not require blood flow measurement) was significantly higher in children (p < 0.05). Therefore, we believe that if blood flow had an effect on protein synthesis it was not due to the mathematical model, i.e., the three-pool model that was used for calculations. Rather, it is possible that increased blood flow increased delivery of absorbed nutrients to the leg, and that the increased synthesis was a response to increased nutrient availability. In addition, higher blood flow could have increased transport of certain anabolic substances, i.e., insulin, to the legs. Insulin is a known anabolic agent that increases muscle protein synthesis; therefore this is a possibility.
Growing children are normally in an anabolic state. Therefore, even if burned children are in a catabolic state acutely, it is expected that they would be more anabolic than adults, who are not in an anabolic state normally. Previously we have shown that the mechanism of the beneficial effect of testosterone treatment in burn-injured patients is distinctly different between adults and children [5, 6]. In adults, testosterone administration decreased the breakdown rate [5], whereas in pediatric patients it stimulated protein synthesis [6]. This difference in response to an anabolic agent supports our general speculation that children have a greater capacity for muscle protein synthesis, even when severely burned, than do adults. The similarities in the breakdown rate of the leg muscle protein in these two groups can be explained by the evidence that regardless of the age, burn-injured patients exert similarities in features of the catabolic conditions, e.g., comparable rates of expression of TNF-α and increased levels of glucocorticoids in both age groups [12–18].
In conclusion, the current results demonstrate that during the acute phase after burn injury muscle protein metabolism has similarities and differences between adult and pediatric patients. In burn injury, the muscle protein breakdown is affected to the same extent in both groups, whereas synthesis is higher in children, resulting in a better net balance in children. Although both patient populations remain in a catabolic state, these results may suggest that during acute hospitalization after burn injury measures to counteract the catabolic response of muscle protein are particularly important in adult patients.
Acknowledgments
The authors wish to thank the study volunteers and their families for their patience and dedication, and the clinical and research staff of Shriners Hospital for Children and Blocker Adult Burn Unit, UTMB, Galveston, TX for their help with conducting the clinical portion of the study; Tara Cocke, Christopher Danesi, Ann Hightower, and Cindy Locklin for excellent technical performance; Guy Jones and Jariwala Guarang for performing GC-MS analyses, and Steve Schuenke for his excellent technical assistance in the preparation of this manuscript. This work was supported, in part, by NIH Grants R01-GM56687, P50-GM60338, R01-GM57295, and T32-GM08256 as well as the Shriners Mass Spectrometry Core Grant 84090. CCF is an ITS Career Development Scholar supported, in part, by NIH KL2RR029875 and NIH UL1RR029876 (ARB).
References
- 1.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, gold WF, Wolfe RR, Herndon DN. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debroy MA, Wolf SE, Zhang XJ, Chinkes DL, Ferrando AA, Wolfe RR, Herndon DN. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999;47:904–910. doi: 10.1097/00005373-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. Acute dichloroacetate administration increases skeletal muscle free glutamine concentrations after burn injury. Ann Surg. 1998;228:249–256. doi: 10.1097/00000658-199808000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–1942. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, Wolfe RR, Herndon DN. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SE, Thomas SJ, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, Herndon DN. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–811. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, Wolfe RR, Herndon DN. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–764. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. Wiley; New Jersey: 2005. [Google Scholar]
- 11.Biolo G, Chinkes DL, Zhang XJ, Wolfe RR. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN. 1992;16:305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR. Metabolic response to burn injury: nutritional implications. Keio J Med. 1993;42:1–8. doi: 10.2302/kjm.42.1. [DOI] [PubMed] [Google Scholar]
- 13.Downey RS, Monafo WW, Karl IE, Matthews DE, Bier DM. Protein dynamics in skeletal muscle after trauma: local and systemic effects. Surgery. 1986;99:265–273. [PubMed] [Google Scholar]
- 14.Fang CH, James JH, Ogle C, Fischer JE, Hasselgren PO. Influence of burn injury on protein metabolism in different types of skeletal muscle tissue and the role of glucocorticoids. J Am Coll Surg. 1995;180:33–42. [PubMed] [Google Scholar]
- 15.Costelli P, Carbo N, Tessitore L, Bagby GJ, Lopez-Soriano FJ, Argilés JM, Baccino FM. Tumor necrosis factor-a mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993;92:2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG, Investigators of the inflammation and the host response glue grant Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, III, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugan AL, Malarkey WB, Schwemberger S, Jauch EC, Ogle CK, Horseman ND. Serum levels of prolactin, growth hormone, and cortisol in burn patients: correlations with severity of burn, serum cytokine levels, and fatality. J Burn Care Rehabil. 2004;25:306–313. doi: 10.1097/01.bcr.0000124785.32516.cb. [DOI] [PubMed] [Google Scholar]