Abstract
Glutathione peroxidase-3 (Gpx3), the extracellular glutathione peroxidase synthesized largely in the kidney, binds to basement membranes of renal cortical epithelial cells. The present study assessed extrarenal expression of Gpx3 using RT-PCR and presence of Gpx3 protein using immunocytochemistry. Gpx3 expression was higher in kidney and epididymis than in other tissues. Gpx3 bound to basement membranes of epithelial cells in the gastrointestinal tract, the efferent ducts connecting the seminiferous tubules with the epididymis, the bronchi, and type II pneumocytes. It was not detected on the basement membrane of type I pneumocytes. Gpx3 was also present in the lumen of the epididymis. Transplantation of Gpx3+/+ kidneys into Gpx3−/− mice led to Gpx3 binding to the same basement membranes to which it bound in Gpx3+/+ mice but not to its presence in the epididymal lumen. These results show that Gpx3 from the blood binds to basement membranes of specific epithelial cells and indicate that the cells modify their basement membranes to cause the binding. They further indicate that at least two Gpx3 compartments exist in the organism. In one compartment, kidney supplies Gpx3 through the blood to specific basement membranes in a number of tissues. In the other compartment, the epididymis provides Gpx3 to its own lumen. Tissues other than kidney and epididymis express Gpx3 at lower levels and may supply Gpx3 to other compartments.
Keywords: Gpx3 compartments, Gpx3 in the epididymis
glutathione peroxidase-3 (Gpx3) is an extracellular selenoprotein that accounts for nearly all the glutathione peroxidase activity in plasma (16). The principal source of plasma Gpx3 is kidney proximal convoluted tubule (PCT) cells (2, 22, 23), which are supplied with selenium by megalin-mediated endocytosis of selenoprotein P (Sepp1) from the glomerular filtrate (17).
Our group recently reported that Gpx3 binds in a specific manner to the basement membrane of renal cortical tubule cells (16). Moreover, the amount of Gpx3 in the kidney was estimated to be greater than the amount circulating in the plasma. These findings indicate that a substantial amount of the enzyme is present in bound form and raise the possibility that such binding might occur in extrarenal tissues and play a role in the function of the enzyme.
To extend our observations, we have assessed the expression of Gpx3 by a number of tissues as well as the presence of Gpx3 protein in them. We have also assessed the ability of the kidney to provide Gpx3 to extrarenal tissues by transplanting Gpx3+/+ kidneys into Gpx3−/− mice. Our results confirm that high expression of Gpx3 mRNA is limited to kidney and epididymis but show that Gpx3 localizes to specific basement membranes in many tissues. Moreover, Gpx3 produced by the kidney can populate those same extrarenal basement membranes but does not account for the Gpx3 in the lumen of the epididymis.
MATERIALS AND METHODS
Animal husbandry.
Separate Gpx3−/− and Gpx3+/+ colonies were established from mice that had been backcrossed three times with C57BL/6 mice (16). The mice were fed pelleted Torula yeast-based diets (Harlan-Teklad, Madison, WI) deficient in selenium or supplemented with 0.25 mg selenium/kg as sodium selenite (8). Food and water were provided ad libitum. The animal room light cycle was 14 h light and 10 h dark.
Mice used for immunocytochemical experiments were euthanized with CO2, and tissues were removed and processed immediately. Mice used for other experiments were anesthetized with isoflurane and exsanguinated via the inferior vena cava using a syringe and needle. Blood was treated with disodium EDTA (1 mg/ml) to prevent clotting, and plasma was separated by centrifugation at 16,000 g for 2 min. Tissues and plasma were stored at −80°C until they were assayed for Gpx3 or selenium. Tissues used for RNA isolation, except for intestines, were frozen immediately in liquid nitrogen and stored at −80°C. Intestines were rinsed with PBS. The intestine was then cut into duodenum (proximal 3 cm), jejunum (proximal half of remaining small intestine), and ileum (distal half of remaining small intestine) sections and frozen in liquid nitrogen. The Vanderbilt University Institutional Animal Care and Use Committee approved all procedures.
Kidney transplantation.
The kidney transplantation protocol has been published (15) and is outlined here. After anesthesia had been established with isoflurane, the abdomen of the donor Gpx3+/+ mouse was opened, and the left kidney was exposed. The renal vein and artery were isolated, and the ureter was dissected down to the bladder without stripping its fat and cut with a small (1–2 mm) bladder patch. After clamping the aorta above the renal artery, a 30-gauge needle was introduced into the aorta, the inferior vena cava was cut, and the graft was perfused with 1 ml of cold saline solution containing 100 μg heparin/ml. After being flushed and cooled with saline at 4°C, the donor kidney was ready for transplant.
After anesthesia had been established in the recipient with isoflurane, the abdomen was opened and the donor Gpx3+/+ kidney was positioned. The donor renal artery, renal vein, and ureter were connected to the aorta, inferior vena cava, and bladder, respectively, through end-to-side anastomoses. On completion of transplantation, the native left Gpx3−/− kidney, renal artery, renal vein, and ureter were ligated and divided. The native left kidney was then removed. The abdominal wall was sutured, and the skin was closed. Thus, in this procedure, one Gpx3+/+ kidney was transplanted to replace one native Gpx3−/− kidney, which was removed, resulting in mice with one Gpx3+/+ kidney and one Gpx3−/− kidney. The mice with transplanted kidneys were allowed to recover in a cage placed on a warming blanket. They were monitored constantly until they were awake and able to right themselves. The diet fed to the transplanted mice was the selenium-supplemented diet. Tissues were removed for biochemical analysis and immunocytochemical study 14 days after the transplant.
A separate experimental protocol carried out at the same time as the present study verified that transplanted kidneys functioned well enough to support life. In that protocol, no results of which are presented here, a Gpx3+/+ mouse received a Gpx3−/− kidney and had one of its native kidneys removed. Two weeks later the other native kidney was removed, leaving the mouse with only the transplanted Gpx3−/− kidney. Mice subjected to this protocol were followed for 6 more wk. Thus the transplanted kidney functioned well enough to support life for at least 6 wk.
RT-PCR.
Tissues were pulverized under liquid nitrogen and homogenized in Trizol Reagent (Invitrogen, Carlsbad, CA). RNA was isolated according to the manufacturer's protocol. Further purification of RNA was accomplished using RNeasy Mini kit (Qiagen, Valencia, CA). RNA concentration was determined by measurement of the A260/A280 ratio. Synthesis of cDNA was carried out using High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA) with 0.1 μg RNA per 20 μl reaction. For real-time PCR, 1 μl of the cDNA was used in 20-μl reactions with Power SYBR GREEN PCR Master Mix (Applied Biosystems). Comparative CT reactions were carried out in an Applied Biosystems StepOnePlus Real Time PCR System using hypoxanthinephosphoribosyltransferase (hprt) amplification as the endogenous control. Oligonucleotides used for qPCR were as follows: Gpx3 forward primer, 5′-ATTTGGCTTGGTCATTCTGG-3′; Gpx3 reverse primer, 5′- CCACCTGGTCGAACATACTTG-3′; hprt forward primer, 5′-TCCTCCTCAGACCGCTTTT-3′; hprt reverse primer, 5′-CCTGGTTCATCGCTAATC-3′. Relative quantitation of the comparative CT PCR results was performed using StepOnePlus analysis software.
Selenium and glutathione peroxidase assays.
Selenium was determined in whole tissues by the method of Koh and Benson (12) as modified by Sheehan and Gao (20). Glutathione peroxidase activity was measured in plasma as previously described using 0.25 mM H2O2 as substrate (13). Protein concentration was determined using bicinchoninic acid reagent (Pierce Chemical, Rockford, IL).
Western detection of Gpx3.
Polyclonal antibody preparation no. 8096 (16) was used for Western analyses and immunocytochemistry experiments. For the Western blots used to assess Gpx3 in transplanted and native kidneys, SDS/PAGE was performed using 10% Bis-Tris NuPAGE Novex acrylamide gels (Invitrogen) as described previously (16). The blots were imaged using an Odyssey infrared imager.
For the Western blots used to quantify Gpx3, proteins were separated on SDS/PAGE gels made with 15% Protogel (National Diagnostics, Atlanta, GA) as described in a previous publication (16). After transfer of the proteins to nitrocellulose, the blots were blocked with 3% BSA in 0.5 M NaCl, 20 mM Tris, and 0.05% Tween 20, pH 7.0. Following sequential incubations with antibody preparation no. 8096 and horseradish peroxidase-conjugated goat anti-rabbit whole-molecule IgG, Gpx3 protein was detected using Western Lighting ECL chemiluminescence reagent (Perkin-Elmer, Boston, MA). Gpx3 protein ECL signal density was quantified using ImageJ software program (NIH).
Immunofluorescence microscopy.
Mouse tissues were fixed and stained as described in detail in a previous publication (16). The primary antibody used for Gpx3 was no. 8096. Hoechst 33258 was used to stain DNA, and Alexa 488-phalloidin (Invitrogen) was used to stain F-actin. Specimens were examined by phase contrast and epifluorescence microscopy. Digital images were made. Each experiment was carried out with at least three mice, and the images shown are representative of all replicates.
RESULTS
Expression of Gpx3 mRNA by tissues.
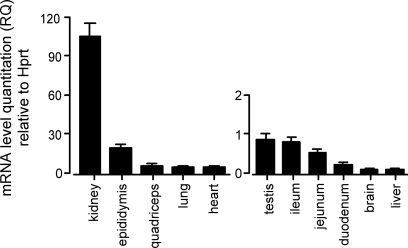
The highest concentration of Gpx3 mRNA has been reported to occur in the kidney (7, 9, 19). We reassessed and quantified its presence in a number of tissues using RT-PCR. Figure 1 shows that kidney had the highest Gpx3 mRNA relative to hprt mRNA concentration of the tissues we studied. The concentration in the epididymis was 18% of that in kidney. Other tissues studied were all less than 6% of that in kidney, with testis, small intestine, brain, and liver less than 1%. Ct values of Gpx3 expression (not shown) generally agreed with the relative values. Thus high expression of Gpx3 mRNA was restricted to kidney and epididymis, but other tissues that we studied expressed it at lower levels.
Fig. 1.
Relative quantities (RQ) of glutathione peroxidase-3 (Gpx3) mRNA in tissues of Gpx3+/+ mice fed the diet supplemented with 0.25 mg selenium/kg. The kidney value was 106 ± 9.5 RQ, n = 3. For other tissues n was 3 or 4. Corresponding Gpx3−/− values did not reach the threshold of significance. Hprt, hypoxanthinephosphoribosyltransferase.
Presence of Gpx3 protein in tissues.
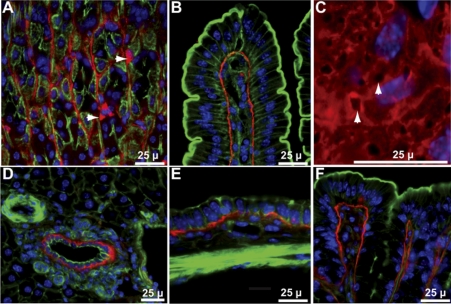
Gpx3 protein was present in a number of tissues, including some with low expression of its mRNA. Figures 2 and 3A show that Gpx3 localized to the position of the basement membrane beneath epithelial cells in the gastrointestinal tract from the stomach to the colon. Additionally, it was detected surrounding the most basal layer of esophageal squamous cells (not shown) and, apparently, inside some cells in stomach glands (arrowheads in Fig. 2A). The same tissues shown in Figs. 2 and 3, but taken from Gpx3−/− mice, did not stain for the enzyme (Fig. 3B and not shown), demonstrating the specificity of the antiserum used to detect Gpx3.
Fig. 2.
Gpx3 in tissues of Gpx3+/+ mouse gastrointestinal tract. A: stomach-arrowheads point to Gpx3-positive cells that have not been identified. B: duodenum. C: en face view of duodenal villus arrowheads point to basement membrane pores. D: liver. E: gall bladder. F: colon. Gpx3 is red; actin is green; nuclei are blue.
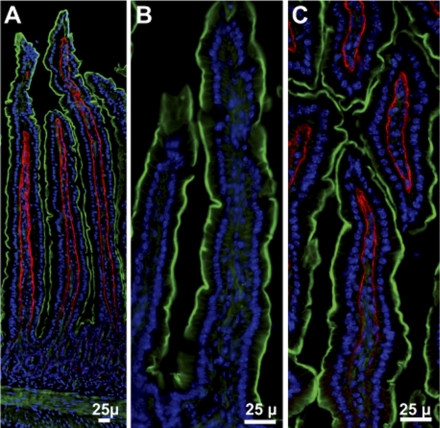
Fig. 3.
Gpx3 in mouse jejunum. A: from Gpx3+/+ mouse. B: from Gpx3−/− mouse. C: from Gpx3−/− mouse 2 wk after transplantation of a Gpx3+/+ kidney. Gpx3 is red; actin is green; nuclei are blue.
Evidence that the Gpx3 observed was attached to basement membrane was provided by an en face view of the basement membrane in duodenum (Fig. 2C) that revealed characteristic pores (14). Additionally, staining of sequential sections of jejunum for collagen IV and Gpx3 demonstrated staining of the two proteins in the same position (not shown). Thus we conclude that Gpx3 bound to certain basement membranes in the gastrointestinal tract as it had been shown to do in the kidney cortex (16).
Not all basement membranes bound Gpx3. This is illustrated by Fig. 2D of liver. Gpx3 was present in the basement membrane of the bile duct but not in blood vessel endothelial cell basement membranes.
In the lung, basement membranes of the bronchioles and type II pneumocytes bound Gpx3 (Fig. 4A). The type I pneumocyte basement membrane did not bind Gpx3 even though it is continuous with the basement membrane of type II pneumocytes.
Fig. 4.
Gpx3 in mouse lung. In A and B Gpx3 is red, actin is green, and nuclei are blue. A: from Gpx3+/+ mouse. B: from Gpx3−/− mouse 2 wk after transplantation of a Gpx3+/+ kidney. C: from Gpx3+/+ mouse. In C, Gpx3 is green, antiprosurfactant protein C (stain for type II pneumocytes) is red, and nuclei are blue.
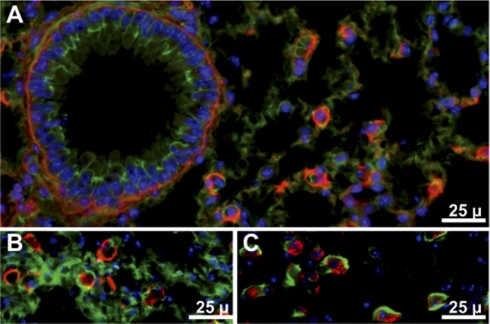
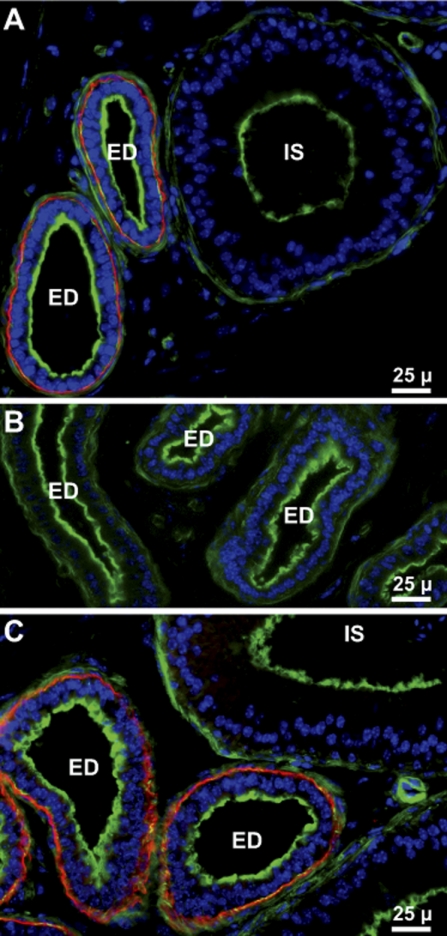
Gpx3 was present in the male reproductive tract. Figure 5A shows that Gpx3 bound to basement membranes in the efferent ducts that connect the seminiferous tubules with the epididymis but did not bind to epithelial cell basement membranes in the epididymis (Figs. 5A and 6, A and C).
Fig. 5.
Gpx3 in mouse efferent ducts and initial segment of the epididymis. A: from Gpx3+/+ mouse. B: from Gpx3−/− mouse. C: from Gpx3−/− mouse 2 wk after transplantation of a Gpx3+/+ kidney. Gpx3 is red; actin is green; nuclei are blue. ED indicates efferent ducts and IS indicates the initial segment of the epididymis.
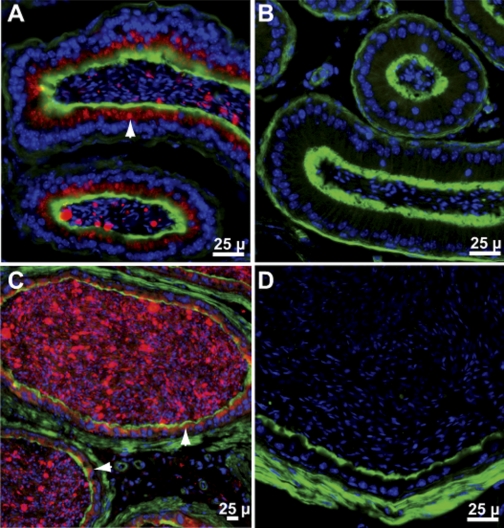
Fig. 6.
Gpx3 in mouse epididymis. A: distal caput from Gpx3+/+ mouse. B: distal caput from Gpx3−/− mouse. C: cauda from Gpx3+/+ mouse. D: cauda from Gpx3−/− mouse 2 wk after transplantation of a Gpx3+/+ kidney. Gpx3 is red; actin is green; nuclei are blue. Arrowheads point to Gpx3 inside cells.
Gpx3 was present between the nucleus and the apical plasma membrane of epithelial cells in the distal epididymal caput (Fig. 6A) and in the cauda (Fig. 6C). It was also detected in the epididymal lumen (Fig. 6, A and C), suggesting that the epithelial cells of the epididymis secrete Gpx3 apically into the lumen.
Several other tissues were stained to determine whether Gpx3 was present in them. No Gpx3 was detected in brain, heart, skeletal muscle, thymus, skin, or spleen (Table 1).
Table 1.
Presence of Gpx3 protein on basement membranes
| Tissue | Gpx3+/+ Mouse | Gpx3−/− Mouse 2 wk After Transplant of Gpx3+/+ Kidney |
|---|---|---|
| Kidney | ||
| cortex | + | + |
| medulla | − | − |
| Gastrointestinal tract | ||
| esophagus | + | n.d. |
| stomach | + | n.d. |
| duodenum | + | + |
| jejunum | + | + |
| ileum | + | + |
| colon | + | + |
| bile ducts | + | n.d. |
| gall bladder | + | + |
| Lungs | ||
| bronchi | + | n.d. |
| type I pneumocytes | − | − |
| type II pneumocytes | + | + |
| Male reproductive tract | ||
| testis | − | − |
| efferent duct | + | + |
| epididymal basement membrane | − | − |
| epididymal lumen | + | − |
| Muscle | ||
| cardiac | − | n.d. |
| quadriceps | − | n.d. |
| intestinal smooth muscle | − | − |
| Others | ||
| brain | − | n.d. |
| thymus | − | n.d. |
| spleen | − | n.d. |
| skin | − | n.d. |
Gpx3, glutathione peroxidase-3; +, Gpx3 present; −, absent; n.d., not determined.
Source of basement membrane-bound Gpx3.
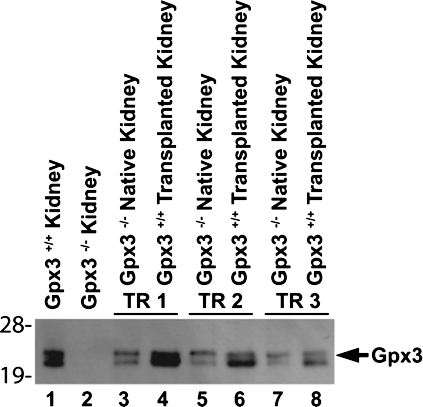
Because kidney is the major source of plasma Gpx3, we carried out experiments to determine whether that organ can supply Gpx3 to basement membranes in extrarenal tissues. One kidney of a Gpx3−/− mouse was replaced by a kidney from a Gpx3+/+ mouse. Two weeks later, tissues were harvested from the Gpx3−/− mouse with the transplanted Gpx3+/+ kidney. Histological examination of transplanted kidneys (not shown) revealed that they were viable. Plasma glutathione peroxidase activity was 38 ± 3 nmol NADPH oxidized·min−1·ml−1 (n = 3) in the transplanted mice and 26 ± 8 nmol NADPH oxidized·min−1·ml−1 (n = 3) in untransplanted Gpx3−/− mice. These values were much lower than those of Gpx3+/+ mice (670 ± 76 nmol NADPH oxidized·min−1·ml−1, n = 3) and they were not significantly different from one another by the Student's t-test. They indicate that the transplanted Gpx3+/+ kidneys were not maintaining Gpx3 at expected levels. Figure 7 shows the effect of transplanting Gpx3+/+ kidneys on the presence of Gpx3 in the native Gpx3−/− kidneys. In each case, Gpx3 synthesized in the Gpx3+/+ kidney appeared in the native Gpx3−/− kidney. This indicates that the transplanted Gpx3+/+ kidneys were synthesizing and secreting Gpx3 even though they did not significantly increase plasma glutathione peroxidase activity.
Fig. 7.
Appearance of Gpx3 in native kidneys of Gpx3−/− mice after transplant of Gpx3+/+ kidneys. Three Gpx3−/− mice received kidney transplants from Gpx3+/+ mice and are indicated by TR1, TR2, and TR3. Homogenates of native and transplanted kidneys were made 2 wk after the transplant and subjected to SDS/PAGE and Western blotting. Lanes 1 and 2 represent kidneys from mice that did not undergo transplant.
Figure 3C shows that the transplant resulted in Gpx3 appearing in the basement membrane of the jejunum in the same pattern seen in Gpx3+/+ mice (Fig. 3A). Similar results were obtained with lung type II pneumocytes (Fig. 4B), efferent duct (Fig. 5C), and other tissues, including the remaining Gpx3−/− kidney (Table 1). Thus the Gpx3 produced by the transplanted Gpx3+/+ kidneys bound to the same basement membranes in the Gpx3−/− mice that bound Gpx3 in the Gpx3+/+ mice even though the low plasma glutathione peroxidase activities indicate that the amount of Gpx3 secreted by transplanted Gpx3+/+ kidneys was significantly less than that secreted by native Gpx3+/+ kidneys. This allows the conclusion that a specific binding site for Gpx3 is present only on certain basement membranes (see Table 1) and that Gpx3 synthesized by the kidney and released into the blood has access to that site.
Source of Gpx3 in the lumen of the epididymis.
Transplantation of a Gpx3+/+ kidney did not cause Gpx3 to appear in the cells of the epididymis or in its lumen (Fig. 6D). Because the epididymis contains a relatively high concentration of Gpx3 mRNA (Fig. 1) and Gpx3 is present in epididymal epithelial cells at the expected position of the Golgi apparatus, these results strongly suggest that Gpx3 is synthesized in the epididymal epithelial cells and secreted apically into the lumen.
Binding of Gpx3 in kidney and small intestine at varying plasma levels.
The transplant experiment showed that Gpx3 produced by kidney can traverse the blood and bind to basement membranes in other tissues. We carried out experiments to characterize the Gpx3 binding in different locations.
Selenium concentration was measured in kidney, small intestine, and liver of Gpx3+/+ and Gpx3−/− mice fed a diet supplemented with 0.25 mg selenium per kg for 4 wk beginning at weaning to assess the amount of Gpx3 present in them. Table 2 shows that deletion of Gpx3 lowered selenium concentration by 280 ng/g in kidney and by 40 ng/g in small intestine. The apparent decrease in liver was not statistically significant. These results suggest that a greater amount of Gpx3 is present in kidney than in intestine.
Table 2.
Tissue selenium concentrations in Gpx3−/− and Gpx3+/+ mice
|
Gpx3+/+ Mice |
|||
|---|---|---|---|
| Tissue | Gpx3−/− Mice | ng/g tissue | Gpx3+/+ - Gpx3−/− |
| Kidney | 1050 ± 61* | 1330 ± 15* | 280 |
| Small intestine | 380 ± 17† | 420 ± 30† | 40 |
| Liver | 1040 ± 89 | 1140 ± 52 | n.s. |
Values are means ± SD, n = 5 in each group. *†Values with the same superscripts are different (P < 0.05) by the Student's t-test. Difference not significant is signified by n.s.
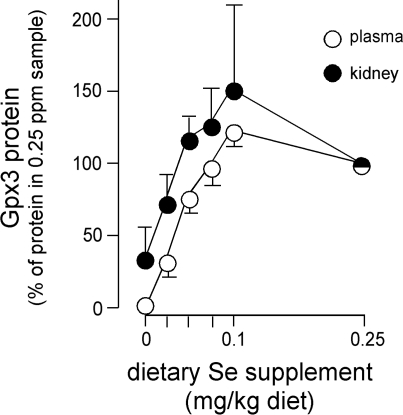
Using quantitative Western blotting, we assessed the amount of Gpx3 in kidney, plasma, and small intestine as a function of selenium nutritional status to determine whether Gpx3 concentrations in these compartments correlate with one another. This experiment was performed because kidney transplant had shown that small intestine basement membranes bound Gpx3 (Fig. 3C) even when plasma glutathione peroxidase activity was not significantly increased (see above), suggesting that binding affinity in the intestine was relatively high. The results presented in Fig. 8 show that selenium nutritional status affected plasma and kidney Gpx3 protein concentrations similarly. The signal from intestine was too small to be quantified in this experiment, and thus we could not compare binding there with binding in the kidney. These results and those in Table 2 indicate that the concentration of Gpx3 in the small intestine was less than that in the kidney. They further indicate that plasma Gpx3 and kidney Gpx3 concentrations vary in a similar manner under these conditions.
Fig. 8.
Gpx3 protein in plasma and kidney from mice of differing selenium nutritional status. Plasma samples and kidney homogenates were subjected to SDS/PAGE, and Western blotting was carried out. Protein (50 μg) in the form of 10% kidney homogenate or 0.5 μl plasma was loaded per lane. Density of the Gpx3 band was determined and compared with the average density from mice fed 0.25 mg selenium/kg diet. Values are means ± SD, n = 5.
DISCUSSION
The results presented here demonstrate that Gpx3 binds to specific basement membranes, not only in the kidney as previously described, but also in the gastrointestinal tract, the lung, and the male reproductive tract. They also show that Gpx3 produced by the kidney can travel through the blood and bind to those same basement membranes, indicating that binding sites for Gpx3 are present on them. In addition, our results show that Gpx3 produced in the kidney does not have access to the epididymal lumen and strongly suggest that Gpx3 present there is secreted by the epididymal epithelium. These findings demonstrate that Gpx3 is distributed within compartments.
Although Gpx3 had been previously observed to be present in the basal extracellular space of kidney tubules (22), we recently showed that it binds to a specific population of kidney basement membranes (16). The present report extends that observation to other tissues and shows that this binding is cell specific. For example, the basement membrane under type II pneumocytes binds Gpx3, whereas the basement membrane continuous with it, but under type I pneumocytes, does not bind the enzyme (Fig. 4A). The same pattern of binding was observed for Gpx3 produced by a transplanted kidney (Fig. 4B), eliminating the possibility that the type II pneumocyte had secreted the Gpx3 attached to its basement membrane. This indicates that the overlying cell produces Gpx3 binding sites on the basement membrane. Thus it can be predicted that these cells, and perhaps other cells, can be induced to modify their basement membranes so that they bind Gpx3 in greater or lesser quantities, according to their need for the enzyme.
The nature of the Gpx3 binding sites on the basement membranes remains unknown. Possibilities include that the overlying cell secretes a unique Gpx3-binding protein that attaches to the basement membrane or that the cell modifies some of the usual constituents of the basement membrane to form the binding site. It is also possible that more than one type of binding site exists, each type perhaps having a different function. Additional study will be needed to characterize the binding sites and to determine their affinity for Gpx3.
Recognition that Gpx3 binds to basement membranes provides a rationale for its function in vivo as a glutathione peroxidase. Enzymatic function of Gpx3 in plasma has been questioned because GSH, its reducing substrate, is present in plasma at concentrations unfavorable to the kinetics of the enzyme (4). Many cells, if not all of them, secrete GSH and thus create a zone of relatively high GSH concentration in proximity to the secreting plasma membrane (6). As a consequence, Gpx3 attached to the basement membrane will be exposed to a higher GSH concentration than Gpx3 in plasma. Moreover, bound Gpx3 will presumably be in equilibrium with unbound enzyme in the adjacent extracellular space, increasing the amount of unbound Gpx3 at this location. Thus we propose that basement membrane binding of Gpx3 increases glutathione peroxidase activity at the basal extracellular aspect of the overlying epithelial cells.
A potential reason for Gpx3 association with certain epithelial cells is a need for regulation of hydroperoxide concentration and antioxidant protection in the extracellular space adjacent to those cells. The epithelial cells that have Gpx3 on their basement membranes are active in absorption and/or secretion. These activities require ATP production by mitochondria, a process that produces O2·− and H2O2 as byproducts. Both these reactive oxygen species can exit the cell, and thus localized Gpx3 may regulate H2O2 concentration outside the cell membrane.
Kidney PCT cells have long been known to produce most of the Gpx3 in plasma (2). On the basis of the decrease in tissue selenium content when Gpx3 was deleted (Table 2), it appears that a greater concentration of the enzyme is present in kidney than in intestine. The experiment depicted in Fig. 8 was designed to determine kidney and intestinal Gpx3 concentrations at varying plasma Gpx3 levels with the aim of comparing binding affinities for Gpx3 in these tissues. We were motivated by the observation that plasma levels of Gpx3 fall sharply in patients with renal failure and hoped to learn whether decreased plasma Gpx3 would lead to a decrease in its concentration in the small intestine. The experiment failed in that objective because we could not quantify intestinal Gpx3. However, kidney and plasma Gpx3 showed good correlation over the range of selenium intakes studied (Fig. 8). This observation is consistent with the kidney basement membranes serving as a reservoir for plasma Gpx3. It seems possible that renal basement membrane-bound Gpx3, a pool of Gpx3 larger than the plasma Gpx3 pool (16), buffers plasma Gpx3 and thereby supports the supply of the enzyme to peripheral tissues via the plasma. The transplant experiments show that remote tissues bind Gpx3 produced by the kidney (Fig. 3) even when plasma levels of the enzyme are quite low (see results). It will thus be important for the understanding of Gpx3 physiology to determine the nature of the binding sites in different tissues and their relative affinities for Gpx3.
Our results demonstrate that Gpx3 is present in the lumen of the epididymis (Fig. 6) and that the epithelial cells in that organ contain Gpx3 at the location of the Golgi apparatus (Figs. 6, A and C). Thus we infer that these cells secrete Gpx3 apically into the lumen. Earlier work using antibody detection suggested that Gpx3 was present in the cytosol of epididymal epithelial cells and did not detect Gpx3 in fluid obtained by perfusion of the epididymis (19). Our demonstration that Gpx3 staining is present in the epididymal lumen of Gpx3+/+ mice but not of Gpx3−/− mice demonstrates the specificity of our antibody preparation and supports our contention that Gpx3 is present in the lumen where spermatozoa are stored.
Our results have identified two Gpx3 compartments in the organism that are separate from one another. One is the combined blood-interstitial space that is supplied with Gpx3 by the kidney, and the other is the epididymal lumen, presumably supplied by the epididymal epithelial cells. Kidney and epididymis both contain high concentrations of Gpx3 mRNA (Fig. 1). Other tissues contain Gpx3 mRNA, albeit at lower concentrations (Fig. 1), so other compartments are likely to exist. In particular, lung appears to secrete Gpx3 into the airways (1). Epithelial cells in the mammary gland, the thyroid gland, and the yolk sac secrete it as well (3, 11, 18). More work will be needed to determine the disposition and function of the Gpx3 expressed by each tissue.
Several pathological conditions increase Gpx3 in the compartment fed by the kidney. The inflammatory colitis caused in mice by dextran sodium sulfate administration raises the concentration of Gpx3 mRNA in kidney and Gpx3 in plasma (21). Exposure of mice to 100% oxygen for 72 h raises plasma and lung Gpx3 concentrations (10). Patients with cirrhosis have increased plasma glutathione peroxidase activity that is presumably due to Gpx3 (5). Oxidative stress occurs in all these conditions and may be the cause of the Gpx3 increase.
Plasma Gpx3 falls in kidney failure (2). Thus it will be important to determine the relationship of the plasma Gpx3 level with Gpx3 function in tissues where the enzyme is bound to basement membranes. Inadequate Gpx3 may play a role in how renal failure affects remote tissues. In addition, characterization of other Gpx3 compartments may uncover functions of Gpx3.
GRANTS
This work was supported by the National Institutes of Health Grants R01 DK82813 and P30 ES00267.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors are grateful to Amy K. Motley for performing the selenium analyses, to Lori M. Austin and Teri D. Stevenson for animal colony management, to Lianli Ma for assistance with kidney transplants, and to William Lawson for providing the antibody for ICC of antiprosurfactant protein C. Drs. John C. Whitin and Harvey J. Cohen of Stanford University produced the Gpx3−/− mice and kindly gave them to us.
REFERENCES
- 1. Avissar N, Finkelstein JN, Horowitz S, Willey JC, Coy E, Frampton MW, Watkins RH, Khullar P, Xu YL, Cohen HJ. Extracellular glutathione peroxidase in human lung epithelial lining fluid and in lung cells. Am J Physiol Lung Cell Mol Physiol 270: L173–L182, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol Cell Physiol 266: C367–C375, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Avissar N, Slemmon JR, Palmer IS, Cohen HJ. Partial sequence of human plasma glutathione peroxidase and immunologic identification of milk glutathione peroxidase as the plasma enzyme. J Nutr 121: 1243–1249, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Björnstedt M, Xue J, Huang W, Åkesson B, Holmgren A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J Biol Chem 269: 29382–29384, 1994 [PubMed] [Google Scholar]
- 5. Burk RF, Early DS, Hill KE, Palmer IS, Boeglin ME. Plasma selenium in patients with cirrhosis. Hepatology 27: 794–798, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Burk RF, Hill KE. Reduced glutathione release into rat plasma by extrahepatic tissues. Am J Physiol Gastrointest Liver Physiol 269: G396–G399, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 79: 3233–3238, 1992 [PubMed] [Google Scholar]
- 8. Hill KE, Zhou J, McMahan WJ, Motley AK, Burk RF. Neurological dysfunction occurs in mice with targeted deletion of selenoprotein P gene. J Nutr 134: 157–161, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann PR, Hoge SC, Li PA, Hoffmann FW, Hashimoto AC, Berry MJ. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res 35: 3963–3973, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim KK, Whitin JC, Sukhova NM, Cohen HJ. Increase in extracellular glutathione peroxidase in plasma and lungs of mice exposed to hyperoxia. Pediatr Res 46: 715–721, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Kingsley PD, Whitin JC, Cohen HJ, Palis J. Developmental expression of extracellular glutathione peroxidase suggests antioxidant roles in deciduum, visceral yolk sac, and skin. Mol Reprod Dev 49: 343–355, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Koh TS, Benson TH. Critical re-appraisal of fluorometric method for determination of selenium in biological materials. J Assoc Off Anal Chem 66: 918–926, 1983 [PubMed] [Google Scholar]
- 13. Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71: 952–958, 1976 [DOI] [PubMed] [Google Scholar]
- 14. Mahida YR, Galvin AM, Gray T, Makh S, McAlindon ME, Sewell HF, Podolsky DK. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol 109: 377–386, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martins PN. Learning curve, surgical results and operative complications for kidney transplantation in mice. Microsurgery 26: 590–593, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Olson GE, Whitin JC, Hill KE, Winfrey VP, Motley AK, Austin LM, Deal J, Cohen HJ, Burk RF. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am J Physiol Renal Physiol 298: F1244–F1253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olson GE, Winfrey VP, Hill KE, Burk RF. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J Biol Chem 283: 6854–6860, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Schmutzler C, Mentrup B, Schomburg L, Hoang-Vu C, Herzog V, Kohrle J. Selenoproteins of the thyroid gland: expression, localization and possible function of glutathione peroxidase 3. Biol Chem 388: 1053–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Schwaab V, Faure J, Dufaure JP, Drevet JR. GPx3: the plasma-type glutathione peroxidase is expressed under androgenic control in the mouse epididymis and vas deferens. Mol Reprod Dev 51: 362–372, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Sheehan TMT, Gao M. Simplified fluorometric assay of total selenium in plasma and urine. Clin Chem 36: 2124–2126, 1990 [PubMed] [Google Scholar]
- 21. Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res 51: 641–646, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Whitin JC, Bhamre S, Tham DM, Cohen HJ. Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. Am J Physiol Renal Physiol 283: F20–F28, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Whitin JC, Tham DM, Bhamre S, Ornt DB, Scandling JD, Tune BM, Salvatierra O, Avissar N, Cohen HJ. Plasma glutathione peroxidase and its relationship to renal proximal tubule function. Mol Genet Metab 65: 238–245, 1998 [DOI] [PubMed] [Google Scholar]