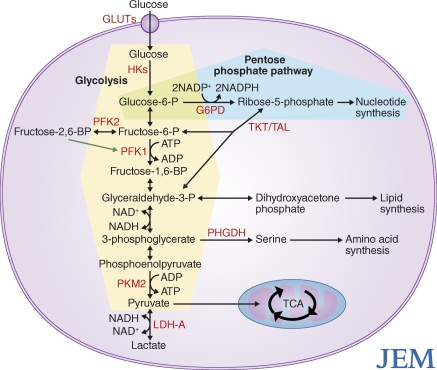
Cancer therapeutic targets found within metabolic pathways.
Abstract
Cellular transformation is associated with the reprogramming of cellular pathways that control proliferation, survival, and metabolism. Among the metabolic changes exhibited by tumor cells is an increase in glucose metabolism and glucose dependence. It has been hypothesized that targeting glucose metabolism may provide a selective mechanism by which to kill cancer cells. In this minireview, we discuss the benefits that high levels of glycolysis provide for tumor cells, as well as several key enzymes required by cancer cells to maintain this high level of glucose metabolism. It is anticipated that understanding which metabolic enzymes are particularly critical for tumor cell proliferation and survival will identify novel therapeutic targets.
Tumor cells exhibit high levels of glycolysis despite the presence of ample oxygen, a phenomenon termed aerobic glycolysis. This observation was first published by Warburg et al. (1924); it has since been supported by multiple studies in a variety of tumor types, and is now exploited in the clinic for diagnostic purposes. Positron emission tomography using 2-deoxy-2(18F)-fluoro-D-glucose, a glucose analogue, demonstrates a significant increase in glucose uptake in tumors compared with adjacent normal tissue (Gambhir, 2002). Warburg’s initial observations led him to hypothesize that cancer is caused by mitochondrial injury, followed by an increase in glycolysis that converts differentiated cells into proliferating cancer cells (Warburg, 1956). However, primary defects in mitochondrial enzymes or complexes within the electron transport chain are not frequently observed in cancer (Frezza and Gottlieb, 2009). Recent studies indicate that the activation of protooncogenes (e.g., Myc), signaling pathways (e.g., PI3K), and transcription factors (e.g., HIF-1), as well as the inactivation of tumor suppressors (e.g., p53), induce the Warburg effect in cancer cells (Vander Heiden et al., 2009)
Glycolysis generates ATP with lower efficiency, but at a faster rate, than oxidative phosphorylation (Pfeiffer et al., 2001). This enhanced rate of ATP generation has been postulated to be beneficial for rapidly proliferating cells. However, this is probably not the main reason why proliferating cells engage in high levels of aerobic glycolysis, as multiple studies have suggested that mitochondria are the major source of cellular ATP in most cancer cell lines and tissues (Zu and Guppy, 2004). Furthermore, it was recognized >30 yr ago that galactose or fructose, which are preferentially shunted into glycolytic subsidiary pathways and do not generate substantial amounts of glycolytic ATP, allow cancer cells to proliferate in the absence of glucose (Reitzer et al., 1979). Thus, high glycolytic rates likely benefit proliferating cells through the production of glycolytic intermediates, which are shunted into subsidiary pathways to fuel metabolic pathways that generate de novo nucleotides, lipids, amino acids, and NADPH (Lunt and Vander Heiden, 2011).
Meeting the biosynthesis needs of proliferating cells
Glycolytic intermediates fuel several biosynthetic pathways that are essential for duplication of biomass during cellular proliferation (Fig. 1). After cellular uptake through glucose transporters (GLUTs), glucose is phosphorylated by hexokinases (HKs), which produces glucose-6-phosphate. Glucose-6-phosphate can either proceed into glycolysis through conversion into fructose-6-phosphate by glucose-6-phosphate isomerase, or it can be shunted into the oxidative branch of the pentose phosphate pathway (PPP) by glucose-6-phosphate dehydrogenase. The oxidative branch of the PPP generates NADPH, which is used for the reduction of cellular glutathione pools to promote redox homeostasis and acts as a reducing agent for lipid, nucleotide, and amino acid biosynthesis. The nonoxidative branch of the PPP generates ribose-5-phosphate, which is used in the biosynthesis of nucleic acids. Back in glycolysis, phosphofructokinase-1 (PFK-1) irreversibly converts fructose-6-phosphate to fructose-1,6-bisphosphate. Fructose-1,6-bisphosphate is converted into glyceraldehyde-3-phosphate or dihydroxyacetone phosphate. The latter is a precursor to glycerol-3-phosphate, which is crucial for the biosynthesis of the phospholipids and triacylglycerols required for generation of cell membranes. Fructose-6-phosphate and glyceraldehyde-3-phosphate can also combine to generate ribose-5-phosphate through transketolases and transaldolases. Further down the glycolytic pathway, 3-phosphoglycerate can undergo oxidation to generate serine and NADH. Serine can be used to generate two critical amino acids, cysteine and glycine, and to generate important signaling molecules such as ceramide.
Figure 1.
Potential targets for cancer therapy found within metabolic pathways involved in glucose metabolism. The PPP is shaded in blue, and glycolysis is shaded in yellow. Red text is used to denote potential therapeutic targets. The green arrow indicates positive regulation of PFK1.
Mechanisms maintaining high glycolytic flux
Cellular glycolytic rates are subject to several negative feedback mechanisms, which proliferating cells must overcome to maintain biosynthesis. PFK-1 is a critical driver of glycolytic flux and is allosterically inhibited by high ATP levels. Proliferating cells up-regulate the expression of PFK-2, which generates fructose-2,6-bisphospate, a potent allosteric activator of PFK-1, thus maintaining high glycolytic flux in the presence of high ATP (Vora et al., 1985; Colombo et al., 2010). High glycolytic flux is also maintained by the overexpression of lactate dehydrogenase (LDH), a transcriptional target of Myc (Shim et al., 1997). LDH generates NAD+ from NADH while reducing pyruvate to lactate. NAD+ regeneration is necessary for continued flux through glycolysis, as NAD+ is required for conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate. The overexpression of LDH is sufficient to increase glycolytic flux (Shim et al., 1997). Although NAD+ can also be regenerated by mitochondria, this requires the transport of NADH into the mitochondria through various shuttles that are likely too slow to meet the high demands of the cytosolic NAD+ required to maintain glycolysis (Locasale and Cantley, 2011).
Emerging studies indicate that pyruvate kinase (PK) plays an essential role in regulating the balance between glycolytic ATP generation and biosynthetic needs for proliferating cells (Christofk et al., 2008b). PK catalyzes the rate-limiting and ATP-producing step of glycolysis in which phosphoenolpyruvate (PEP) is converted to pyruvate. Cancer cells express higher levels of M2 isoform (PKM2) over the more catalytically active M1 isoform (PKM1), and cancer cells engineered to express PKM1 instead of PKM2 exhibit reduced tumor-forming ability (Christofk et al., 2008a; Bluemlein et al., 2011). PKM2 alternates between a dimer that exhibits low catalytic activity and a highly active tetramer that is driven by allosteric binding of fructose-1,6-bisphosphate (Spoden et al., 2009). PKM2 is phosphorylated on tyrosine 105 downstream of growth factor signaling, disrupting tetramer assembly required for PKM2 activity (Hitosugi et al., 2009). Furthermore, high glucose consumption triggers acetylation of PKM2 at lysine 305 to further reduce its activity (Lv et al., 2011). This inhibition of PKM2 allows diversion of glycolytic intermediates upstream of pyruvate into biosynthetic pathways. Under these conditions, PEP can be converted to pyruvate through alternative, non–ATP-producing pathways, allowing for lactate and NAD+ generation (Vander Heiden et al., 2010).
Targets for cancer therapy
Targeting metabolic pathways for cancer therapy seems appealing at first glance, as enzymes are attractive molecular targets. However, to be an attractive candidate for cancer therapy, there must be a significant difference in the requirement for a given enzyme’s activity between cancer cells and normal proliferating cells. A few potential candidates that are overexpressed in certain cancer types include GLUT1, HKII, phosphoglycerate dehydrogenase (PHGDH), and LDH-A (Fig. 1).
GLUT1 is overexpressed in many cancer types, including renal cell carcinomas (RCCs) that exhibit loss of the von Hippel-Lindau (VHL) tumor suppressor gene (Younes et al., 1996; Chan et al., 2011). A recent study identified a series of small molecules that inhibit GLUT1 and selectively kill VHL-deficient RCCs (Chan et al., 2011).
HKs catalyze the first step of glycolysis and are another potentially attractive target. There are four mammalian HKs (HKI–IV). HKI is the ubiquitously expressed isoform, whereas HKII is expressed in insulin-sensitive tissues such as muscle and adipose (Robey and Hay, 2006). Many tumor cells overexpress HKII, and preclinical studies demonstrate that HKII inhibition could be an effective cancer therapy (Jae et al., 2009).
PHGDH converts 3-phosphoglycerate into 3-phosphohydroxypyruvate, a rate-limiting step in the conversion of 3-phosphoglycerate into serine. Two recent studies reported that subsets of human melanoma and breast cancers have high levels of PHGDH, and these cancer cells are dependent on these enzymes for growth (Locasale et al., 2011; Possemato et al., 2011).
LDH-A was the first metabolic target demonstrated to be directly regulated by an oncogene (MYC), and genetic or pharmacologic inhibition of LDH-A diminishes MYC-dependent tumors (Shim et al., 1997; Le et al., 2010). Although these are promising preliminary studies, GLUT1, HKII, PHGDH, and LDH-A are expressed in normal tissues, and it remains to be seen whether inhibition of these enzymes will be effective in diminishing tumors without imparting significant toxicity to normal tissues.
The finding that PKM2 expression provides a proliferative advantage for cancer cells raised the possibility that PKM2 could be an attractive target for cancer therapy. Tumors express higher levels of PKM2 than normal control tissues (Bluemlein et al., 2011); however, inhibiting PKM2 could also allow glycolytic intermediates to accumulate and feed biosynthetic pathways, resulting in tumor promotion. Remarkably, there is data to suggest that either inhibiting or activating PKM2 in cancer cells diminishes tumor growth. Anastasiou et al. (2011) have recently demonstrated that PKM2 is regulated by cellular oxidative stress. PKM2 is specifically oxidized on cysteine 358, inhibiting its activity and promoting diversion of glycolytic intermediates into the PPP, producing NADPH and promoting redox balance. Expression of a nonoxidizable PKM2 mutant reduced flux through the PPP, increased oxidative stress, and inhibited tumor growth (Anastasiou et al., 2011). These results indicate that PKM2 activators could be viable cancer therapeutics, especially when used in conjunction with radiation or chemotherapeutic agents known to promote oxidative stress. Paradoxically, in this issue Goldberg and Sharp have demonstrated that inhibition of PKM2 activity using small interfereing RNA (siRNA) increases apoptosis in cell culture, and can also inhibit tumor cell growth. Work by this group demonstrates that in vivo delivery of siRNA molecules targeting PKM2 causes tumor regression in mouse xenograft models. This is potentially promising, as recent advances in nanotechnology are attempting to target siRNA delivery specifically to tumor cells in vivo.
Although seemingly irreconcilable with the data from Anastasiou et al., 2011, these disparate results may be explained by cellular responses to varying degrees of hypoxia. Moderate hypoxia (1.5% O2) promotes mitochondrial generation of hydrogen peroxide (H2O2), which activates signaling pathways critical for the cellular response to hypoxia. Under these conditions, PKM2 becomes oxidized, which inhibits its activity, promotes flux through the PPP, and promotes redox balance (Anastasiou et al., 2011). This prevents accumulation of aberrantly high levels of cellular H2O2 and oxidative cellular damage. Under severe hypoxia (<0.5% O2), however, oxygen becomes limiting for the mitochondrial electron transport chain and production of mitochondrial ATP and H2O2 ceases. Under these conditions, cells become dependent on PK activity for ATP production. Thus, PKM2 inhibitors and activators could both be therapeutically beneficial for different reasons. PKM2 inhibitors would prevent ATP production in severely hypoxic cells, whereas PKM2 activators would promote oxidative damage in moderately hypoxic cells. As most tumors exhibit a range of oxygen concentrations depending on tumor size and vascularization, whether PKM2 activation or inhibition is most therapeutically effective may be tumor-dependent. It is important to note that insufficient inhibition of PKM2 activity, which allows for glycolytic ATP production during severe hypoxia, would promote flux through biosynthetic pathways and possibly accelerate tumor growth. It will also be important to study the effects of PKM2 modulation in relatively well-oxygenated tissues, such as the lung.
Another mechanism by which PKM2 inhibition by siRNA could potentially diminish tumor growth is by inhibiting other nonglycolytic functions of PKM2. Goldberg and Sharp (2012) posit that PKM2 inhibition by siRNA selectively induces caspase-dependent cell death, suggesting that PKM2 might regulate apoptosis through yet to be defined mechanisms. Interestingly, PKM2 has recently been shown to bind to and enhance the transcriptional activity of the hypoxia-inducible factor-1 (HIF-1; Luo et al., 2011). HIF-1 is a critical mediator of cellular adaptation to hypoxic stress through transcription of diverse targets including GLUTs and glycolytic enzymes such as HKII and LDH-A. It will be important to determine whether pharmacological inhibition of PKM2 can inhibit HIF-1 transcriptional activity or whether inhibition of PKM2 expression is required. Similarly, PKM2 has been show to associate with OCT4 and β-catenin, two transcription factors associated with tumor progression (Lee et al., 2008; Yang et al., 2011). Going forward, it will be important to decipher whether PKM2 inhibition or activation affects nonglycolytic functions of PKM2, and whether PKM2 interaction with nonglycolytic proteins is important for tumorigenesis.
Conclusions and future directions
The genetic revolution brought with it the discovery of genes and signaling pathways that promote or repress cellular transformation, and a genetic basis for understanding tumorigenesis. More recent work has focused on deciphering interactions between these proliferative pathways and cellular metabolic pathways. Currently, there are three major issues that need to be addressed to determine whether targeting glucose metabolism is a viable strategy for cancer therapy.
First, to date, the genetic or pharmacologic interventions performed have primarily been done using human cancer cells injected subcutaneously into nude mice. It will be important for future experiments to make use of genetically engineered mouse cancer models or orthotopic models with human cancer cells to assess whether glucose-dependent pathways are promising targets for cancer therapy.
Second, the toxic effects of inhibiting metabolic enzymes in normal cells need to be deciphered. Aside from cancer cells, immune cells and stem cells also display aerobic glycolysis. Unless antimetabolic therapies can distinguish between malignant and nonmalignant cells with self-renewal capacity, then therapeutically targeting metabolism could be complicated by the same types of toxicity that plague conventional cytotoxic chemotherapeutic regimens.
Third, cancer cells display metabolic plasticity. Thus, it is conceivable that cancer cells could develop resistance to inhibition of a particular metabolic pathway through expression of alternate isoforms or up-regulation of alternate pathways, such as gluconeogenesis. Adjacent cells such as adipocytes may also provide precursors for the biosynthetic needs of tumor cells (Nieman et al., 2011). Still, these are exciting times for the metabolism field as the links between gene expression, epigenetics, cell proliferation, differentiation, and metabolic pathways are being uncovered.
Acknowledgments
We thank Ralph Deberardinis for his helpful suggestions.
This work was supported by a National Institutes of Health Grant R01CA123067-05 to N.S. Chandel.
References
- Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S., et al. 2011. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 334:1278–1283 10.1126/science.1211485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluemlein K., Grüning N.M., Feichtinger R.G., Lehrach H., Kofler B., Ralser M. 2011. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D.A., Sutphin P.D., Nguyen P., Turcotte S., Lai E.W., Banh A., Reynolds G.E., Chi J.T., Wu J., Solow-Cordero D.E., et al. 2011. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 3:ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk H.R., Vander Heiden M.G., Harris M.H., Ramanathan A., Gerszten R.E., Wei R., Fleming M.D., Schreiber S.L., Cantley L.C. 2008a. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 452:230–233 10.1038/nature06734 [DOI] [PubMed] [Google Scholar]
- Christofk H.R., Vander Heiden M.G., Wu N., Asara J.M., Cantley L.C. 2008b. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 452:181–186 10.1038/nature06667 [DOI] [PubMed] [Google Scholar]
- Colombo S.L., Palacios-Callender M., Frakich N., De Leon J., Schmitt C.A., Boorn L., Davis N., Moncada S. 2010. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc. Natl. Acad. Sci. USA. 107:18868–18873 10.1073/pnas.1012362107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza C., Gottlieb E. 2009. Mitochondria in cancer: not just innocent bystanders. Semin. Cancer Biol. 19:4–11 10.1016/j.semcancer.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Gambhir S.S. 2002. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer. 2:683–693 10.1038/nrc882 [DOI] [PubMed] [Google Scholar]
- Goldberg M.S., Sharp P.A. 2012. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J. Exp. Med. 209:217–224 10.1084/jem.20111487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitosugi T., Kang S., Vander Heiden M.G., Chung T.W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G.Z., et al. 2009. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2:ra73 10.1126/scisignal.2000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae H.J., Chung J.W., Park H.S., Lee M.J., Lee K.C., Kim H.C., Yoon J.H., Chung H., Park J.H. 2009. The antitumor effect and hepatotoxicity of a hexokinase II inhibitor 3-bromopyruvate: in vivo investigation of intraarterial administration in a rabbit VX2 hepatoma model. Korean J. Radiol. 10:596–603 10.3348/kjr.2009.10.6.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. 2010. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA. 107:2037–2042 10.1073/pnas.0914433107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kim H.K., Han Y.M., Kim J. 2008. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int. J. Biochem. Cell Biol. 40:1043–1054 10.1016/j.biocel.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Locasale J.W., Cantley L.C. 2011. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 14:443–451 10.1016/j.cmet.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J., Heffron G., Metallo C.M., Muranen T., Sharfi H., et al. 2011. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43:869–874 10.1038/ng.890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt S.Y., Vander Heiden M.G. 2011. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27:441–464 10.1146/annurev-cellbio-092910-154237 [DOI] [PubMed] [Google Scholar]
- Luo W., Hu H., Chang R., Zhong J., Knabel M., O’Meally R., Cole R.N., Pandey A., Semenza G.L. 2011. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 145:732–744 10.1016/j.cell.2011.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Li D., Zhao D., Lin R., Chu Y., Zhang H., Zha Z., Liu Y., Li Z., Xu Y., et al. 2011. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell. 42:719–730 10.1016/j.molcel.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S., et al. 2011. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17:1498–1503 10.1038/nm.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer T., Schuster S., Bonhoeffer S. 2001. Cooperation and competition in the evolution of ATP-producing pathways. Science. 292:504–507 10.1126/science.1058079 [DOI] [PubMed] [Google Scholar]
- Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K., Sethumadhavan S., Woo H.K., Jang H.G., Jha A.K., et al. 2011. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 476:346–350 10.1038/nature10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L.J., Wice B.M., Kennell D. 1979. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 254:2669–2676 [PubMed] [Google Scholar]
- Robey R.B., Hay N. 2006. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 25:4683–4696 10.1038/sj.onc.1209595 [DOI] [PubMed] [Google Scholar]
- Shim H., Dolde C., Lewis B.C., Wu C.S., Dang G., Jungmann R.A., Dalla-Favera R., Dang C.V. 1997. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA. 94:6658–6663 10.1073/pnas.94.13.6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoden G.A., Rostek U., Lechner S., Mitterberger M., Mazurek S., Zwerschke W. 2009. Pyruvate kinase isoenzyme M2 is a glycolytic sensor differentially regulating cell proliferation, cell size and apoptotic cell death dependent on glucose supply. Exp. Cell Res. 315:2765–2774 10.1016/j.yexcr.2009.06.024 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Locasale J.W., Swanson K.D., Sharfi H., Heffron G.J., Amador-Noguez D., Christofk H.R., Wagner G., Rabinowitz J.D., Asara J.M., Cantley L.C. 2010. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 329:1492–1499 10.1126/science.1188015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Halper J.P., Knowles D.M. 1985. Alterations in the activity and isozymic profile of human phosphofructokinase during malignant transformation in vivo and in vitro: transformation- and progression-linked discriminants of malignancy. Cancer Res. 45:2993–3001 [PubMed] [Google Scholar]
- Warburg O., Posener K., Negelein E. 1924. Über den Stoffwechsel der Carcinomzelle. Biochem. Zeitschr. 152:309–344 [Google Scholar]
- Warburg O. 1956. On the origin of cancer cells. Science. 123:309–314 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Yang W., Xia Y., Ji H., Zheng Y., Liang J., Huang W., Gao X., Aldape K., Lu Z. 2011. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 480:118–122 10.1038/nature10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M., Lechago L.V., Somoano J.R., Mosharaf M., Lechago J. 1996. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 56:1164–1167 [PubMed] [Google Scholar]
- Zu X.L., Guppy M. 2004. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 313:459–465 10.1016/j.bbrc.2003.11.136 [DOI] [PubMed] [Google Scholar]