Abstract
Cholestatic liver disorders are accompanied by the hepatic accumulation of cytotoxic bile acids that induce cell death. Increases in cAMP protect hepatocytes from bile acid-induced apoptosis by a cAMP-guanine exchange factor (cAMP-GEF)/phosphoinositide-3-kinase (PI3K)/Akt pathway. The aim of these studies was to identify the downstream substrate in this pathway and to determine at what level in the apoptotic cascade cytoprotection occurs. Since inhibitory phosphorylation of glycogen synthase kinase-3 (GSK) occurs downstream of PI3K/Akt and this phosphorylation has been implicated in cell survival, we conducted studies to determine whether GSK was downstream in cAMP-GEF/PI3K/Akt-mediated cytoprotection. Our results show that treatment of hepatocytes with the cAMP-GEF-specific analog, 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cAMP, results in PI3K-dependent phosphorylation of GSK. Direct chemical inhibition of GSK in rat hepatocytes or human HUH7-NTCP cells with several structurally and functionally distinct inhibitors including bromoindirubin-3′-oxime (BIO), maleimides (SB216763, SB415286), thiadiazolidine derivatives, and LiCl attenuates apoptosis induced by glycochenodeoxycholate (GCDC). In addition, genetic silencing of the GSK β isoform with small interfering RNA attenuates GCDC apoptosis in HUH7-NTCP cells. Adenoviral inhibition of the Rap1 blocks both cAMP-GEF-mediated cytoprotection against GCDC-induced apoptosis and Akt/GSK3β phosphorylation. GCDC-induced phosphorylation of the proapoptotic kinase, c-Jun NH2-terminal kinase (JNK) is inhibited by GSK inhibition or cAMP-GEF activation. GCDC-induced apoptosis is accompanied by phosphorylation of the endoplasmic reticulum stress markers pIEF2α and IRE-1, and pretreatment with the cAMP-GEF analog or GSK inhibitors prevents this phosphorylation. Collectively, our results support the presence of a cAMP/cAMP-GEF/Rap1/PI3K/Akt/GSKβ survival pathway in hepatocytes that inhibits bile acid-induced JNK phosphorylation.
Keywords: EPAC, phosphoinositide-3-kinase, Rap, cholestasis, endoplasmic reticulum stress
cholestasis, which is defined by diminished bile flow, accompanies many chronic human hepatopathies (89). The reduction in bile formation in these cholestatic disorders results in the hepatic accumulation of toxic intermediates normally excreted into bile. Among these toxic substances are bile acids. This hepatic accumulation of bile acids promotes progressive hepatic injury (51, 65). The pathophysiological implication of increased intracellular bile acid concentrations is illustrated in children with progressive familial intrahepatic cholestasis (PFIC type 2), a disorder marked by progressive hepatic injury due to a mutation in the canalicular bile salt transporter (45). This mutation leads to the accumulation of bile acids within hepatocytes. Despite the pathological importance of bile salt-induced hepatic injury, the mechanisms behind its toxic effects have not been fully characterized, thus hampering the development of therapeutic strategies to protect against their deleterious effects.
Increases in cAMP protect hepatocytes from apoptosis are due to several stimuli including bile acids, lipopolysaccharide, Fas, and TNF-α (16, 22, 25, 67, 71, 80). Despite cAMP's important role in maintaining hepatocyte health, the downstream signaling events involved in its prosurvival signaling have not been fully characterized. cAMP transduces intracellular signals through three pathways: 1) the serine/threonine protein kinase A (PKA), 2) cAMP-gated channels in the heart and brain, and 3) the cAMP binding guanine nucleotide exchange factors [cAMP-GEFs, also known as exchange proteins regulated by cAMP (EPACs)] (24, 33). We and others have shown that the cAMP cytoprotection in hepatocytes is, in part, transduced through cAMP-GEF activation (16, 22, 67, 80). cAMP-GEFs function as GEFs for the small GTPase, Rap (11, 24, 33, 37). cAMP/cAMP-GEF/Rap signaling pathways exist in hepatocytes, and we have shown that cAMP-GEF activation protects hepatocytes against bile acid-induced apoptosis through activation of phosphoinositide-3-kinase (PI3K)/Akt (16, 22). The PI3K/Akt signaling is a well-known cellular survival signaling pathway in many cell types including hepatocytes and cholangiocytes (13, 16, 18, 22, 23, 67, 75, 77, 96). The downstream effector of the cAMP-GEF/PI3K/Akt cytoprotective pathway in hepatocytes, however, has not been determined.
Glycogen synthase kinase-3 (GSK) was initially discovered decades ago as a kinase that reduces glycogen synthase activity. However, it is now known that GSK is a multifunctional kinase, playing a role not only in glycogen metabolism but also in cell proliferation, growth, and death (20, 38, 41, 70). GSK is constitutively active in resting cells, and phosphorylation results in inactivation. GSK is present as two isoforms, α and β. Phosphorylation and inhibition of the GSK3β has been implicated in protection from cell death in cardiac myocytes, neurons, and renal epithelial cells (1, 39, 48, 58, 90). Most recently, inhibition of GSK3β activity has been shown to be cytoprotective from acetaminophen, ischemia-reperfusion, endotoxin, and free fatty acid injury in hepatocytes (17, 35, 78, 87). Several upstream kinases can phosphorylate GSK3β, including PKA and PI3K/Akt. Thus it is logical to speculate that cAMP cytoprotection in bile acid-induced injury might depend on phosphorylation and inactivation of GSK3β.
The goal of these studies was to determine whether GSK3β inhibition might be the downstream mechanism involved in a cAMP/cAMP-GEF/PI3K/Akt cytoprotective pathway in hepatocytes. Our results indicate that cAMP-GEF activation results in PI3K-dependent phosphorylation of GSK3β and that inhibitors of GSK protect hepatocytes from bile acid-induced apoptosis. Protection is accompanied by inhibition of both bile acid-induced phosphorylation of the proapoptotic kinase c-Jun NH2-terminal kinase (JNK) and bile acid-mediated endoplasmic reticulum (ER) stress.
MATERIALS AND METHODS
Reagents.
Collagenase, Hoechst 33258, glycochenodeoxycholate (GCDC), wortmannin, LY294002, 8-(4-chlorophenylthio)-cAMP (CPT-cAMP), tunicamycin, and all tissue culture reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO). The GSK inhibitors lithium chloride (LiCl), 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione (SB415286), and 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763) were from Sigma Aldrich Chemical. Additional GSK inhibitors 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD), (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO), its negative control compound 1-methyl-BIO (ME-BIO), the Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), and its inactive control 4-amino-7-phenylpyrazol [3,4-d]pyrimidine (PP3), the JNK inhibitor 1,3-benzothiazol-2-yl-(2-((2-(3 pyridinyl)ethyl)amino)-4-pyrimidinyl)acetonitrile (AS601645), and antibodies to actin were from EMD Bioscience (San Diego, CA). The cAMP-GEF-specific cAMP analog 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (CPT-2-Me-cAMP), the specific PKA inhibitor Rp-8-(4-chlorophenylthio)-cAMP (Rp-CPT-cAMP), and Fas ligand were from Axxora (San Diego, CA). The PI3Kγ-specific inhibitors, AS604850, was from Calbiochem (San Diego, CA) [3H]taurocholate was from Perkin Elmer (Boston, MA).
Phosphospecific antibodies to Aktser473, GSK3βser9, JNKthr183,tyr185, and pIEF2αser51 and antibodies to cleaved caspase 3 and total Akt, GSK3β, and BAX were obtained from Cell Signaling Technology (Beverly, MA). Small interfering RNA (siRNA) targeted to human GSK3β, random oligonucleotide siRNA controls and antibodies to GADPH, Mcl-1 and cytochrome c were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to inositol-requiring enzyme (IRE)-1ser724 and β-catenin were from Abcam (Cambridge, MA). The adenoviral control AD5LacZ and dominant-negative AD5delta-FADD constructs were a generous gift of Dr. David Brennar (University of California San Diego Health Sciences) and have been previously described (10). The adenoviral control green fluorescent protein (GFP) adenovirus and GFP-tagged Rap1GAP-GFP were provided by Erika Wittchin (University of North Carolina at Chapel Hill) (95).
Cell cultures.
Primary rat hepatocytes were isolated from male Wistar rats (200–250 g) as previously described (16, 22). Hepatocytes were plated at 5 × 105 cells/cm2 on tissue culture dishes or coverslips coated with type I rat tail collagen in minimal Eagle's medium (MEM) with l-glutamine, 100 nM insulin, and 10% heat-inactivated fetal calf serum and incubated at 37°C in a humidified atmosphere of 5% CO2 for 1 h. Medium was changed to MEM without supplements and, after an additional 3 h, apoptosis was initiated by the addition of 50 μM GCDC, 50 ng/ml Fas ligand or 2 μg/ml tunicamycin. Unless otherwise noted, modulators were added at the indicated concentration 30 min prior to the addition of the apoptotic stimulus. All animals received humane care according to the criteria outlined in the “Guide for the Care and use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health, and all animal study protocols were submitted to and approved by an institutional animal use and care committee.
HUH7-NTCP cells, a human hepatoma cell line, that stably overexpresses the human bile salt transporter were a generous gift of Dr. Greg Gores (Mayo Clinic, Minneapolis, MN) (30). The cells were cultured in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 100,000 U/l penicillin, 100 mg/l streptomycin, and 0.5 μg/ml of genistein at 37°C in a 5% CO2-95% O2 air incubator. For experiments, cells were serum starved overnight and then treated with 200 μM GCDC for 3–4 h. Modulators were added 30 min prior to GCDC.
Assessment of apoptosis.
Morphological evaluation of apoptotic cell death in rat hepatocytes was conducted 2 or 4 h after the addition of GCDC or tunicamycin or Fas ligand and 4 h after the addition of GCDC to HUH7-Ntcp cells as previously described (16, 22). Briefly, coverslips were stained with Hoechst 33258 and apoptosis was evaluated with fluorescent microscopy. Apoptotic cells were identified as those whose nucleus exhibited brightly staining condensed chromatin or nuclear fragmentation. Five hundred cells were counted by an observer blinded to the treatment conditions and the number of apoptotic cells was expressed as a % of the total number of cells counted. The presence of the p17-kDa cleavage product of caspase 3 was used as a biochemical indicator of hepatocyte apoptosis. Cell lysates were prepared from hepatocytes treated with GCDC (2 h) or Fas (4 h) in cell lysis buffer, 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mm EGTA, 1% Triton, 2 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM glycerolphosphate, 1 mM phenylmethylsulfonyl fluoride, 100 nM okadaic acid, 1 mM sodium orthovanadate, and 10 μg/ml of leupeptin, aprotinin, and pepstatin, separated on SDS-PAGE, and the proteins were transferred to polyvinylidene difluoride (PVDF) membranes. Immunoblotting was performed with a caspase 3 antibody and equal protein loading was verified by stripping and probing with an actin antibody.
Determination of kinase phosphorylation.
Rat hepatocytes or HUH7-Ntcp cells were treated with indicated bile acids for 60 min in with and without prior treatment with 20 μM CPT-2-Me-cAMP or with the indicated concentrations of GSK or PI3Kγ inhibitors for 30 min. Cells were lysed in ice-cold cell lysis buffer and equal amounts of protein were separated in SDS-PAGE, transferred to PVDF membranes and probed with antibodies AktSer473, JNKThr183/Tyr185, GSK3βser9, pIEF2αser51, or IRE-1ser724. Membranes were developed with chemiluminescence after incubation with a peroxidase-conjugated second antibody. Stripped membranes were reprobed with antibodies for the unphosphorylated form of the kinase or with actin or GADPH to verify equal protein loading. Blots were digitalized and subjected to densitometric analysis. Transfections.
HUH7-NTCP cells at 40–50% confluency were transfected with 50 nmol/l of a control siRNA or siRNA targeted to GSK3β by using Lipofectamine (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Cells were used for experiments 48 h later. Downregulation of GSK3β was verified by immunoblot analysis. For adenoviral studies primary hepatocytes were transfected in serum-free media for 2 h with gentle rocking at a multiplicity of infection of 10. The cells were cultured overnight in MEM, exposed to 100 μM GCDC or 50 ng/ml Fas ligand, and analyzed morphologically and biochemically for the presence of apoptosis as described above.
Preparation of mitochondrial and cytosolic fractions.
Cytosolic and mitochondrial fractions were prepared from rat hepatocyte cultures treated for the indicated times with GCDC, as previously described (93), with slight modifications. Briefly, cells were scraped in mannitol-sucrose buffer [200 mM mannitol, 70 mM sucrose, 1 mM EGTA, and 10 mM HEPES (pH=7.5) supplemented with 200 mM dithiothreitol, 0.1 mM PMSF, and 10 μg/ml leupeptin, pepstatin, and aprotinin] and then Dounce homogenized. The homogenate was spun at 400 g to remove nuclear debris. The supernatant was spun again at 10,000 g, and the pellet, which represented the mitochondrial fraction, was resuspended in mannitol-sucrose buffer containing 1% Nonidet P-40. The supernatant from the mitochondrial spin was centrifuged at 100,000 g, and the supernatant from this high-speed spin was saved as the cytosolic fraction.
Mitochondrial and cytosolic fractions were also prepared by a selective digitonin permeabilization as originally described by Leist et al. (46) and modified by Higuchi et al. for hepatocytes (32). This protocol was used to avoid possible artifacts due to potential mechanical breakage of the outer mitochondrial membrane of apoptotic cells by Dounce homogenization and to facilitate experiments in which multiple treatments were required. Briefly, at the desired time points, the culture medium was replaced with permeabilization buffer (210 mm d-mannitol, 70 mm sucrose, 10 mm HEPES, 5 mm succinate, 0.2 mm EGTA, 0.15% bovine serum albumin, 80 μg/ml digitonin, pH 7.2). The cells were incubated in this buffer for 5 min on ice and then centrifuged for 10 min at 13,000 g. The supernatant was saved as the cytosolic extract. Pellets were washed three times with ice-cold PBS, centrifuged, and solubilized in digitonin buffer with 1% Triton X-100 and used as the mitochondrial fraction. Purity of the fractions was determined by immunoblotting the cytosolic and mitochondrial preparations with the mitochondrial protein anti-cytochrome c oxidase (ACCO; Molecular Probes, Eugene, OR) or the cytosolic protein GADPH (Santa Cruz Biotechnology), respectively. The mitochondrial release of cytochrome c in cytosolic fractions and the movement of Bcl-2 like proteins to the mitochondria was monitored by immunoblotting. Cytosolic and mitochondrial proteins (30 μg) were separated on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies to cytochrome c, GADPH, ACCO, BAX, or Mcl-2.
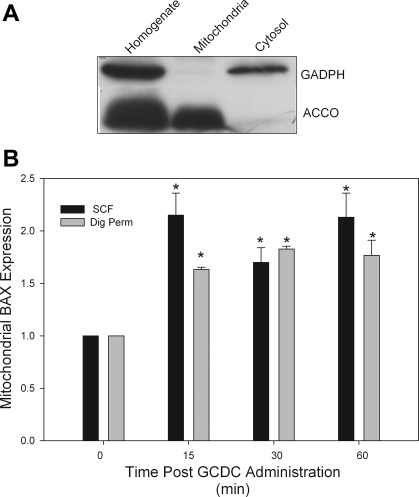
To validate this technique, we compared the preparation of mitochondrial membranes using this method to standard subcellular fractionization techniques. Since we have previously demonstrated that treatment of hepatocytes with GCDC results in translocation of BAX to the mitochondria using the subcellular fractionization method, we did side-by-side experiments comparing the amount of BAX in mitochondrial fractions prepared by both methods at different points after treatment of rat hepatocytes with GCDC. The results show that both techniques give almost identical results (Fig. 1). To further verify the integrity of the digitonin technique we immunoblotted mitochondrial and cytosolic fractions with GAPDH and cytochrome c oxidase. These studies showed that the mitochondrial fractions prepared by digitonin permeabilization were clear of the cytosolic marker GADPH and enriched in the mitochondrial marker ACCO (Fig. 1).
Fig. 1.
Mitochondrial fractions prepared from subcellular fractionization and digitonin permeabilization are equivalent. A: rat hepatocytes were solubilized with digitonin as indicated in materials and methods to isolate a digitonin-soluble fraction (called cytosol) and a digitonin-resistant fraction that was subsequently solubilized in Triton X-100 (mitochondria). Equal aliquots of each preparation were immunoblotted with the cytosolic marker protein GADPH and the mitochondrial marker cytochrome c oxidase (ACCO). B: rat hepatocytes were treated with glycochenodeoxycholate (GCDC) for the indicated times and then a mitochondrial fraction was prepared by either subcellular fractionization (SCF) or selective digitonin permeabilization (Dig Perm). Then the amount of the proapoptotic protein, BAX, in each mitochondrial fraction was determined by immunoblotting. *Amount is significantly different than that in control untreated cultures.
Bile acid uptake.
The 30-min accumulation of radiolabeled bile acid, [3H]taurocholate (Perkin Elmer, Boston, MA), in hepatocyte cultures was determined as previously described (16).
Statistical evaluation.
All results are expressed as means ± SD of at least three separate experiments. Results were analyzed for statistical significance with Student's t-test or one-way ANOVA. A P value of < 0.05 was considered significant.
RESULTS
PKA- and PI3K-dependent phosphorylation of GSK3β by cAMP.
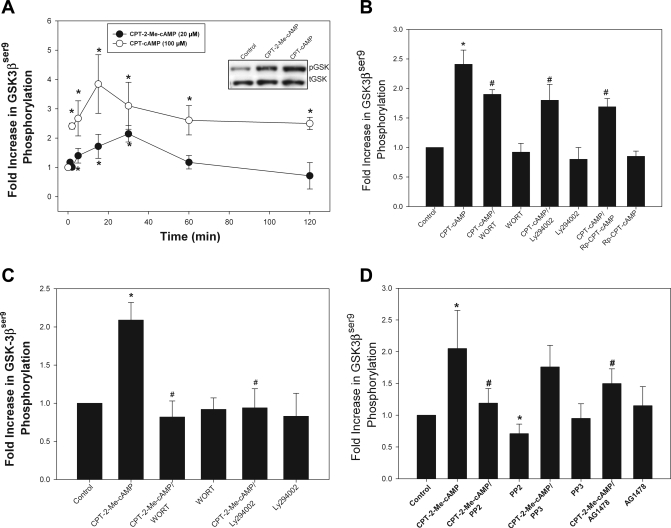
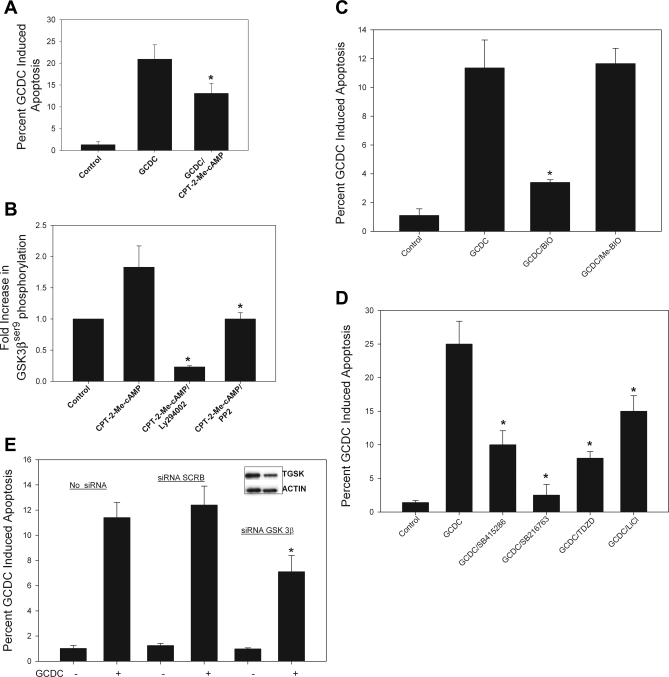
GSK3β can be phosphorylated and thus inactivated by a number of kinases including PKA and PI3K/Akt (20, 41, 70). Since cAMP can activate both PKA and PI3K/Akt in hepatocytes (16), we hypothesized that cAMP might phosphorylate GSK3β via this pathway. We have previously demonstrated that the CPT-cAMP analog activates both PKA and cAMP-GEFs, whereas CPT-2-Me-cAMP is specific for cAMP-GEF activation (16). Thus we studied the effect of both CPT-cAMP and CPT-2-Me-cAMP to determine the effects mediated via PKA and cAMP-GEF. Time course studies showed that CPT-cAMP caused a rapid (within 2 min) and sustained phosphorylation (60 min) of GSK3β whereas CPT-2-Me-cAMP caused a more gradual and transient rise in GSK3β phosphorylation (Fig. 2A). To determine the kinase responsible for cAMP-mediated GSK3β phosphorylation, we pretreated cultures with the PI3K/Akt inhibitors Ly294002 or wortmannin or the PKA inhibitor Rp-cAMP for 30 min prior to addition of the cAMP analogs. The PI3K/Akt and PKA inhibitors each decreased CPT-cAMP-induced GSK3β phosphorylation by 40 and 50%, respectively (Fig. 2B), indicating that the effect of CPT-cAMP is mediated via both PI3K/Akt and PKA. On the other hand, PI3K/Akt inhibitors completely blocked GSK3β phosphorylation in response to CPT-2-Me-cAMP (Fig. 2C) whereas the PKA inhibitor had no effect (data not shown). Thus cAMP-GEF-mediated phosphorylation GSK3β is mediated entirely through PI3K/Akt.
Fig. 2.
Phosphorylation of glycogen synthase kinase (GSK)-3 (GSK3β) by cAMP analogs in rat hepatocytes. A: whole cell lysates were prepared from rat hepatocytes treated with 8-(4-chlorophenylthio)-cAMP (CPT-cAMP; 100 μM) or 4-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (CPT-2-Me-cAMP; 20 μM) for the indicated time period, and the amount of GSK3βser9 phosphorylation was determined by immunoblotting with phospho-specific antibodies. Equal protein loading was verified by reprobing for total GSK3β expression. Results are expressed as the fold increase in the amount of GSK3βser9 phosphorylation compared with untreated controls. Inset: representative blot of the amount of phosphorylated (pGSK) and total GSK3β (tGSK) in hepatocytes cultures treated with the indicated concentration of the analogs for 30 min. *Significantly different than the amount in untreated control cultures. B: downstream mediators of cAMP-induced phosphorylation of GSK3βser9 were determined by immunoblotting of cell lysates prepared from cultures treated with CPT-cAMP for 15 min in the presence and absence of phosphoinositide-3-kinase (PI3K) inhibitors wortmannin (WOT; 50 nM) or Ly294002 (20 μM) or 500 nM of the protein kinase A inhibitor Rp-8-(4-chlorophenylthio)-cAMP (Rp-CPT-cAMP). *Significantly different than control. #Significantly different from CPT-cAMP-treated hepatocytes. The downstream mediators of cAMP-GEF-induced phosphorylation GSK3βser9 were determined by immunoblotting of cell lysates from cultures treated with CPT-2-Me-cAMP for 15 min in the presence and absence of PI3K inhibitors wortmannin (50 nM) or Ly294002 (20 μM) (C) or epidermal growth factor inhibitor AG1478 (5 μM) or 10 μM of the Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) or its inactive control compound 4-amino-7-phenylpyrazol [3,4-d]pyrimidine (PP3) (D). *Significantly different than control. #Significantly different from CPT-2-Me-cAMP-treated hepatocytes.
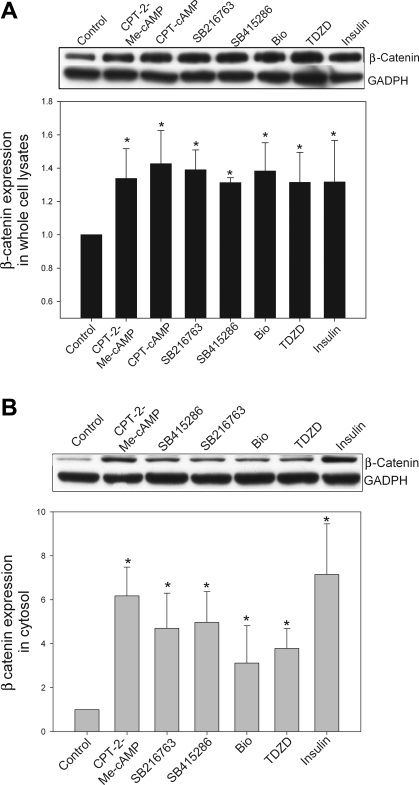
To verify that cAMP-GEF activation and use of the GSK inhibitors were associated with inhibition of GSK, we examined the effect of these treatments on the downstream GSK substrate β-catenin. In the basal state when GSK is constitutively active and unphosphorylated, β-catenin is phosphorylated (61). Phosphorylated β-catenin is targeted for degradation by the proteosome and the β-catenin that remains in the cell is primarily localized in the membrane/cytoskeleton. When GSK3β is inactivated β-catenin is dephosphorylated and protected from degradation. Thus protein expression increases and β-catenin moves into the cytoplasm and nucleus. Therefore treatment with cAMP-GEF, GSK inhibitors, or insulin, all of which inactivate GSK, should lead to an increase in cytosolic β-catenin. When we treated rat hepatocytes with the cAMP analogs or GSK inhibitors we observed a modest (∼30%) but significant increase in the expression of β-catenin in whole cell lysates (Fig. 3). However, there was a substantial increase (3- to 6-fold) in the amount of cytosolic β-catenin with all treatments (Fig. 3).
Fig. 3.
cAMP-GEF activation and GSK inhibition increase β-catenin levels in hepatocytes. Rat hepatocyte cultures were treated for 2 h with CPT-2-Me-cAMP (20 μM), SB415286 (25 μM), SB216763 (10 μM), bromoindirubin-3′-oxime (BIO; 10 μM), 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD; 40 μM), or insulin (50 nM). Whole cell lysates and cytosol extracts were prepared. Aliquots of each preparation were immunoblotted with antibodies to β-catenin, and equal protein loading was verified with immunoblotting for GADPH. Representative blots are shown in A and B along with graphical depiction of the results of 3 separate experiments. *Significantly different from control cells treated with only vehicle.
In a previous study we showed that the activation of PI3K/Akt pathway by cAMP-GEF is dependent on Src and epidermal growth factor receptor (EGFR) activity (22). To determine whether cAMP-GEF/Src/EGFR/PI3K/Akt pathway is involved in GSK3β phosphorylation, we tested the effect of the EGFR inhibitor AG1478 and the Src inhibitor PP2 and its inactive analog PP3 on cAMP-GEF-stimulated GSK3β phosphorylation. Both PP2 and AG1478 inhibited CPT-2-Me-cAMP-mediated phosphorylation of GSK3β (Fig. 2D). These results suggest that GSK3β phosphorylation involves a cAMP-GEF/Src/EGFR/PI3K/Akt/GSK3β pathway and raises the possibility that this pathway may be involved in the cytoprotective effect of cAMP against GCDC-induced apoptosis.
We have previously demonstrated that the compounds we used to inhibit kinase activity upstream of GSK3β, namely wortmannin, RP-cAMP, LY294002, PP2, and AG1478, do indeed inhibit their target kinases in rat hepatocytes (16, 22, 34, 93).
cAMP-GEF-mediated PI3K/Akt/GSK3β phosphorylation and hepatocyte cytoprotection are Rap1 dependent.
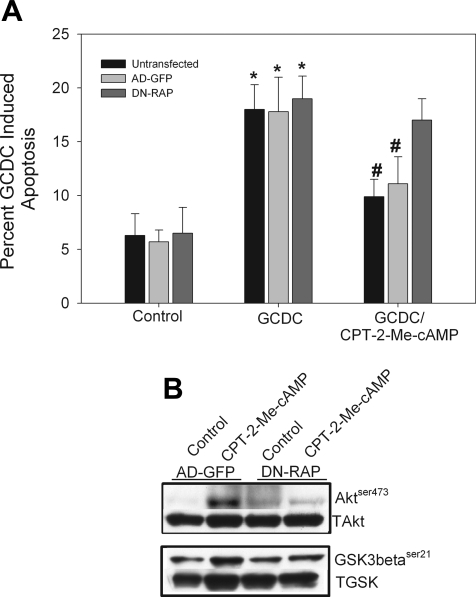
We had previously shown that cAMP-GEF activation in hepatocytes leads to activation of both Rap1 and Rap2 (16, 22). Although Rap1 and Rap2 share structural homology, they are not functionally redundant (11, 33, 37). To determine which isoform is necessary for cAMP-GEF-mediated PI3K/Akt phosphorylation and hepatocyte cytoprotection, we used an adenoviral Rap1GAP construct that prevents GTP loading of Rap1 to inhibit Rap1 activity (93). Transfection with Rap1GAP attenuated both the cytoprotective effect of CPT-2-Me-cAMP in bile acid-induced apoptosis (Fig. 4A) and the ability of the analog to phosphorylate Akt and GSK3β (Fig. 4B). These results suggest that Rap1 activation is necessary for cAMP-GEF PI3K/Akt/GSK3β-mediated cytoprotection from bile acid-induced apoptosis.
Fig. 4.
cAMP-GEF-mediated cytoprotection and activation of PI3K/Akt is Rap1 dependent. Rat hepatocytes were transfected with a green fluorescent protein (GFP) adenovirus (AD-GFP) or a Rap1GAP-GFP adenovirus (DN-RAP) for 24 h and then treated with GCDC (100 μM) for 2 h in the presence and absence of 20 μM CPT-2-Me-cAMP. Apoptosis was monitored morphologically in Hoechst-stained cells (A). Rat hepatocytes were transfected with a control GFP adenovirus or a Rap1GAP-GFP adenovirus for 24 h then treated for 15 min with CPT-2-Me-cAMP and the amount of phosphorylated Aktser473 and GSK3βser9 determined and normalized to total Akt (TAkt) or GSK (TGSK). by immunoblotting. B: representative immunoblots from 3 separate experiments are shown.
GSK inhibition protects against GCDC-induced apoptosis.
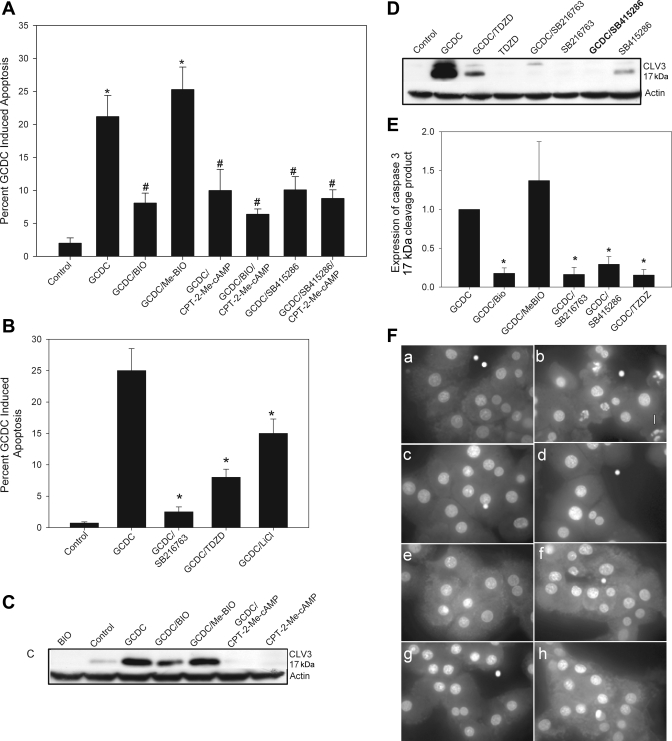
The next series of studies were conducted to determine the role of GSK in GCDC-induced apoptosis. If cAMP-GEF/Src/EGFR/PI3K/Akt/GSK3β pathway is involved in the cytoprotective effect of cAMP, inhibition of GSK3β should reverse GCDC-induced apoptosis. This hypothesis was tested by determining the effect of chemical inhibitors of GSK on GCDC-induced apoptosis. To ensure the specificity of our observations, we utilized several structurally distinct GSK inhibitors with different modes of action. BIO and the SB compounds (SB216763 and SB415286) are indirubin-3′-oxime and maleimide derivatives, respectively, that inhibit GSK in an ATP-competitive manner (5, 14, 15, 53, 81). ME-BIO is a methylated inactive analog of BIO. LiCl is the classic, although somewhat nonspecific, GSK inhibitor, which works in an ATP-noncompetitive manner. The thiadiazolidinone TDZD is also a non-ATP-competitive inhibitor that exhibits specificity for the GSK3β isoform (66). Its inhibitory activity is due to covalent modification of a cysteine residue in GSK3β ATP binding site.
Pretreatment with either BIO or SB415286 attenuated morphological (Hoechst staining) and biochemical (cleavage of caspase 3) evidence of GCDC-induced apoptosis (Fig. 5, A, C, E, and F). There was no additional protective effect when the cAMP-GEF analog, CPT-2-Me-cAMP, was combined with either of these GSK inhibitors (Fig. 5, A and C), indicating a common pathway. The GSK inhibitors (BIO, ME-BIO, or SB415286) alone had no effect on baseline apoptosis (data not shown). The other GSK inhibitors SB216763, TDZD, and LiCl (Fig. 5, B, D–F) also attenuated GCDC-induced apoptosis. Thus it appears that active GSK3β is necessary for GCDC-induced apoptosis and that inactivation of GSK3β is a putative downstream effector in the cAMP-GEF/PI3K/Akt cytoprotective pathway.
Fig. 5.
Inhibition of GSK attenuates bile acid-induced apoptosis in rat hepatocytes. Hepatocyte cultures were treated with GCDC (50 μM) for 2 h in the presence or absence of the GSK inhibitors SB415286 (25 μM), 10 μM of BIO, or its inactive analog ME-BIO (A) or with TDZD (40 μM) or SB216763 (10 μM) (B). In some experiments 20 μM of CPT-2-Me-cAMP was also added. The amount of apoptosis was determined by morphological examination of Hoechst stained cells (A and B) or biochemical analysis for the presence of the 17-kDa cleavage fragment of caspase (CLV3) (C–E). Equal protein loading was verified by reprobing CLV3 blots with actin. Representative blots are shown in C and D with the quantification of 3 separate experiments in E. The composite photomicrograph of Hoechst-stained cells (F) shows control hepatocytes (a) and hepatocytes treated with GCDC (50 μM) alone (b) or after 30-min pretreatment with CPT-2-Me-cAMP (20 μM, c), SB216763 (5 μM, d), SB415286 (5 μM, e), BIO (5 μM, f), TDZD (40 μM, g), or the JNK inhibitor AS601645 (5 μM, h). Magnification ×100. *Significantly different than untreated control cultures. #Significantly different from GCDC-treated cultures.
To determine whether the effect of GSK inhibition extended to human liver cells, we investigated the effect of GSK inhibition on GCDC-induced apoptosis in the HUH7-NTCP cell line. We and others have previously used the HUH7-Ntcp cell line, a model system that is amenable to genetic manipulation, to study GCDC-induced apoptosis (34, 74, 76). First, we verified that the cAMP-GEF analog, CPT-2-Me-cAMP, was cytoprotective in HUH-NTCP cells and that it induced PI3K and Src kinase-dependent phosphorylation of GSK3β (Fig. 6, A and B). Thus HUH-NTCP cell line was considered to be an appropriate model. Next we showed that GSK inhibitors were cytoprotective. BIO attenuated GCDC-induced apoptosis whereas the inactive analog, ME-BIO, had no effect (Fig. 6C). Similar inhibition of GCDC apoptosis was obtained by pretreatment with SB415286, SB 216786, TDZD, and LiCl (Fig. 6D). None of the GSK inhibitors themselves had any effect on the baseline level of apoptosis in HUH7-NTCP cells (data not shown). The above studies with GSK inhibitors suggest that inhibition of GSK leads to cytoprotection against GCDC-induced apoptosis. However, these results do not necessarily suggest that the effect is mediated via GSK3β isoform. Although BIO, SB216763, SB415286, and LiCl inhibit both the α and β isoforms of GSK, TDZD is more specific for the β isoform. Thus our results showing that TDZD inhibited GCDC apoptosis in rat hepatocytes and HUH7-NTCP cells suggest that it is the phosphorylation (inhibition) of GSK3β that may be involved in the cytoprotective effect. To test this hypothesis more directly, we determined the effect of GSK3β knockdown on GCDC-induced apoptosis. This was done only in the HUH7-NTCP cells because siRNA knockdown of GSK3β requires at least 48 h. In this time frame, primary hepatocytes in culture lose the expression of bile salt transporter and thus are resistant to bile acid-induced apoptosis. Transfection with siRNA to GSK3β resulted in a decrease in the level of GSK3β by ∼60% by 48 h (Fig. 6E, inset). Apoptosis in response to GCDC in the GSK3β siRNA- but not scrambled siRNA-transfected cells was significantly attenuated (Fig. 6E). Collectively these results suggest that inhibition of GSK3β is necessary for cytoprotection against GCDC-induced apoptosis. Since cAMP-GEFs inhibits GSK3β, it is highly likely that the cytoprotective effect of cAMP-GEFs against GCDC-induced apoptosis is mediated via inhibition of GSK3β.
Fig. 6.
Activation of cAMP-GEF and inhibition of GSK protect against bile acid-induced apoptosis in human hepatoma cells. A: HUH7-NTCP cells were pretreated with CPT-2-Me-cAMP for 30 min and then exposed to GCDC (200 μM) for 3 h. Apoptosis was determined by morphological evaluation of Hoechst-stained cells. B: HUH7-NTCP cells were treated with CPT-2-Me-cAMP for 30 min in the presence or absence of Ly294002 (20 μM) or PP2 (10 μM) and the amount of GSK3βser9 phosphorylation was determined in cell lysates. HUH7-NTCP cells were pretreated with the GSK inhibitor BIO (10 μM) or it inactive control analog ME-BIO (10 μM) (C) or with SB415286 (25 μM), SB216746 (10 μM), or TDZD (25 μM) (D) and then exposed to GCDC (200 μM) for 3 h. Apoptosis was determined by morphological evaluation of Hoechst-stained cells. E: HUH7-NTCP cells were transfected with small interfering RNA (siRNA) to GSK3β or a scrambled siRNA (siRNA SCRB) for 48 h and then exposed to GCDC for 4 h, and the percent apoptosis was determined morphologically. *Significantly different than GCDC (A, C–E)- or CPT-2-Me-cAMP (B)-treated cells.
As we have previously shown that cAMP-GEF activation protects against death receptor-mediated apoptosis stimulated by ligation of the Fas receptor (22), if we hypothesize that the protective effect of cAMP-GEF is mediated by GSK phosphorylation, then direct chemical inhibition of GSK should also attenuate Fas ligand-mediated apoptosis. To test this hypothesis we pretreated rat hepatocytes with BIO, Me BIO, SB216763, or TDZD prior to exposure to Fas ligand. Fas ligand-induced apoptosis in hepatocytes (20 ± 2.4%) and BIO (13 ± 3.4%), SB216763 (12.5 ± 1.8%), and TDZD (14 ± 2.9%). significantly attenuated this apoptosis (P < 0.05), supporting our observation that the cAMP-GEF survival effect may involve GSK3β phosphorylation.
GSK inhibition attenuates GCDC-induced JNK activation.
The mechanism by which inhibition of GSK3β leads to cytoprotection against GCDC is not known. Recent studies demonstrated that the protective effect of GSK3β inhibition in hepatocytes was associated with attenuation of acetaminophen and fatty acid-induced JNK phosphorylation (35, 78). Several studies have shown that bile acids induce sustained phosphorylation of JNK in hepatocytes and that chemical or genetic inhibition of this activation attenuates bile acid-induced apoptosis (25, 26, 34, 56, 69, 72). This observation coupled with our data from previous studies showing that cAMP-GEF activation can attenuate lipopolysaccharide-induced JNK phosphorylation in hepatocytes (67) led us to hypothesize that cAMP-GEF-induced cytoprotection against GCDC-induced apoptosis may be due to GSK3β inhibition of GCDC-induced JNK activation. The following studies were conducted to test this hypothesis.
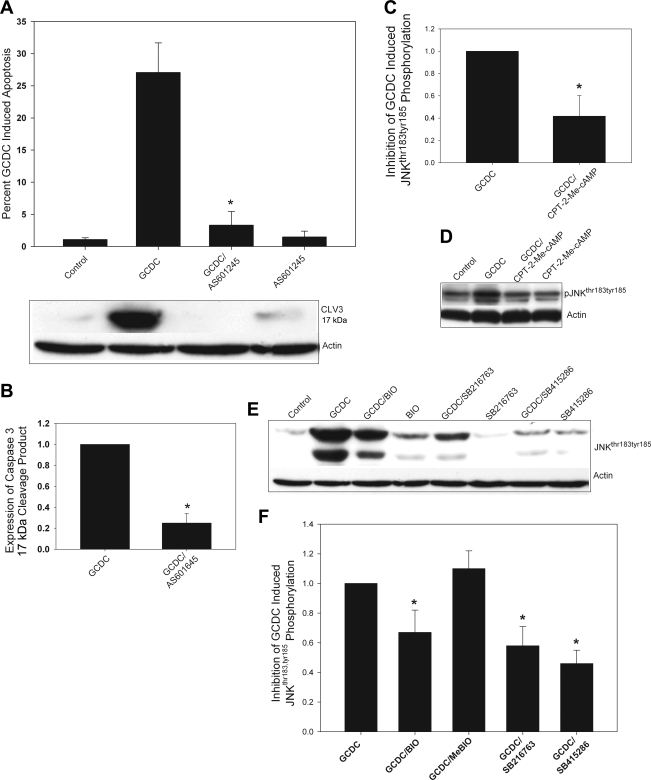
We have recently demonstrated that chemical inhibition of JNK with SP600125 prevents GCDC-induced apoptosis (34). However, this inhibitor is somewhat nonspecific for JNK (3) and can interfere with bile acid uptake into hepatocytes (data not shown). Thus to confirm the role of JNK we tested the effect of a more specific JNK inhibitor, AS601245, on bile acid uptake and GCDC-induced apoptosis in hepatocytes (12). Results show that AS601245 prevents morphological and biochemical evidence of apoptosis in primary rat hepatocytes after exposure to GCDC (Fig. 7, A and B) and has no effect on bile acid uptake (data not shown), confirming GCDC-induced apoptosis requires JNK activation.
Fig. 7.
GSK inhibition prevents bile acid-induced phosphorylation of JNK. A and B: rat hepatocytes were treated with GCDC (50 μM) for 1 h in the presence or absence the JNK inhibitor. AS601245 (10 μM) and the amount of apoptosis determined by morphological evaluation of Hoechst-stained cells or Western blotting for the active 17-kDa cleaved fragment of caspase 3 (CLV3; A). B–D: quantification of 3 separate experiments. Rat hepatocytes were treated with GCDC (50 μM) for 1 h in the presence or absence of the CPT-2-Me-cAMP (20 μM) and the amount of phosphorylated JNKthr183,tyr185 (pJNKthr183,tyr185) determined by immunoblotting. A representative blot is shown in C and the quantification of immunoblots from 4 separate experiments is shown in D. Results are expressed as the change in the amount of phosphorylated JNK compared with that seen with GCDC. Rat hepatocytes were treated with GCDC (50 μM) for 1 h in the presence and absence of the GSK inhibitors BIO (10 μM), SB216746 (10 μM), or SB415286 (25 μM) and the amount of phosphorylated JNKthr183,tyr185 was determined by immunoblotting. A representative blot is shown in E, and the quantification of immunoblots from 5 separate experiments shown in F. *Significantly different from GCDC-treated hepatocytes.
Next, we determined whether activation of cAMP-GEF affects GCDC-induced JNK phosphorylation. Activation of cAMP-GEFs by CPT-2-Me-cAMP inhibited GCDC-induced JNK phosphorylation without affecting basal level of JNK phosphorylation (Fig. 7, C and D), raising the possibility that this inhibition may be-mediated via cAMP-GEF-mediated inhibition of GSK3β. This possibility was tested by studying the effect of GSK inhibitors on GCDC-induced JNK phosphorylation. All of the GSK inhibitors tested (SB216763, SB415286, and BIO) attenuated GCDC-induced JNK activation in hepatocytes (Fig. 7, E and F), indicating that inhibition of GSK3β leads to inhibition of GCDC-induced JNK activation. Taken together, these results are consistent with our hypothesis that cytoprotection against GCDC-induced apoptosis involves cAMP-GEF-induced inhibition of GSK3β followed by inhibition of GCDC-induced JNK activation.
GSK inhibition prevents GCDC-induced mitochondrial-mediated apoptosis.
Although previous studies have suggested that bile acid-induced apoptosis is mediated via death receptor signaling (51), accumulating data support a more prominent role for the intrinsic apoptotic pathway in bile acid injury. This evidence includes the observations that 1) hepatocytes from mice lacking death receptors or Bid still undergo death in vivo after bile duct ligation or in culture after exposure to bile acids despite blockage of death receptor pathways and 2) blockage of death receptor signaling with genetic inactivation of the Fas-associated protein with death domain (FADD) has no effect on bile acid-induced apoptosis in both rat hepatocytes and human hepatoma cells (17, 31, 59, 69, 74, 76). FADD is an adaptor molecule that bridges death receptors to caspase-8 and is required for execution of apoptosis by all known death receptors.
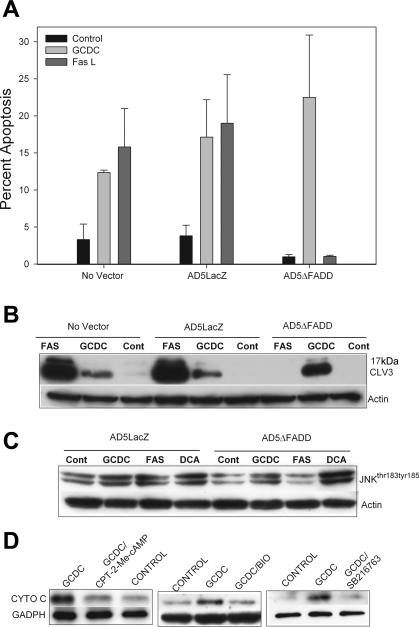
To further address this controversy and to determine how bile acid injury proceeds in our experimental model, we examined the effect of transfection with an adenoviral dominant-negative FADD construct on GCDC and Fas ligand-induced apoptosis in rat hepatocytes. Adenoviral transfection of rat hepatocytes with a dominant-negative FADD construct had no effect on GCDC-induced apoptosis but totally blocked Fas ligand-mediated apoptosis (Fig. 8, A and B). Furthermore, transfection of dominant-negative FADD had no effect on proapoptotic phosphorylation of JNK by bile acids but inhibited Fas-mediated JNK phosphorylation (Fig. 8C). This suggests that bile acid-induced apoptosis in our hepatocyte cultures is mediated primarily by a death receptor independent pathway.
Fig. 8.
Bile acid-induced apoptosis and JNK activation in rat hepatocytes is death receptor independent. Rat hepatocytes were transfected with an adenoviral control AD5LacZ and dominant-negative AD5delta-FADD construct and then treated with GCDC (50 μM), deoxycholate (DCA, 100 μM) for 2 h, or Fas ligand (Fas L; 50 ng/ml) for 4 h. Apoptosis was monitored morphologically by examination of Hoechst-stained cells (A) or biochemically by immunoblotting for CLV3 (B). Cont, control. Equal protein loading was verified by immunoblotting for actin. The amount of phosphorylated JNKthr183,tyr185 was determined by immunoblotting (C). D: rat hepatocytes were pretreated with CPT-2-Me-cAMP (20 μM), BIO (10 μM), or with SB216763 (10 μM) and then exposed to GCDC for 2 h. Cytosolic fractions were prepared by subcellular fractionization as described in materials and methods. The amount of cytochrome c (CYTO C) released into cytosol was determined by immunoblotting. *Significantly different than in the presence of GCDC.
Consistent with our demonstration of the importance of mitochondrial dysfunction in bile acid-induced apoptosis, we noted that the treatment of rat hepatocytes with GCDC was accompanied by the release of cytochrome c from the mitochondria (Fig. 8D). Pretreatment with CPT-2-Me-cAMP or the GSK inhibitors BIO or SB216763 prevented GCDC release of cytochrome c (Fig. 8D). These studies suggest that the protective effect of cAMP-GEF activation and/or GSK inhibition is associated with stabilization of mitochondrial function.
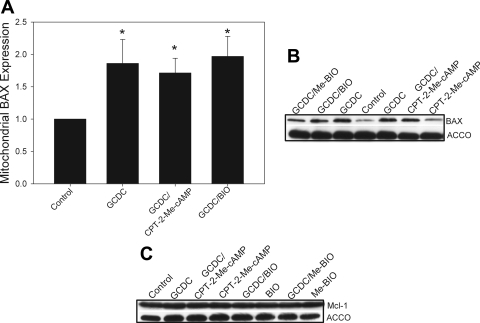
The protective effect of GSK3β inhibition on apoptosis in cardiac myocytes and neurons can be mediated by regulation of Bcl-2 proteins. In these cells GSK3β inhibition can prevent proteosomal degradation of the antiapopototic Bcl-2 protein Mcl-1 and inhibit the translocation of the proapoptotic Bcl-2 protein BAX to the mitochondrial membrane (49, 52, 64). We and others have previously shown that bile acid-induced apoptosis is accompanied by movement of BAX to the mitochondria, and a recent study has demonstrated that genetic knockdown of BAX inhibits bile acid-associated apoptosis (73, 93, 98). Thus we hypothesized that cAMP-GEF activation might protect against bile acid-induced apoptosis by modulating mitochondrial levels of BAX or Mcl-1. To test these hypotheses, we pretreated hepatocytes with CPT-2-Me-cAMP or BIO and then with GCDC for 1 h and then prepared mitochondrial fractions using the digitonin permeabilization method. Our results show that GCDC treatment translocates BAX to the mitochondrial membrane, but pretreatment with CPT-2-Me-cAMP or BIO had no effect on this translocation event (Fig. 9, A and B). However, we saw no effect on the mitochondrial levels of Mcl-1 after GCDC treatment or following pretreatment of hepatocytes with BIO or CPT-2-Me-cAMP (Fig. 9C). These results demonstrate that GSK inhibition of bile acid-induced apoptosis is not associated with changes in the mitochondrial expression of BAX or Mcl-1.
Fig. 9.
Cytoprotection by cAMP-GEF activation or GSK inhibition is independent of BAX or Mcl-1. Rat hepatocytes were pretreated with CPT-2-Me-cAMP (20 μM) or the GSK inhibitors BIO (10 μM) or it inactive analog ME-BIO (10 μM) for 30 min and then exposed to GCDC for 60 min. Mitochondrial fractions were prepared by selective digitonin permeabilization as described in materials and methods. Mitochondrial fractions were immunoblotted for BAX (A and B) or Mcl-1 (C). Representative gels are shown in B and C with quantification of the BAX expression in A. Equal protein loading was verified by immunoblotting for the mitochondrial specific protein ACCO. *Significantly different than the amount of protein in control untreated cells.
cAMP activation and GSK inhibitors blunt bile acid-induced ER stress in rat hepatocytes.
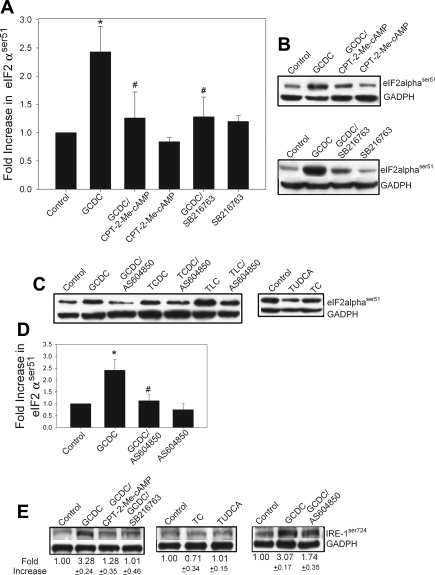
Since bile acid can initiate ER stress in hepatocytes (6, 9, 54, 83, 86) and ER stress can lead to JNK activation (50, 79), we examined the role of ER stress in bile acid apoptosis and JNK phosphorylation in rat hepatocytes. ER stress is associated with initiation of the unfolded protein response which is mediated by transmembrane protein sensors including inositol-requiring enzyme (IRE-1) and RNA-dependent protein kinase-like ER kinase. The latter phosphorylates and inactivates peIF2α. To determine whether bile acids-induced ER stress in our cultures, we looked at the phosphorylation of peIF2αser51 and IRE-1ser724 as indicators of ER stress. Treatment with the hydrophobic bile acids GCDC, taurochenodeoxycholate, or taurolithocholate, but not with the hydrophilic and nontoxic bile acids tauroursodeoxycholate or taurocholate, caused an increase in phosphorylation of both peIF2α and IRE-1 (Fig. 10, A–C, F). GCDC-induced phosphorylation of both compounds was inhibited by pretreatment with CPT-2-Me-cAMP or SB216763. Further evidence for a role of GSK in preventing ER stress apoptosis comes from our observation that both the CPT-2-Me-cAMP analog and SB216763 attenuate tunicamycin-mediated hepatocyte apoptosis by 75 and 90%, respectively (data not shown). Tunicamycin inhibits protein glycosylation and is frequently used to induce ER stress.
Fig. 10.
Bile acid-induced apoptosis and activation of JNK involves induction of the endoplasmic reticulum stress response. Rat hepatocytes were treated with hydrophobic cytotoxic bile acids GCDC (50 μM), taurochenodeoxycholate (TCDC, 100 μM), or taurolithocholate (TLC, 25 μM) or the hydrophobic cytoprotective bile acids tauroursodeoxycholate (TUDCA, 100 μM) or taurocholate (TC, 100 μM) for 1 h in the presence or absence the 20 μM CPT-2-Me-cAMP, 10 μM SB216763, or 10 μM AS604850, and the amounts of phosphorylated peIF2αser51 (A–D) and phosphorylated IRE-1ser724 (E) were determined by immunoblotting. Equal protein loading was verified by immunoblotting for actin or GADPH. Representative immunoblots are shown in B, C, and E, and the quantification of immunoblots from 3 separate experiments is presented in A and D. *Significantly different from control cells treated; #significantly different from bile acid-treated cells.
As we have previously shown that bile acid-induced activation of JNK proceeds through selective activation of PI3Kγ (34), we tested the effect of PI3Kγ inhibitor AS604850 on bile acid-induced ER stress (Fig. 10, D and E). These results show that inhibition of PI3Kγ attenuates bile acid-induced phosphorylation of both peIf2α and IRE-1.
Collectively our results suggest that bile acids activate an ER stress response in hepatocytes and that cytoprotection due to GSK or PI3Kγ inhibition is accompanied by attenuation of these phosphorylation events.
Effect of modulators on bile acid uptake.
None of the inhibitors used in these studies (10 μM SB216763, 25 μM SB415286, 10 μM BIO, 10 μM Me-BIO, 40 μM TDZD, 5 μM AG1478, or 5 μM AS610245) had any effect on the accumulation of radiolabeled taurocholate in rat hepatocytes (data not shown). We have previously documented that the cAMP analogs and the Src, EGF receptor, and PI3K inhibitors have no effect on bile acid uptake (16, 22).
DISCUSSION
Previously we have shown that cAMP protects hepatocytes from bile acid-induced apoptosis through a cAMP-GEF/PI3K/Akt pathway (16, 22). The present studies were designed to provide additional mechanistic insight into this survival pathway by delineating its downstream mediator and the site in the apoptotic pathway it intercepts. Our studies show that 1) cAMP phosphorylates GSK3β by PKA- and PI3K-dependent mechanisms and that the latter is associated with specific activation of cAMP-GEF, 2) genetic or chemical inhibition of GSK3β with known GSK inhibitors or by activation of cAMP-GEFs protects rat hepatocytes and/or human hepatoma cells from apoptosis due to cytotoxic bile acids, 3) cAMP-GEF cytoprotection and PI3K/Akt/GSK3β phosphorylation is Rap1 dependent, and 4) GSK inhibition or activation of cAMP-GEFs prevent bile acid-induced phosphorylation of the proapoptotic kinase JNK that is accompanied by concurrent attenuation of bile acid-induced phosphorylation of the ER stress markers peIF2α and IRE-1.
We have previously demonstrated the presence of a cAMP-cAMP-GEF/PI3K/Akt signaling pathway in hepatocytes (16, 22). This signaling pathway is not unique to hepatocytes; others have demonstrated its presence in cardiac and skeletal muscle, pancreatic β cells, neurons, macrophages, and mesangial cells (4, 43, 44, 55, 60, 62, 97). The present studies now establish GSK3 as a downstream substrate in this pathway in hepatocytes. We show that cAMP phosphorylates GSK3β and that this phosphorylation occurs through both cAMP/PKA- and cAMP/cAMP-GEF/PI3K-dependent pathways. The finding that cAMP phosphorylates GSK3 through PKA mechanism has been demonstrated in nonhepatic cells, but ours is the first demonstration that cAMP can also phosphorylate GSK3β in a cAMP-GEF/PI3K-dependent manner.
Our prior studies demonstrate that activation of a cAMP-GEF/PI3K/Akt signaling pathway is associated with cell survival (16, 22). Several observations in the present studies suggest that GSK3β is also a downstream effector in this survival pathway. First, we demonstrate that multiple structurally and functionally distinct chemical inhibitors of GSK3 protect rat hepatocytes and human hepatoma cells from bile acid-induced apoptosis. As GSK3 is constitutively active under resting conditions and phosphorylation on ser9 results in inactivation, specific activation of cAMP-GEFs that phosphorylate GSK would be expected to also inhibit GSK3. Since we have demonstrated that direct GSK3 inhibition is cytoprotective, this ability of cAMP-GEF activation to inhibit GSK would be expected to be cytoprotective as well. Further support for a role of GSK in cAMP-GEF cytoprotection comes from the finding that there is no additional protective effect when cAMP-GEF activation is coupled with direct chemical inhibition of GSK. Finally, we demonstrate that cAMP-GEF-mediated GSK3β phosphorylation is sensitive to Src tyrosine kinase and EGFR kinase inhibition, similar to our observations with the cAMP-GEF/PI3K/Akt cytoprotective pathway (22).
GSK3 exists in two isoforms encoded by separate genes, GSK3α and GSK3β. Although structurally related, the isoforms are not functionally equivalent, with most studies implicating the GSK3β isoform in cell survival (39, 41, 58, 70). Our data showing that protection occurs with the relatively specific GSK3β inhibitor, TDZD, in rat hepatocytes and with siRNA to GSK3β in human hepatoma cells suggest that it is the β isoform that is important for its protective action.
In nonhepatic cells the survival effect of GSK3β inactivation is mediated through several mechanisms including inactivation of proapoptotic kinase JNK, regulation of Bcl-2 proteins, and inhibition of the mitochondrial membrane permeability transition (39, 52, 58, 82). Less is known in hepatocytes about the cytoprotective action of GSK inactivation. Silencing of GSK3β has been associated with inhibition of acetaminophen and fatty acid-mediated JNK activation and cell death (35, 78). The role of JNK as a proapoptotic kinase in bile acid-induced apoptosis is well established. Several studies show that cytotoxic bile acids result in sustained phosphorylation of JNK and that chemical or genetic inhibition of JNK can attenuate bile acid-induced apoptosis (25, 26, 56, 69, 72). In these studies we now demonstrate that inhibition of GSK with chemical inhibitors or by phosphorylation by cAMP-GEF inhibits GCDC from phosphorylating JNK. Since direct inhibition of JNK protects hepatocytes from bile acid apoptosis, it is likely that GSK3β inhibition of GCDC-induced JNK phosphorylation is important in hepatocyte cell survival. A recent study demonstrating that GSK inhibition attenuates lipoapoptosis in hepatocytes verifies the importance of this signaling pathway in hepatocyte survival (35).
The mechanism through which bile acids cause induce sustained phosphorylation of JNK is not fully understood. Bile acids can induce ER stress in hepatocytes (6, 9, 54, 83, 86), and ER stress is known to result in phosphorylation of JNK (50, 79). Our demonstration that hydrophobic cytotoxic but not hydrophilic cytoprotective bile acids result in phosphorylation of peIF2α and IRE-1, two mediators of the unfolded protein response in the ER (50, 79), suggests that ER stress may be involved in bile acid toxicity. Further support for a role of ER stress comes from our observation that cytoprotection by cAMP-GEF activation or GSK inhibition blocks GCDC-induced phosphorylation of these markers. Recently we presented evidence that JNK activation by cytotoxic bile acids proceeds through the isoform-specific activation of PI3Kγ (34). Cytotoxic bile acids, but not cytoprotective bile acids, preferentially activate PI3Kγ and chemical or genetic inactivation of PI3Kγ promotes hepatocyte survival after exposure to toxic bile acids (34). As our present studies show that PI3K inhibition blocks bile acid-induced ER stress, it is tempting to speculate that the cytotoxic bile acid/PI3Kγ pathway proceeds through ER stress-mediated phosphorylation of JNK. In the pancreas, bile acid activation of PI3Kγ blocks sarco(endo)plasmic reticulum Ca2+-ATPase, causing calcium depletion in the ER, a known stimulus for ER stress (19). Experiments are underway to determine the exact mechanism whereby a bile acid/PI3Kγ may stimulate ER stress in hepatocytes.
GSK3β also modulates apoptosis by controlling mitochondrial Bcl-2 proteins. GSK inactivation prevents the phosphorylation of the antiapoptotic protein Mcl-1 and thus precludes the protein from being targeted for degradation (52). Sine we failed to see any change in mitochondrial levels of Mcl-1 after cAMP-GEF activation or GSK inhibition, modulation of this protein is an unlikely downstream effector in a cAMP-GEF/GSK3β survival pathway. In neurons, GSK inactivation can lead to phosphorylation of the proapoptotic Bcl-2 effector protein BAX, which in turn prevents BAX's translocation to the mitochondria (49). Although we and others have demonstrated that GCDC-induced apoptosis is associated with translocation of BAX to the mitochondria (73, 93), in these studies reported in this manuscript we failed to see any effect of cAMP-GEF activation or direct GSK inhibition on this event. However, since BAX activation requires not only translocation to mitochondria, but also a conformational change and integration into the mitochondrial membrane (21), our studies do not preclude a role for GSK inhibition in cAMP-GEF/GSK3β cytoprotection. Indeed in nonhepatic cells GSK inhibition has been reported to prevent BAX conformation changes and thus prevent its proapoptotic action (49). This is an area that will require additional study.
The finding that increasing cAMP results in PI3K- and PKA-dependent phosphorylation of GSK has implications beyond bile acid-induced hepatobiliary disease. GSK has a prominent role in carbohydrate and lipid metabolism (20, 29, 70). Traditionally, increases in cAMP that occur downstream of glucagon signaling in hepatocytes were felt to be anti-insulin. However, this may be an oversimplification since we show that cAMP itself can trigger PI3K/Akt-dependent activation of GSK similar to insulin. It is possible that the insulin- and cAMP-dependent PI3K/Akt/GSK pathways differentially modulate separate pools of GSK leading to divergent downstream events. This phenomenon has been demonstrated for adrenaline signaling in skeletal muscle (36). GSK is a known as a promiscuous kinase whose downstream actions are mediated in a cell- and stimulus-specific manner (7, 8, 36, 38). GSK's signaling is made more complex in that all GSK substrates must be primed by phosphorylation by another kinase for GSK recognition (20, 38, 70). Future experiments to determine the downstream events controlled by cAMP/PKA/GSK3β and cAMP-GEF/PI3K/Akt/GSK3β pathways in hepatocytes are needed.
Dysregulation of GSK is implicated in various human disease processes including cardiac myocyte ischemic injury, neurogeneration, and diabetes mellitus (2, 28, 29, 58, 88). The investigation of the role of GSK3 in hepatobiliary disorders is still in its early stages. In previous studies GSK inhibition has been implicated in ischemia-reperfusion, endotoxin, free fatty acid, and acetaminophen-mediated cell death in hepatocytes (17, 35, 78, 87). We now report its association with bile acid-induced apoptosis in hepatocytes and thus its possible role in hepatocyte cell death during cholestatic liver injury.
GSK inhibitors are currently under development for their therapeutic potential in neuronal and cardiac disease (2, 29, 68, 88). These inhibitors and/or targeting of GSK inactivation with cAMP-GEF analogs may also have therapeutic potential in hepatobiliary disease. GSK inhibition may ultimately be a better way to intercept proapoptotic JNK signaling pathway than direct targeting of JNK. Numerous studies have documented the pathological significance of sustained JNK signaling in hepatocyte cell death. Thus inhibition of bile acid-induced JNK activation should be cytoprotective. In the liver JNK is encoded by two genes, JNK1 and JNK2. Studies using genetic strategies to inactivate the JNK isoforms or in mice with knockdown of either JNK1 and/or JNK2 have shown distinct and often opposing functions of JNK1 and JNK2 in hepatocyte injury depending on the pathological stress (27, 40, 42, 47, 69, 84, 85, 91). The molecular basis for these differences are not well characterized. It does appear, however, that for clinical application JNK isoform-specific inhibitors will be necessary. The currently available JNK inhibitors (SP600125 and AS601645), however, are not isoform specific. In contrast to JNK inhibition, GSK inhibition seems to have a consistent hepatoprotective effect, and although most GSK inhibitors work on both GSKα and GSKβ, there is no literature to support the observation that they have opposing effects in cell death (1, 2, 15, 20, 30, 34, 35, 41, 48, 58, 68, 78, 87, 90). Thus inhibition of GSK3β may be a more targeted way to decrease JNK activation. Moreover, since GSK is involved in the pathogenesis of other human disease including Type II diabetes, cardiac myocyte ischemia, Alzheimers disease, and stroke, there is already a significant body of information showing the preclinical efficacy of GSK inhibitors in in vivo models (2, 17, 58, 88).
GRANTS
This research was supported National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK065975 to C.R.L. Webster and R01 DK-33436 to M.S. Anwer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aghdam SY, Barger SW. Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res 4: 21–31, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Avila J, Wandosell F, Hernández F. Role of glycogen synthase kinase-3 in Alzheimer's disease: pathogenesis and glycogen kinase-3 inhibitors. Expert Rev Neurother 10: 703–710, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baviera AM, Zanon NM, Navegantes LC, Kettelhut IC. Involvement of cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Mol Cell Endrocrinol 315: 104–112, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Beauchard A, Ferandin Y, Frère S, Lozach O, Blairvacq M, Meijer L, Thiéry V, Besson T. Synthesis of novel 5-substituted indirubins as protein kinases. Bioorg Med Chem 14: 6434–6443, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bernstein H, Payne CM, Bernstein C, Schneider J, Beard SE, Crowley CL. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol Lett 108: 37–46, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Beurel E, Jope RS. The paradoxical pro- and antiapoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79: 173–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly active in nuclei and mitochondria. Neuroreport 14: 2415–2419, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med 14: 828–836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol 18: 6353–6364, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breckler M, Berthouze M, Laurent AC, Crozatier B, Morel E, Lezoualc'h F. Rap-linked cAMP signaling Epac proteins: Compartmentation, functioning and disease implications. Cell Signal 23: 1257–1266, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Carboni S, Hiver A, Szyndralewiez C, Gaillard P, Gotteland JP, Vitte PA. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): a c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J Pharmacol Exp Ther 310: 25–32, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Cleary JM, Shapiro GI. Development of phosphoinositide-3 kinase pathway inhibitors for advanced cancer. Curr Oncol Rep 12: 87–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecular inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7: 793–803, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem 77: 94–102, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 287: G334–G343, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Dugo L, Collin M, Allen DA, Patel NS, Bauer I, Mervaala EM, Louhelainen M, Foster SJ, Yaqoob MM, Thiemermann C. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med 33: 1903–1912, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Engelman JA. Targeting PI3K signaling in cancer: opportunities, challenges, and limitations. Nat Rev Cancer 9: 550–562, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Fischer L, Gukovskaya AS, Penninger JM, Mareninova OA, Friess H, Gukovsky I, Pandol SJ. Phosphatidylinositol 3-kinase facilitates bile acid-induced Ca2+ responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 292: G875–G886, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci 64: 1930–1944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 10: 481–494, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 β/α in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G764–G774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, Marzioni M, Mancino MG, Phinizy JL, Reichenbach R, Fava G, Summers R, Venter J, Alpini G. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol 290: G813–G826, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50: 355–375, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Graf D, Reinehr R, Kurz AK, Fischer R, Häussinger D. Inhibition of taurolithocholate 3-sulfate induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A dependent and independent mechanism. Arch Biochem Biophys 415: 34–42, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Grambihler A, Higuchi H, Bronk SF, Gores GJ. cFLIP-L inhibits p38 MAPK activation: an additional anti-apoptotic mechanism in bile acid-mediated apoptosis. J Biol Chem 278: 26831–26837, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut 56: 982–990, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hernández F, de Barreda EG, Fuster-Matanzo A, Goñi-Oliver P, Lucas JJ, Avila J. The role of GSK3 in Alzheimer's disease. Brain Res Bull 80: 248–250, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Hernández F, Nido JD, Avila J, Villanueva N. GSK3 inhibitors and disease. Mini Rev Med Chem 9: 1024–1029, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El-Deiry W, Gores GJ. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem 276: 38610–36808, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Higuchi H, Miyoshi H, Bronk SF, Zhang H, Dean N, Gores GJ. Bid antisense attenuates bile acid induced apoptosis and cholestatic liver injury. J Pharmacol Exp Ther 299: 866–873, 2001 [PubMed] [Google Scholar]
- 32. Higuchi H, Yoon JH, Grambihler A, Werneburg N, Bronk SF, Gores GJ. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. J Biol Chem 278: 454–461, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Holz GG, Chepurny OG, Schwede F. Epac selective cAMP analogues: new tools with which to evaluate the signal transduction properties of cAMP-regulated quinine nucleotide exchange factors. Cell Signal 20: 10–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hohenester S, Gates A, Wimmer R, Beuers U, Anwer MS, Rust C, Webster CR. Phosphatidylinositol-3-kinase p110 gamma contributes to bile salt induced apoptosis in primary rat hepatocytes and human hepatoma cells. J Hepatol 53: 918–926, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, Billadeau DD, Gores GJ. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol 54: 765–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen J, Brennesvik EO, Lai YC, Shepherd PR. GSK-3beta regulation in skeletal muscles by adrenaline and insulin: evidence that PKA and PKB regulate different pools of GSK-3. Cell Signal 19: 204–210, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Jeyaraj SC, Unger NT, Chotani MA. Rap1 GTPases: an emerging role in the cardiovasculature. Life Sci 88: 645–652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29: 95–102, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Juhaszova M, Zoroy DB, Yaniv Y, Nuss HB, Wang S, Sollot SJ. Role of glycogen synthase kinase 3-beta in cardioprotection. Circ Res 104: 1240–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 138: 347–359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase 3—an overview of an over-achieving kinase. Curr Drug Targets 7: 1377–1388, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Antiapoptotic effect of c-Jun N-terminal kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology 136: 1423–1434, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Kwak HJ, Park KM, Choi HE, Chung KS, Lim HJ, Park HY. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal 20: 803–814, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML. cAMP dose dependently prevents palmitate induced apoptosis by both protein kinase A and cAMP guanine nucleotide exchange factor dependent pathways in beta-cells. J Biol Chem 279: 8938–8945, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Lam P, Soroka CJ, Boyer JL. The bile salt export pump: clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin Liver Dis 30: 125–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leist M, Volbracht C, Fava E, Nicotera P. 1-Methyl-4-phenylpyridinium induces autocrine excitotoxicity, protease activation, and neuronal apoptosis. Mol Pharmacol 54: 789–801, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Liedtke C, Trautwein C. The role of JNK2 in toxic liver injury. J Hepatol 45: 762–764, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3β, mood stabilizers and neuroprotection. Bipolar Disord 4: 137–144, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase 3 beta phosphorylates Bax and promotes it mitochondrial localization during neuronal apoptosis. J Neurosci 24: 9993–10002, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 54: 795–809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev 90: 1165–1194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21: 749–760, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3 selective inhibitors derived from Tyrian purple indirubins. Chem Biol 10: 1255–1266, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Mencin A, Seki E, Osawa Y, Kodama Y, De Minicis S, Knowles M, Brenner DA. Alpha-1 antitrypsin Z protein (PiZ) increases hepatic fibrosis in a murine model of cholestasis. Hepatology 46: 1443–1452, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal 20: 1459–1470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitchell C, Park MA, Zhang G, Han SI, Harada H, Franklin RA. 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther 6: 618–632, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Muira T, Miki T. GSK-3 beta, a therapeutic target for cardiomyocyte protection. Circ J 73: 1184–1192, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Nalapareddy P, Schüngel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3 protein bid does not mediate death receptor induced liver injury in obstructive cholestasis. Am J Pathol 175: 1077–1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Namkoong S, Kim CK, Cho YL, Lee H, Ha KS, Choe J, Kim PH, Won MH, Kown YG, Shim EB, Kim YM. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal 21: 906–915, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol 21: 44–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nijholt IM, Dolga AM, Ostroveanu A, Luiten PG, Schmidt M, Eisel UL. Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal 20: 1715–1724, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Nitta T, Kim JS, Mohunczy D, Behrns KE. Murine cirrhosis induces hepatocytes epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology 48: 909–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohori K, Miura T, Tanno M, Miki T, Sato T, Ishikawa S, Horio Y, Shimamoto K. Ser9 phosphorylation of mitochondrial GSK3β is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant induced apoptosis. Am J Physiol Heart Circ Physiol 295: H2079–H2086, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Perez MJ, Briz O. Bile acid induced cell injury and protection. World J Gastroenterol 15: 1677–1689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Perez DI, Conde S, Perez C, Gil S, Simon D, Wandosell F, Moreno FJ, Gelpi JL, Lugue FJ, Martinez A. Thienylhalomethylketones: irreversible glycogen synthase kinase 3 inhibitors as useful pharmacological tools. Bioorg Med Chem 57: 6914–6925, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Ponzetti K, King M, Gates A, Anwer SA, Webster CRL. Cyclic AMP-guanine exchange factor activation inhibits JNK dependent lipopolysaccharide induced apoptosis in rat hepatocytes [Online]. Hepatic Medicine: Evidence and Research http://www.dovepress.com/cyclic-amp-guanine-exchange-factor-activation-inhibits-jnk-dependent-l-peer-reviewed-article-HMER, [3 July 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN. GSK3beta: a role in therapeutic landscape and development of modulators. Br J Pharmacol 60: 1–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W, Molkentin J, Schmidt-Ullrich R, Fisher PB, Grant S, Hylemon PB, Dent P. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen activated protein kinase signaling kinase signaling module enhances DCA induced apoptosis. Mol Biol Cell 12: 2629–2645, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3; more than a namesake. Br J Pharmacol 156: 885–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reinehr R, Häussinger D. Inhibition of bile salt induced apoptosis by cyclic AMP involves serine/threonine phosphorylation of CD95. Gastroenterology 126: 249–262, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Reinehr R, Becker S, Wettstein M, Häussinger D. Involvement of the Src family kinase yes in bile salt induced apoptosis. Gastroenterology 127: 1540–1557, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species. Mol Med 4: 165–178, 1998 [PMC free article] [PubMed] [Google Scholar]
- 74. Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid induced apoptosis via activation of survival pathways. Bile acid induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem 284: 2908–2916, 2009 [DOI] [PubMed] [Google Scholar]
- 75. Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jensen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 39: 1563–1573, 2004 [DOI] [PubMed] [Google Scholar]
- 76. Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol 39: 153–161, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Büchler P, Müller M, Krammer PH. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology 39: 645–654, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing of glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid leukemia sequence 1. J Biol Chem 285: 8244–8255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 23: 1–7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, Drucker DJ. Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology 135: 2096–2106, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Smith DG, Buffet M, Fenwick AE, Haigh D, Ife RJ, Saunders M, Slingsby BP, Stacey R, Ward RW. 3-Anilino-4-arylmaleimides: potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg Med Chem Lett 11: 635–639, 2001 [DOI] [PubMed] [Google Scholar]
- 82. Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem 279: 39541–39554, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol 294: G498–G505, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Theruvath TP, Czerny C, Ramshesh VK, Zhong Z, Chavin KD, Lemasters JJ. C-Jun N-terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am J Transplant 8: 1819–1828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Theruvath TP, Snoddy MC, Zhong Z, Lemasters JJ. Mitochondrial permeability transition in liver ischemia and reperfusion: role of c-Jun N-terminal kinase 2. Transplantation 85: 1500–1504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tsuchiya S, Tsuji M, Morio Y, Oguchi K. Involvement of endoplasmic reticulum in glycochenodeoxycholic acid-induced apoptosis in rat hepatocytes. Toxicol Lett 166: 140–149, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Varela AT, Simoes AM, Teodoro JS, Gomes AP, Palmeira CM, Rolo AP. Indirubin-3′-oxime prevents hepatic I/R damage by inhibiting GSK-3β and mitochondrial permeability transition. Mitochondrion 10: 456–463, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Wada A. GSK-3 inhibitors and insulin receptor signaling in health, disease and therapeutics. Front Biosci 14: 1558–1570, 2009 [DOI] [PubMed] [Google Scholar]
- 89. Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol 51: 565–580, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, Lin CF. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br J Pharmacol 157: 1004–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem 281: 15258–15267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Webster CR, Anwer MS. Phosphoinositide 3-kinase, but not mitogen-activated protein kinase, pathway is involved in hepatocyte growth factor-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology 33: 608–615, 2001 [DOI] [PubMed] [Google Scholar]
- 93. Webster CR, Usechak P, Anwer MS. cAMP inhibits bile acid induced apoptosis by blocking caspase activation and cytochrome C release. Am J Physiol Gastrointest Liver Physiol 283: G727–G738, 2002 [DOI] [PubMed] [Google Scholar]
- 94. Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP and cell swelling stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem 277: 28578–28583, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280: 11675–11682, 2005 [DOI] [PubMed] [Google Scholar]
- 96. Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20: 87–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng YT. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem 282: 18819–18830, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E, Graf M, Rahmani M, Gupta S, Hylemon PB, Fisher PB, Grant S, Dent P. Multiple cyclin kinase inhibitors promote bile acid induced apoptosis and autophagy in primary hepatocytes via p53-CD95 dependent signaling. J Biol Chem 283: 24343–24358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]