Abstract
Background
Rituximab, a monoclonal antibody that selectively targets CD20-positive B-lymphocytes, is used for the treatment of patients with rheumatoid arthritis (RA) with an inadequate response or tolerance to tumor necrosis factor inhibitors. The objective of this study was to investigate the effects of rituximab treatment on the serum concentrations of vitamin D, interleukin (IL) 2, IL-6, IL-7, and IL-10 in patients with rheumatoid arthritis (RA).
Methods
Forty-five patients, aged 25–78 years, were enrolled into a cohort prospective study. All patients were treated with intravenous rituximab. Disease activity score-28 (DAS-28) and serum concentrations of rheumatoid factor (RF), C-reactive protein (CRP), anticyclic citrullinated peptide (anti-CCP), erythrocyte sedimentation rate (ESR), health assessment questionnaire (HAQ), vitamin D, ILs 2, 6, 7, and 10 were estimated in the patients before and after treatment with rituximab.
Results
DAS-28, HAQ score, and serum concentrations of CRP, RF, anti-CCP, IL-2, IL-6, IL-7, IL-10, and ESR significantly decreased after treatment. All 45 patients had vitamin D deficiency before treatment and this did not significantly change after treatment. However no significant association was found among serum vitamin D concentration and any of the ILs.
Conclusion
We concluded from this study that although rituximab treatment of patients with RA significantly reduced their disease activity and serum concentrations of IL-2, IL-6, IL-7, and IL-10, it did not significantly alter their vitamin D status. Furthermore, no significant association was found among serum vitamin D concentration and any of the ILs.
Keywords: vitamin D, rituximab treatment, rheumatoid arthritis, interleukins
Introduction
Vitamin D, or cholecalciferol, is a steroidal hormone whose main function is the regulation of calcium homeostasis and bone formation and reabsorption.1 Recently, there has been a plethora of data on the noncalcitropic effects of vitamin D deficiency, separate from its known associations with increased fracture risk.2,3 As an example, vitamin D has been shown to alter the expression of more than 200 genes that affect cellular functions such as proliferation, differentiation, apoptosis, and angiogenesis.4 Thus vitamin D deficiency has been associated with various cancers.5–8
There is also a growing body of evidence that vitamin D is important in the initiation and propagation of a range of autoimmune diseases.9–11 This vitamin inhibits antibody secretion and autoantibody production in B cells.12 It has also been reported that synovium and lymphocytes from patients with rheumatoid arthritis (RA) express vitamin D receptor. The metabolically active form of the vitamin 1, 25 dihydroxyvitamin D3 (1, 25-OHD) inhibits T-cell proliferation and prevents the release of Th-1 cytokines such as interleukin (IL)-2, interferon-γ and tumor necrosis factor (TNF)-α.13
Thus, some but not all studies, have implicated low vitamin D intake as a risk factor in the development of RA. There are reports linking low vitamin D levels with increased disease activity and severity in patients with inflammatory arthritis.14–18 A number of studies have also demonstrated an inverse association between vitamin D and disease activity in patients with inflammatory arthritis but the results of these studies were not similar.19–21
Rituximab is a monoclonal antibody that selectively targets CD20-positive B lymphocytes.22 It is approved for the treatment of patients with RA with an inadequate response or tolerance to TNF inhibitors. The drug has been reported to provide significant and clinically meaningful improvements in disease activity in patients with longstanding RA who had been resistant to one or more anti-TNF therapies.22 Rituximab also inhibits the progression of structural damage in RA patients.23 Since this drug is a B-cell depleter, its use offers an opportunity to study the relationship between vitamin D status and some cytokines. The objectives of this study were therefore (1) to study the effect of rituximab treatment on vitamin D status in patients with RA and (2) to find out if there are any significant associations among serum vitamin D concentrations and proinflammatory cytokines (IL-2, IL-6, and IL-7) and anti-inflammatory cytokines (IL-10) to justify the acclaimed link of vitamin D to cytokine production.
Patients and methods
Study patients
Consecutive patients attending the Rheumatology Outpatient Clinic of Al-Amiri Teaching Hospital, Kuwait, who fulfilled the American College of Rheumatology (formerly the American Rheumatism Association) 1987 revised criteria24 were recruited for this study.
All patients first received one course of intravenous (IV) rituximab 1000 mg and a second IV infusion of 1000 mg 2 weeks later after IV methylpredinosolone 100 mg premedication. There was a washout period of at least 3 months before giving rituximab to those who had been on TNF-α blockers. All the patients were assessed before rituximab treatment and 6 months after the second dose of rituximab. The assessment consisted of counting the number of tender and swollen joints, estimation of the erythrocyte sedimentation rate (ESR) by the Westergren method, measurement of serum concentrations of C-reactive protein (CRP) by the nephelometric method, and evaluation of disease activity by the Disease Activity for 28 Joint Indices Score (DAS-28).25 Functional disability was measured by using the Arabic version of the Health Assessment Questionnaire (HAQ).26 All patients gave written informed consents and the study protocol was approved by the local ethics committee.
Measurement of serum 25-OH-D concentrations
Banked serum samples collected from the patients before and after treatment with rituximab were analyzed for 25-OH-D3 using the commercially available Immunodiagnostic Systems RIA kit (Immunodiagnostic Systems Ltd, Boldon Tyne and Wear, UK). This kit is a liquid phase radioimmunoassay kit for the quantitation of 25-hydroxy vitamin D and other hydroxylated metabolites in human serum or plasma. The sensitivity of the assay in our laboratory is <3 nmol/L while the intra assay and inter assay coefficient of variations were 5.0 and 8.1%, respectively. Insufficiency of 25-OH-D was defined as <70 nmol/L (<30 ng/mL) and deficiency as <50 nmol/L (<20 ng/mL). The normal range in our laboratory is 70–100 nmol/L.
Measurement of serum IL-2, IL-6, IL-7, and IL-10 concentrations
The serum concentrations of these ILs in patients with RA before and after treatment with rituximab were estimated by using commercially available ELISA kits from IMMUNOTECH SAS (Marsielle, France). The instructions of the manufacturer of the kits were followed.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences Software ([SPSS] v17.0; SPSS Inc, Chicago, IL). The variables were examined for normality with the Kolmogorov-Smirnov test, and descriptive statistics presented as appropriate. The differences between pretreatment and post-treatment values were compared using nonparametric Mann–Whitney or Kruskal–Wallis tests. Vitamin D concentrations were tested for any relationship with IL concentrations using Spearman correlation coefficient. The two-tailed probability P < 0.05 was considered statistically significant.
Results
Demographic characteristics
A total of 45 patients with active RA were enrolled into the study. Of these, 34 (75.6%) were females and 11 (24.4%) were males (Table 1). The mean age of the patients was 48.9 ± 1.78 years with a range of 25–78 years. The mean age at disease onset was 36.6 ± 11.4 years ranging from 14–70 years. The median RA duration was 10 years ranging from 6 months to 28 years. None of the patients had any significant dietary change that might affect vitamin D intake during the study. None of the patients had abnormal liver or renal functions that might affect the serum vitamin D concentrations.
Table 1.
Demographic and clinical characteristics of patients with active RA before treatment with rituximab
| Characteristics | n = 45 |
|---|---|
| Gender ratio (M:F) | 1.1:3.4 |
| Age (years) | |
| Mean ± SD | 48.9 ± 1.78 |
| Range | (25–78) |
| Age at onset (years) | |
| Mean ± SD | 36.6 ±11.4 |
| Range | (14–70) |
| RA duration (months) | |
| Median (interquartile) | 120 |
| Range | (6–336) |
| Treatment | |
| Prednisolone (%) | 20.7 |
| DMARDs (%) | |
| One | 62.1 |
| ≥2 | 13.8 |
| Previous anti-TNF (%) | |
| 1 anti-TNF | 41.3 |
| 2 anti-TNFs | 26.1 |
| 3 anti-TNFs | 6.5 |
Abbreviations: RA, rheumatoid arthritis; M, male; F, Female; SD, standard deviation; DMARDs, disease modifying anti-rheumatic drugs; TNF, tumor necrosis factor.
Clinical and laboratory parameters
Table 2 shows a significant decrease in DAS-28, HAQ, serum concentrations of RF, CRP, anti-CCP, and ESR following treatment of RA patients with rituximab, suggesting that the drug was effective in reducing the inflammation of RA.
Table 2.
Clinical and laboratory parameters in patients with RA before and after treatment with rituximab
| Parameter | Before treatment | After treatment | P value |
|---|---|---|---|
| DAS-28 | 6.28 ± 0.24 | 3.84 ± 0.30 | 0.001 |
| HAQ | 2.89 ± 0.83 | 1.52 ± 0.61 | 0.05 |
| Mean serum RF | 721.84 ± 51.5 | 93.32 ± 30.3 | 0.001 |
| Mean ESR (mm/hr) | 35.5 ± 5.21 | 18 ± 6.35 | 0.001 |
| Mean serum CRP (mg/dL) | 27.21 ± 3.76 | 11.54 ± 1.92 | 0.001 |
| Mean serum anti-CCP (U/mL) | 563.34 ± 93.64 | 350.75 ± 113.25 | 0.001 |
Abbreviations: RA, rheumatoid arthritis; DAS, disease activity score; HAQ, health assessment questionnaire; RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; anti-CCP, anti-cyclic citrullinated peptide.
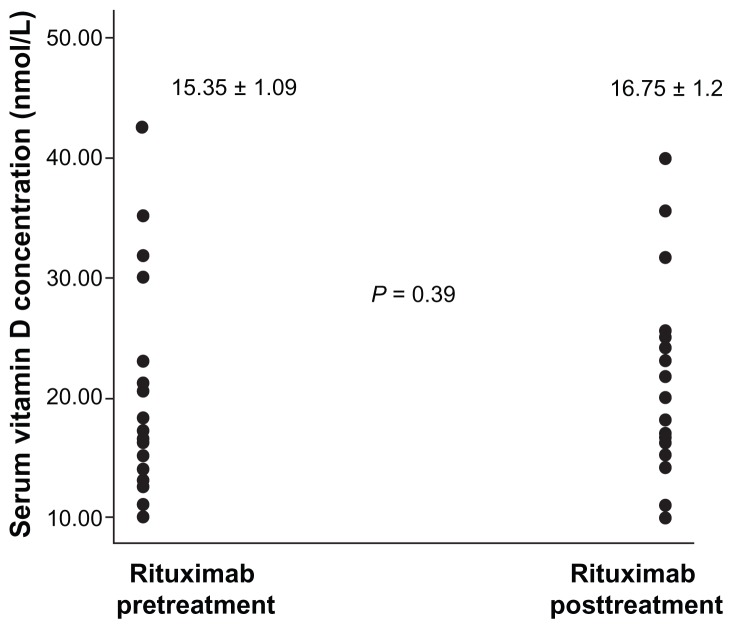
Serum concentrations of vitamin D, IL-2, IL-6, IL-7, and IL-10
Figure 1 is a scatter plot showing the distribution of serum vitamin D concentrations in patients with RA before and after treatment with rituximab. All patients, before and after treatment, had serum vitamin D concentrations of less than 50 nmol/L, the deficiency value in our laboratory. We did not include controls in this study because our aim was not to compare the prevalences of vitamin D deficiencies between patients with RA and the healthy population but to demonstrate the effect of rituximab treatment on vitamin D status. Figure 1 also shows that rituximab treatment did not significantly alter the status of vitamin D in patients with RA.
Figure 1.
Scatter plot of vitamin D in patients with rheumatoid arthritis before and after treatment with rituximab.
Table 3 shows that the mean serum concentrations of IL-2, IL-6, IL-7, and IL-10 significantly fell in patients with RA following treatment with rituximab. For example, serum IL-2 concentration fell from 52.56 ± 6.29 pg/mL before treatment to 8.77 ± 1.81 pg/mL after treatment while IL-7 fell from 146.63 ± 9.23 pg/mL before treatment to 68.71 ± 11.63 pg/mL after treatment.
Table 3.
Serum concentrations of vitamin D, IL-2, IL-6, IL-7, and IL-10 in patients with RA before and after treatment with rituximab
| Analyte | Before treatment (mean ± SD) | After treatment (mean ± SD) | P value |
|---|---|---|---|
| Serum vitamin D (nmol/L) | 15.35 ± 1.09 | 16.75 ± 1.2 | 0.39 |
| Serum IL-2 (pg/mL) | 52.56 ± 6.29 | 8.77 ± 1.81 | 0.0001 |
| Serum IL-6 (pg/mL) | 34.56 ± 5.01 | 21.32 ± 2.99 | 0.01 |
| Serum IL-7 (pg/mL) | 146.63 ± 9.23 | 68.71 ± 11.63 | 0.001 |
| Serum IL-10 (pg/mL) | 32.26 ± 6.01 | 17.21 ± 2.84 | 0.01 |
Abbreviations: IL, interleukin; RA, rheumatoid artritis; SD, standard deviation.
Association among serum vitamin D concentration and serum ILs before and after treatment with rituximab
Table 4 shows that there were no significant associations among serum vitamin D concentration and serum IL-2, IL-6, IL-7, and IL-10 in patients with RA before and after treatment with rituximab.
Table 4.
Association among serum vitamin D concentration and serum ILs in patients with RA before treatment with rituximab
| Serum ILs | Associations with serum vitamin D | Comments |
|---|---|---|
| IL-2 |
r = 0.05 P = 0.75 |
No significant association |
| IL-6 |
r = 0.036 P = 0.82 |
No significant association |
| IL-7 |
r = −0.003 P = 0.98 |
No significant association |
| IL-10 |
r = −0.03 P = 0.85 |
No significant association |
Abbreviations: IL, interleukin; RA, rheumatoid arthritis.
Discussion
Although our objectives in this study did not include the investigation of the prevalence of vitamin D deficiency in patients with RA (and this was why we did not include any healthy controls), it is important to note that all 45 patients with RA had vitamin D deficiency before treatment. This finding is in accord with the almost pandemic vitamin D hypovitaminosis reported all over the world in general26,27 and in patients with RA in particular.28–30
The second important result from this study was that treatment of RA patients with rituximab did not alter their vitamin D status. To the best of our knowledge, this is the first report of the effect of rituximab treatment on serum vitamin D concentrations in patients with RA. It was surprising that rituximab did not affect vitamin D status since vitamin D has been reported to inhibit antibody secretion and T cell proliferation.12,13 Rituximab is known to deplete B cells that produce antibodies. Therefore one would have expected that rituximab treatment would cause a significant reduction in serum vitamin D concentration. Why this was not so is not clear but further investigations on the relationship between vitamin D, antibody production by B cells, and cytokine serum concentrations need to be carried out on a large population.
The third important result of this study was that rituximab treatment of patients with RA significantly reduced their disease activity and their serum concentrations of RF, CRP, anti-CCP, and ESR. In this study, we found significant reductions in the serum concentrations of proinflammatory interleukins such as IL-2, IL-6, and IL-7. The reduction in disease activity in RA after treatment with rituximab might be due to the reductions in these proinflammatory ILs. It was surprising to find that treatment with rituximab in patients with RA also reduced their serum concentrations of IL-10, an anti-inflammatory IL. This might be due to the fact that rituximab depletes all B lymphocytes. The long term effect of this reduction in patients treated with rituximab is not known.
Perhaps the most important finding in this study was that the serum concentration of vitamin D was not significantly associated with serum concentration of either IL-2, IL-6, IL-7, or IL-10. These findings contradict the reports that vitamin D downregulates the production of several cytokines such as IL-2, IL-6, IL-12, interferon-γ, TNF-α, and TNF-β in in vitro studies.31,32 Our results, however, were in agreement with those of Vilarrasa et al33 who recently reported that no significant associations were found amongst 25-OHD and plasma concentrations of IL-18 and other cytokines. Further studies on larger sample sizes of healthy populations are needed to investigate the associations among serum vitamin D and IL concentrations.
Conclusion
We discovered from our study that treatment of RA patients with rituximab did not significantly alter their already depleted vitamin D status, although it significantly reduced their indices of inflammation and their serum concentrations of IL-2, IL-6, IL-7, and IL-10.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherniack EP, Levis S, Troen BR. Hypovitaminosis D: a widespread epidemic. Geriatrics. 2008;63:24–30. [PubMed] [Google Scholar]
- 4.Cutolo M. Vitamin D and autoimmune rheumatic diseases. Rheumatology. 2009;48:210–212. doi: 10.1093/rheumatology/ken394. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan AV, Trump DL, Johnson CS, Feldman D. The role of vitamin D in cancer prevention and treatment. Endocrinol Metab Clin North Am. 2010;39:401–418. doi: 10.1016/j.ecl.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010;91:1699–1707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson S, Olausson J, Lundh D, et al. Vitamin D and prostate cancer: the role of membrane initiated signaling pathways in prostate cancer progression. J Steroid Biochem Mol Biol. 2010;121:413–416. doi: 10.1016/j.jsbmb.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 8.Tse AK, Zhu GY, Wan CK, Shen XL, Yu ZL, Fong WF. 1alpha, 25-Dihydroxyvitamin D3 inhibits transcriptional potential of nuclear factor kappa B in breast cancer cells. Mol Immunol. 2010;47:1728–1738. doi: 10.1016/j.molimm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 10.Cutolo M, Otsa K, Palino S, Yprus M, Veldi T, Seriolo B. Vitamin D involvement in rheumatoid arthritis and systemic lupus erthymatosus. Ann Rheum Dis. 2009;67:446–447. doi: 10.1136/ard.2008.093476. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-López FR. Vitamin D and its implications for musculoskeletal health in women: an update. Maturitas. 2007;58:117–137. doi: 10.1016/j.maturitas.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Ritterhouse LL, Crowe SR, Niewold TB, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erthematosus. Ann Rheum Dis. 2011;(70):1569–1574. doi: 10.1136/ard.2010.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranganathan P. Genetics of bone loss in rheumatoid arthritis – role of vitamin D receptor polymorphisms. Rheumatology. 2009;48(4):342–346. doi: 10.1093/rheumatology/ken473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21(3):279–283. doi: 10.1097/BOR.0b013e32832a2e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielen MM, van Schaardenburg D, Lems WF, et al. Vitamin D deficiency does not increase the risk of rheumatoid arthritis. Arthritis Rheum. 2006;54:3719–3720. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 16.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and arthritis in women. Ann Rheum Dis. 2008;67:530–535. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56:2143–2149. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 19.Turhanoğlu AD, Güler H, Yönden Z, Aslan F, Mansuroglu A, Ozer C. The relationship between vitamin D and disease activity and functional health status in rheumatoid arthritis. Rheumatol Int. 2010;31:911–914. doi: 10.1007/s00296-010-1393-6. [DOI] [PubMed] [Google Scholar]
- 20.Craig SM, Yu F, Curtis JR, et al. Vitamin D status and its associations with disease activity and severity in African Americans with recent-onset rheumatoid arthritis. J Rheumatol. 2010;37:275–281. doi: 10.3899/jrheum.090705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun-Moscovici Y, Toledano K, Markovits D, Rozin A, Nahir AM, Balbir-Gurman A. Vitamin D level: is it related to disease activity in inflammatory joint disease? Rheumatol Int. 2011;31:493–499. doi: 10.1007/s00296-009-1251-6. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 23.Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216–221. doi: 10.1136/ard.2007.085787. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 26.Shehab D, Al-Jarallah K, Moussa MA. Validation of the Arabic version of the Health Assessment Questionnaire (HAQ) in patients with rheumatoid arthritis. Rev Rhum Engl Ed. 1998;65:387–392. [PubMed] [Google Scholar]
- 27.Gannagé-Yared MH, Tohmé A, Halaby G. Hypovitaminosis D: a major worldwide public health problem. Presse Med. 2001;(30):653–658. [PubMed] [Google Scholar]
- 28.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zold E, Szodoray P, Gaal J, et al. Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res Ther. 2008;10:R123. doi: 10.1186/ar2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damanhouri LH. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi Med J. 2009;30:1291–1295. [PubMed] [Google Scholar]
- 31.Mauricio D, Mandrup-Poulsen T, Nerup J. Vitamin D analogues in insulin-dependent diabetes mellitus and other autoimmune diseases: a therapeutic perspective. Diabetes Metab Rev. 1996;12:57–68. doi: 10.1002/(SICI)1099-0895(199603)12:1<57::AID-DMR155>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Lemire JM. J Immunomodulatory actions of 1, 25-dihydroxyvitamin D3. Steroid Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 33.Vilarrasa N, Vendrell J, Maravall J, et al. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin résistance in both a healthy and morbidly obese population? Endocrinology. 2010;38:235–242. doi: 10.1007/s12020-010-9379-4. [DOI] [PubMed] [Google Scholar]