Abstract
Objectives
To establish the incidence of, and risk factors for, SCD in pediatric DCM.
Background
The incidence of SCD in children with DCM is unknown. The ability to predict patients at high risk for SCD will help define who may benefit most from ICDs.
Methods
The cohort was 1803 children in the PCMR diagnosed with DCM from 1990-2009. Cumulative incidence competing-risks event rates were estimated. We achieved risk stratification using CART methodology.
Results
Five-year incidence rates were 29% for heart transplant, 12.1% non-sudden cardiac death (non-SCD), 4.0% death from unknown cause, and 2.4% for SCD. Of 280 deaths, 35 were SCD and cause was unknown for 56. The 5-year rate for SCD incorporating a subset of the unknown deaths is 3%. Patients receiving anti-arrhythmic medication were at higher risk of SCD (hazard ratio 3.0, 95% CI 1.1-8.3, p =0.025). A risk stratification model based on most recent echocardiographic values had 86% sensitivity and 57% specificity. Thirty of 35 SCDs occurred in patients who met all of these criteria: LV end-systolic dimension z score > 2.6, age at diagnosis <14.3 years, and ratio of LVPWT:EDD <0.14. Sex, ethnicity, cause of DCM, and family history were not associated with SCD.
Conclusions
The 5-year incidence of SCD in children with DCM is 3%. A risk stratification rule (86% sensitivity) included diagnosis age < 14.3 years, LV dilation, and LV posterior wall thinning. Patients who consistently meet these criteria should be considered for ICD placement.
Keywords: death, sudden; cardiomyopathy; pediatrics; heart failure
INTRODUCTION
In adults, SCD accounts for substantial mortality in non-ischemic cardiomyopathy, with deaths from CHF and SCD occurring in near equal numbers (1). Large randomized trials have demonstrated a survival benefit with the use of ICDs in this population (2-4). AHA/ACC guidelines recommend ICD placement in adults with non-ischemic DCM who have a LV ejection fraction < 35% and are in NYHA class II or III (5).
The estimated annual incidence of DCM in children is 0.57 cases per 100,000, and the overall prognosis is poor, with 40% of children undergoing cardiac transplant or dying within 5 years after diagnosis (6). However, the incidence of SCD is low based on single-center reports, yet little information is available on risk factors for SCD (7,8). Hence, there are no established criteria for the use of ICDs for primary prevention of SCD in children with DCM.
We determined the incidence and risk factors for SCD using data in a large multicenter cohort of children with DCM. We analyzed the association of demographic, clinical, and echocardiographic characteristics with SCD. We sought to identify the characteristics of children who may benefit from ICD placement for primary prevention of SCD.
METHODS
Study Design
The PCMR has enrolled over 3,500 infants, children, and adolescents with cardiomyopathy < 18 years of age at diagnosis from nearly 100 pediatric cardiac centers in North America. Children were enrolled retrospectively if they were diagnosed with cardiomyopathy between 1990 and 1995 and prospectively thereafter (9,10). Annual reporting continues until death or heart transplantation.
All centers obtained Institutional Review Board approval for participation in the PCMR.
Study Sample
All enrolled patients with DCM in the PCMR met at least one of the following criteria (10):
Strict echocardiographic criteria for DCM: LV dilation (i.e., LV end-diastolic dimension ≥ 2 SD above normal for body-surface area) and depressed LV systolic function (LV fractional shortening or LVEF ≥ 2 SD below normal for age); or
Pathologic findings consistent with DCM at autopsy or by endomyocardial biopsy; or
Other clinical evidence of DCM provided by the cardiologist.
Children with specific secondary causes of myocardial abnormalities were excluded, including, but not limited to, associated congenital heart disease, endocrine disorders known to cause myocardial damage, a history of chemotherapy or of pharmacologic-associated cardiotoxicity, chronic arrhythmia, pulmonary parenchymal or vascular disease, and immunologic disease.
Sudden Cardiac Death Definition
SCD was defined as an unexpected death occurring < 1 hour after the onset of a symptomatic cardiac event. The circumstances of death were abstracted from the medical record. Three pediatric cardiologists (EP, CEC, SDC) reviewed the autopsy report where available and the abstracted notes for all deaths to ensure consistent classification. All deaths were classified as either SCD, cardiac death that was non-sudden (non-SCD), or unknown.
Measurements
Demographic information, clinical evidence of CHF, NYHA class, family history of cardiomyopathy, medication classes, and other therapies were recorded from the time of cardiomyopathy diagnosis and annually. Echocardiographic measurements were collected from the clinical study performed at the time of presentation and from the most recent clinical echocardiogram performed during each annual reporting period. These included LV end-diastolic dimension, LV end-systolic dimension, LV fractional shortening, LV septal and LV posterior wall thicknesses, LV mass, and the presence of tricuspid or mitral regurgitation. Information regarding the use of medications other than anti-congestive therapy, ICD implantation, valvar regurgitation grade, atrial enlargement, and electrocardiographic (ECG) and Holter findings were primarily collected on retrospectively enrolled patients. In addition, LVEF data collection was limited.
Statistical Methods
All data were analyzed by the Data Coordinating Center at the New England Research Institutes, Watertown, MA. Descriptive statistics include counts and percentages for categorical data, median and interquartile range for highly skewed data, and mean±standard deviation for normally distributed data. We used mean imputation for all echocardiographic values except LVEF that was missing in two-thirds of patients. Echocardiographic z-scores were calculated relative to body-surface area (LV end-diastolic dimension, LV end-systolic dimension, LV end-diastolic posterior wall and septal thicknesses, and M-mode-derived LV mass) or relative to age (LV fractional shortening and LVEF) (11). ECG and Holter data, available in less than one-third of subjects, were used in univariate analysis only without imputation.
The primary outcome was SCD. The cumulative incidence rates of SCD, non-SCD, unknown cause of death and transplant were estimated using competing risks methodology (12). The 56 deaths of unknown cause were excluded from risk factor analysis. Cox proportional hazards regression modeling was used to identify univariate risk factors for SCD. For risk factor modeling, the survival times of all children not experiencing SCD were censored at the date of last known date alive, non-SCD, or transplant. Candidate predictors included measures from the time of cardiomyopathy diagnosis as well as echocardiographic measurements from the latest available echocardiogram. We developed risk prediction models using recursive partitioning (CART), for the presence versus absence of SCD (13). We adopted this approach rather than multivariable regression modeling, where the patient subgroups required to construct interaction terms would require pre-specification. CART creates non-parametric discriminating trees by dividing patients repeatedly into subgroups, each representing subjects with a low versus high risk of SCD.
Alpha was set at 0.05 and all tests were two-tailed. Analyses were conducted using the Statistical Analysis System, Version 9.1 (SAS Institute Incorporated, Cary, NC), and R and S-PLUS 8.0 (Insightful Corporation, Seattle, WA).
RESULTS
As of February 2009, the PCMR included 1803 children diagnosed with DCM since 1990. Causes of DCM determined at presentation were: idiopathic disease (n = 1286), myocarditis (n = 255), neuromuscular disorder (n = 136), malformation syndrome (n = 10), familial isolated cardiomyopathy (n = 78), and inborn error of metabolism (n = 38). Mean age at diagnosis was 5.3±6.1 years. Mean LV end-diastolic dimension z-score was 4.3±2.7, LV fractional shortening was 16±9%, and LVEF (n = 597) was 28±14%. Median follow-up in patients with no death or transplant event was 2.6 years (interquartile range, 0.8 to 5.3 years; maximum, 16.7 years).
Event Rates
Of 280 deaths, the type of death was SCD in 35 (13%), non-SCD in 189 (68%), and unknown in 56 (20%). Thus, amongst patients with a known mode of death, 16% were SCD (35/224). Amongst the 1747 survivors and those with a known cause of death, SCD comprised 1.9%. The majority of SCDs, 74% (n = 26), occurred < 2 years after presentation. Most non-SCDs were caused by CHF.
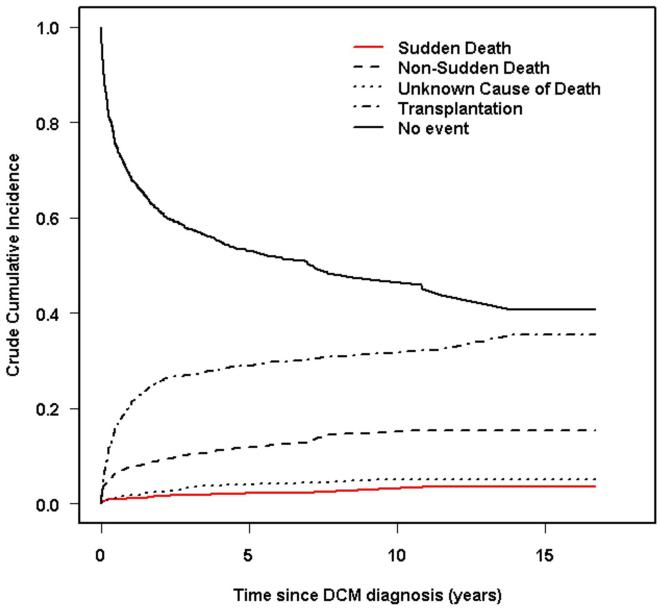
The incidence rate of SCD was low, and the rates of transplant and non-SCD were high. The 1-, 3-, and 5-year cumulative incidence rates were 1.3%, 2.0%, and 2.4% (95% CI 1.7% to 3.4%) for SCD. The 1-, 3-, and 5-year cumulative incidence rates for non-SCD were 8.1%, 10.8%, and 12.1%; and for heart transplant, 22%, 27%, and 29% for heart transplant (Figure 1). In addition, the 5-year incidence of death from unknown type (56 patients) was 4%. If the proportion of patients who had SCD in the group with an unknown cause of death is similar to that in the patients with a known cause of death (16%; 32/225), then we estimate that 9 additional patients died of SCD, and the 5-year cumulative incidence of SCD is 3.0%. This group of 56 patients is not included in risk factor analyses.
Figure 1.
Competing risks analysis for sudden cardiac death, non-sudden cardiac death, unknown cause of death and cardiac transplantation among 1803 children with dilated cardiomyopathy (DCM) listed in the Pediatric Cardiomyopathy Registry. The 3, 5, and 10-year cumulative incidence rates (95% CI) of sudden cardiac death are estimated to be 2.0% (1.4%-2.8%), 2.4% (1.7%-3.4%), and 2.7% (1.8%-3.9%); non-sudden cardiac death 10.8% (9.3%-12.4%), 12.1% (10.4%-13.9%) and 14.9% (12.6%-17.3%); heart transplant 27% (25%-29%), 29% (27% - 32%), and 31% (28% - 34%). The rate of death from unknown cause (95% CI) was 3.4% (2.6%-4.5%), 4.0% (3.0%-5.2%), and 5.2% (3.6%-7.3%).
Risk Factors for Sudden Cardiac Death: Predictors from the Time of Cardiomyopathy Diagnosis
SCD was not associated with sex, race/ethnicity, or cause of DCM (Table 1). Children who experienced SCD were more likely to present with CHF (86%) at diagnosis than children who did not have SCD (73%; HR, 2.84; p = 0.03). In the first month after diagnosis, 47% of the SCD group and 22% of all other children were on anti-arrhythmic therapy (HR = 3.00; 95% CI 1.08 to 8.30, p = 0.03). Family history of SCD, family history of cardiomyopathy, NYHA class, and use of anticongestive or beta-blocker therapy in the first month after diagnosis were not associated with SCD. Among the subset of 548 patients who had information on ICD therapy, only 9 had an ICD, and none experienced SCD.
Table 1. Patient Characteristics by Sudden Cardiac Death Status and Univariate Cox Regression Results.
| Sudden Cardiac Death |
All Others | Hazard Ratio (95% CI) |
p Value | |
|---|---|---|---|---|
| n | 35 | 1712 | ||
| Retrospective cohort | 28.6% | 25.3% | 1.13 (0.53, 2.42) | 0.752 |
| Age at diagnosis, years | 4.7±5.6 | 5.3±6.1 | 0.99 (0.94, 1.05) | 0.738 |
| Male | 54.3% | 53.5% | 1.05 (0.54, 2.05) | 0.880 |
| Race/Ethnicity | 0.649 | |||
| White | 62.9% | 55.3% | 2.42 (0.33, 17.93) | |
| Black | 22.9% | 21.0% | 2.55 (0.32, 20.37) | |
| Hispanic | 11.4% | 16.9% | 1.46 (0.16, 13.04) | |
| Other | 2.9% | 6.8% | -- | |
| Idiopathic | 77.1% | 71.6% | 1.55 (0.70, 3.41) | 0.279 |
| CHF at diagnosis | 85.7% | 72.6% | 2.84 (1.10, 7.35) | 0.031 |
| NYHA class IV | 0.139 | |||
| Yes | 34.3% | 23.8% | 1.79 (0.70, 4.56) | |
| No | 20.0% | 19.6% | Ref | |
| Unknown | 45.7% | 56.5% | 0.85 (0.35, 2.07) | |
| Family history of cardiomyopathy |
0.551 | |||
| Yes | 11.4% | 12.1% | 0.74 (0.25, 2.18) | |
| No | 51.4% | 44.9% | Ref | |
| Unknown | 37.1% | 43.0% | 0.68 (0.33, 1.39) | |
| Family history of sudden death |
0.905 | |||
| Yes | 5.7% | 6.0% | 0.86 (0.20, 3.70) | |
| No | 54.3% | 52.7% | Ref | |
| Unknown | 40.0% | 41.3% | 0.86 (0.43, 1.72) | |
| Anti-congestive therapy | ||||
| Yes | 88.6% | 82.2% | 1.60 (0.57, 4.55) | 0.375 |
| No | 11.4% | 14.3% | Ref | |
| Unknown | 0 | 3.5% | -- | |
| Anti-arrhythmic therapy | 0.025 | |||
| Yes | 20.0% | 12.1% | 3.00 (1.08, 8.30) | |
| No | 22.9% | 41.4% | Ref | |
| Unknown | 57.1% | 46.5% | 3.00 (1.31, 6.86) | |
| ACE inhibitor | 0.023 | |||
| Yes | 20.0% | 38.3% | 0.38 (0.14, 1.06) | |
| No | 22.9% | 18.5% | Ref | |
| Unknown | 57.1% | 43.2% | 1.30 (0.56, 2.98) | |
| Beta blocker | 0.090 | |||
| Yes | 2.9% | 7.0% | 0.30 (0.04, 2.27) | |
| No | 40.0% | 48.0% | Ref | |
| Unknown | 57.1% | 45.0% | 2.05 (1.02, 4.11) | |
|
Echocardiographic
Measurements at Cardiomyopathy Diagnosis |
||||
| LV end-diastolic dimension z-score |
4.2±2.3 | 4.3±2.4 | 1.01 (0.88, 1.16) | 0.928 |
| LV end-systolic dimension z-score |
5.9±2.2 | 6.0±2.5 | 1.01 (0.88, 1.16) | 0.883 |
| LV fractional shortening z- score |
-8.8±2.5 | -8.5±3.4 | 0.95 (0.85, 1.06) | 0.332 |
| LV end-diastolic posterior wall thickness z-score |
-1.1±2.8 | -0.5±2.0 | 0.86 (0.74, 1.00) | 0.047 |
| LV end-diastolic septal wall thickness z-score |
-1.1±1.1 | -0.8±1.5 | 0.84 (0.68, 1.05) | 0.123 |
| LV mass z-score | 2.0±2.7 | 2.3±2.8 | 0.98 (0.84, 1.14) | 0.766 |
| LV ejection fraction z- score* |
-6.9±2.5 | -6.0±2.4 | 0.76 (0.55, 1.05) | 0.094 |
| Raw LV ejection fraction, %* |
23.3±14.6 | 28.5±13.7 | 0.95 (0.90, 1.01) | 0.073 |
| LV ejection fraction < 35%* | 8 (80.0%) | 330 (67.8%) | 2.69 (0.57, 12.69) | 0.213 |
| Raw LV fractional shortening, % |
15.3±6.2 | 16.0±8.3 | 0.97 (0.93, 1.02) | 0.298 |
| LV fractional shortening < 18% |
27 (77.1%) | 1249 (73.0%) | 1.55 (0.70, 3.41) | 0.282 |
| Log ratio of LV posterior wall thickness:end-diastolic dimension |
-2.24±0.38 | -2.13±0.32 | 0.28 (0.10, 0.79) | 0.016 |
| Moderate-severe tricuspid regurgitation |
0.078 | |||
| Yes | 8.6% | 3.7% | 4.73 (1.13, 19.80) | |
| No | 14.3% | 26.3% | Ref | |
| Unknown | 77.1% | 69.9% | 2.46 (0.93, 6.51) | |
| Moderate-severe mitral regurgitation |
0.176 | |||
| Yes | 14.3% | 9.3% | 3.33 (0.89, 12.40) | |
| No | 11.4% | 20.9% | Ref | |
| Unknown | 74.3% | 69.9% | 2.39 (0.82, 6.95) |
Imputation was not used for LV ejection fraction. Raw LV ejection fraction n=10 for SCD group and 487 for all others. Left ventricular ejection fraction z-score n=10 for SCD group and 485 for all others. CHF = congestive heart failure; LV = left ventricular; CI = confidence interval; Ref = reference group for hazard
We examined echocardiographic parameters obtained at presentation (Table 1). Compared to patients without SCD, patients with SCD had a lower (log-transformed) LVPWT:EDD ratio, an index of ventricular remodeling that is a surrogate for LV end-diastolic wall stress, with a low ratio indicating insufficient LV hypertrophy (p = 0.02). A larger LV posterior wall thickness z-score was protective against SCD (HR = 0.86, p = 0.047). Fractional shortening z-score, was not a risk factor (p = 0.33).
We also examined ECG and Holter findings from the latest available follow-up. Of the 35 SCD cases, 13 had an ECG but only 5 had quantitative data, with a mean QTc interval of 449±72 msec. None of the 5 had any degree of AV block. One of the cases had a notation for wide complex tachycardia. These findings were qualitatively very similar to those in the 531 non-SCD patients who had QTc data (428±50 msec), with 5% (21/441) having AV block (none third-degree). Holter data were available for 5 of 35 SCD cases, with 3/5 (60%) having ventricular tachycardia and 60% having ventricular couplets. In the non-SCD group, 18% (52/294) had ventricular tachycardia and 25% (75/295) had ventricular couplets. No patients in either group had third-degree heart block or AV block. These limited data were not used in multivariable analysis.
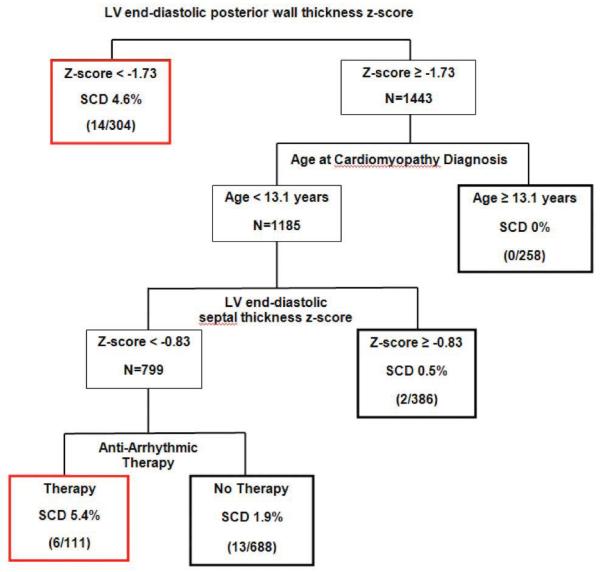
Multivariable CART analysis based on predictors from the time of DCM diagnosis demonstrated that the LV posterior wall thickness z-score, age at diagnosis and LV septal thickness z-score and antiarrhythmic therapy are the most important discriminators between SCD and non-SCD (Figure 2). Overall, 2% of subjects had SCD. Two of the five subgroups in the regression tree have at least twice the risk of SCD (e.g., > 4 %). These include:
Patients with LV posterior wall thickness z-score < −1.7; and
Patients with LV posterior wall thickness z-score ≥ −1.7, age at diagnosis < 13.1 years, septal thickness z-score < −0.8 and who were prescribed antiarrhythmic therapy within a month of presentation with DCM.
This model classified 24% of patients as high-risk, i.e., 20/35 deaths occurred in these groups, yielding 57% sensitivity and 78% specificity. Due to the very low prevalence of SCD, positive predictive value (% SCD among those identified as high risk) was only 5%, while negative predictive value (% non-SCD among those identified as lower risk) was 99%.
Figure 2.
Classification and Regression Tree for Sudden Cardiac Death (SCD): Predictors from Time of Cardiomyopathy Diagnosis (Total N=1747, 35 sudden cardiac deaths). Red boxes denote high-risk patient groups. Bold black boxes denote lower-risk patient subgroups. Sensitivity=57%, specificity=78%. False positive rate=95%, false negative rate=1.1%.
Risk Factors for Sudden Cardiac Death: Predictors from Last Available Follow-up
Since treatment decisions are made based on the most current status of the patient, we also examined the predictive strength of the latest available measures of LV size and function. Over three-quarters (78%) of subjects had at least one follow-up measurement (range, 1 to 17). For the remainder, their value from the time of diagnosis was used.
Univariate analysis (Table 2) shows that a higher hazard of SCD was significantly associated with all echocardiographic parameters except for LV posterior wall and septal thicknesses. For LV end-diastolic dimension and mass, a unit increase in z-score was associated with a 1.2- to 1.3-fold increase in risk. Similarly, a 5-unit decrease in LV fractional shortening (%) or LVEF (%) imparted a 1.4- to 1.5-fold increase in risk of SCD. The LVPWT:EDD ratio was again highly predictive (p < 0.001). At least moderate mitral (3% of patients) or tricuspid (8% of patients) regurgitation was also associated with SCD.
Table 2. Patient Characteristics by Sudden Cardiac Death Status using Measurements from The Last Follow-up and Univariate Cox Regression Results.
| Sudden Cardiac Death |
All Others | Hazard Ratio (95% CI) |
p value | |
|---|---|---|---|---|
| CHF | 77.1% | 50.6% | 7.89 (3.47, 17.96) | 0.002 |
|
Echocardiographic
Measurements |
||||
| LV end-diastolic dimension z-score |
4.8±2.6 | 3.3±2.8 | 1.31 (1.17, 1.48) | <0.001 |
| LV end-systolic dimension z-score |
6.3±2.4 | 4.4±3.5 | 1.30 (1.17, 1.44) | <0.001 |
| LV fractional shortening z-score |
−8.2±3.5 | −5.4±4.8 | 0.78 (0.71, 0.86) | <0.001 |
| LV end-diastolic posterior wall thickness z-score |
−0.6±2.2 | −0.7±2.1 | 1.05 (0.91, 1.23) | 0.494 |
| LV end-diastolic septal thickness z- score |
−1.1±1.1 | −0.9±1.7 | 0.94 (0.76, 1.15) | 0.532 |
| LV mass z-score | 2.6±2.3 | 1.6±2.4 | 1.21 (1.10, 1.32) | <0.001 |
| LV ejection fraction z-score* |
−6.3±1.6 | −5.1±2.4 | 0.69 (0.57, 0.83) | <0.001 |
| Raw LV ejection fraction, %* |
27.0±8.8 | 33.7±14.1 | 0.94 (0.91, 0.97) | <0.001 |
| LV ejection fraction < 35%* |
11 (73.3%) |
336 (42.6%) |
8.67 (2.71, 27.72) | <0.001 |
| Raw LV fractional shortening, % |
16.2±8.5 | 22.1±12.0 | 0.92 (0.88, 0.95) | <0.001 |
| LV fractional shortening < 18% |
25 (71.4%) |
789 (46.1%) |
5.63 (2.64, 11.97) | <0.001 |
| Log (ratio of LV posterior wall thickness:end- diastolic dimension) |
−2.2±0.30 | −2.1±0.3 | 0.19 (0.08, 0.46) | <0.001 |
| Moderate-severe tricuspid regurgitation | 0.001 | |||
| Yes | 17.1% | 7.9% | 9.86 (2.76, 18.94) | |
| No | 11.4% | 33.1% | Ref | |
| Unknown | 71.4% | 58.9% | 6.26 (2.07, 18.94) | |
| Moderate-severe mitral regurgitation |
0.005 | |||
| Yes | 5.7% | 2.8% | 6.44 (1.32, 31.29) | |
| No | 20.0% | 38.3% | Ref | |
| Unknown | 74.3% | 59.0% | 4.09 (1.66, 10.07) |
Raw LV ejection fraction n = 15 for SCD group and 789 for all others. Left ventricular ejection fraction z-score n = 15 for SCD group and 787 for all others.
CHF = congestive heart failure; LV = left ventricular; Ref = reference group for hazard ratio.
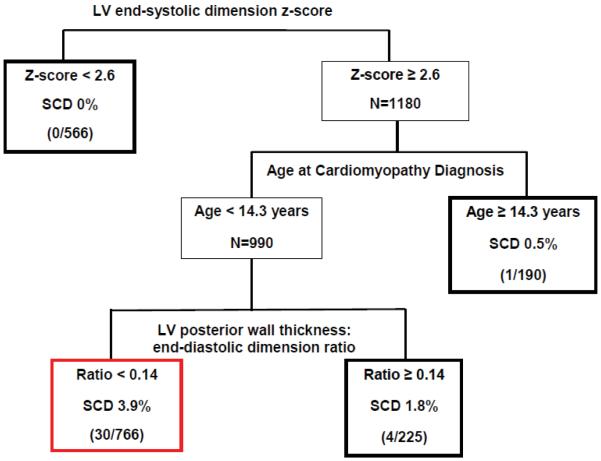
Multivariable CART analysis that considered echocardiographic measurements from the most recent follow-up (except for LVEF, see Methods) in addition to age and presence of CHF at diagnosis demonstrated that LV end-systolic dimension z-score, age at diagnosis and ratio of LVPWT:EDD are the most important discriminators between SCD and non-SCD (Figure 3). The tree had four terminal nodes. Three nodes had a below-average rate of SCD (0% to 1.8%). A single node captured 30 of the 35 SCD. This subgroup (44% of patients) with the highest SCD rate (3.9%, 30/766) met all three of the following criteria: 1) LV end-systolic dimension z-score > 2.6; 2) DCM diagnosis at age < 14.3 years, and 3) LVPWT:EDD ratio < 0.14. This patient subset produced high sensitivity of 30/35 = 86%, and specificity of 981/1712 = 57%; albeit with a positive predictive value of 4% and a negative predictive value of 99%.
Figure 3.
Classification and Regression Tree for Sudden Cardiac Death (SCD): Predictors from Time of Last Follow-up (Total N=1747, 35 sudden cardiac deaths). Red box denotes the high-risk patient group. Bold black boxes denote lower-risk patient subgroups. Sensitivity=86%, Specificity=57%. False positive rate=96%, false negative rate=0.6%.
Despite LVEF from follow-up being available for only 46% of patients, we examined the predictive strength of a commonly used threshold, LVEF < 35%, and assessed its validity by comparing it to the equivalent value of LV fractional shortening < 18%. The results for these two thresholds were similar (Table 2). If LVEF < 35% is used as an indication for ICD placement, sensitivity is only 73% (compared to the regression tree result in Figure 3 with 86% sensitivity). Specificity using LVEF < 35% was the same as that yielded by the regression tree (57%).
DISCUSSION
Incidence of Sudden Cardiac Death
Our first goal was to determine the incidence of SCD in a large cohort of well-characterized children with DCM. We found that the 5-year cumulative incidence of known SCD in patients with DCM is 2.4%. If the proportion of SCD in the patients who died of unknown causes was similar to the proportion in the patients who died of known causes, the estimated 5-year incidence rate of SCD is 3%. In a series of 85 children with DCM (mean LVEF 25%), only one child (1%) died suddenly (7). In Rhee et al.’s multicenter study of SCD in 2392 children with DCM and congenital heart disease listed for transplant, the incidence of SCD was low (1.3%), and only those patients with ischemic cardiomyopathy had an increased risk of SCD (relative risk, 6.92) (14).
The incidence of SCD is well below the rates reported in adults with DCM (15). In the DEFINITE trial, SCD occurred in 7.4% of 458 adults with DCM (with LVEF < 36%); the mortality rate at 2 years was 14.1% in the standard therapy group (annual mortality of 7%), compared to 7.9 % in the ICD group (2). Thus, in children with DCM, in contrast to adults, SCD is rare, and death from progressive CHF is more common (7,15,16). The reason for this lower incidence in SCD is unclear, but may be due to several factors. Children have fewer ventricular arrhythmias documented by ambulatory Holter monitoring than do adults with idiopathic DCM (1-3,5,7,8). Fibrosis has been shown to correlate with ventricular arrhythmias and SCD (17), and children with DCM may have less fibrosis than adults with DCM due to age-related factors as well as age-dependent co-morbidities such as diabetes and hypertension. These comorbities in adults lead to diastolic dysfunction that may have additive effects that lead to more ventricular arrhythmias in adults with DCM, thus DCM in children may have a different natural history than that in adults. Our study has shown that deaths in children with DCM are typically from CHF, or graft loss occurring from transplantation, rather than SCD, suggesting a different pathophysiology than in adults. More research with biomarkers and genotype-phenotype correlations will be necessary to better define the natural history which differentiates DCM presenting in children from that observed in adults.
Risk Factors for Sudden Cardiac Death: Factors Known at Diagnosis
Our second goal was to develop a risk stratification rule to identify children at high-risk for SCD, to aid clinicians in identifying who may benefit from ICD placement. Sex, race/ethnicity, cause of DCM, family history and LV fractional shortening were not independent risk factors. Our multivariable CART analysis showed that 24% of children fell into a high-risk group based solely on factors known at presentation. One high-risk group was defined solely by a thin-walled LV at presentation (LV posterior wall thickness z-score < −1.7), and the other comprised patients with LV wall thickness above this cutoff, but who were diagnosed before 13.1 years, had a thin LV septum and who were prescribed anti-arrhythmic therapy in the month that DCM was diagnosed. These findings stress the value of intraventricular septal thickness and LV posterior wall thickness observed early in the course of DCM in children. Basing ICD implantation decisions on these criteria captured over half of patients who went on to have SCD.
The use of antiarrhythmic medications within 30 days of presentation was a risk factor for SCD in the CART analysis tree as well as in the univariate analysis. The type of anti-arrhythmic agent was not collected for most subjects. Arrhythmia in adults with poor LV function is a risk factor for SCD and has been used as an indication for placing ICDs, and children with a history of sustained ventricular tachycardia or ventricular fibrillation are more likely to undergo ICD placement. In our study, arrhythmia data were not consistently collected and 24-hour Holter data were not routinely available, so these could not be examined as risk factors. A single-center study of 63 children with DCM found 46% had arrhythmias; the majority were atrial, and only 6 had ventricular tachycardia (8). Death occurred in 4 of the 29 children with known arrhythmias; however, only 1 of these died suddenly.
Of note, moderate to severe tricuspid and mitral insufficiency at latest follow-up were more frequently identifiable in the SCD cohort in the univariate analysis. Tricuspid regurgitation typically correlates with more severe CHF and is likely a surrogate for pulmonary hypertension (18).
Risk Factors for Sudden Cardiac Death: Last Available Follow-up
With the use of clinical information from the last available follow-up, we constructed a risk stratification model with high sensitivity (86%) and moderate specificity (57%). A single node of the CART captured 30 of the 35 SCDs. These patients had an elevated systolic ventricular size (LV end-systolic dimension z-score > 2.6) indicating abnormal dilation, were less than 14 years old at diagnosis, and had a diminished LVPWT:EDD ratio (< 0.14). This prediction rule is easy to use and based on measurements performed (or calculable) from a routine echocardiogram. A lower LVPWT:EDD ratio was also found in univariate analyses to be associated with the composite endpoint of all-cause mortality and transplant in the PCMR DCM cohort (6). We hypothesize that patients with a low LVPWT:EDD ratio may have an insufficient LV hypertrophic response to compensate for the LV dilation caused by the cardiomyopathy. Monitoring of disease progression and associated changes in risk for SCD is recommended, as a patient’s echocardiographic measurements of LV size may worsen or improve over time.
This risk stratification model may be used independently, or in conjunction with the first “at diagnosis” tree, to identify children with DCM at higher risk for SCD for whom increased monitoring is appropriate. If the first tree (rule) is used, then 24% of children with DCM would receive an ICD at the time of diagnosis of cardiomyopathy. With the use of the second tree, eventually 44% of subjects might receive an ICD, with only 4% of those implanted truly at risk of an event—that is, 26 patients would undergo ICD implantation to prevent one event of SCD. Considerable tradeoffs therefore continue to exist with respect to ICD placement, but our estimated low incidence of SCD combined with the ability to moderately discriminate risk levels suggests that universal ICD implantation in the pediatric DCM population is probably not warranted.
Whether or not ICDs could be beneficial in higher-risk children is unclear. In a review of ICD databases at nine heart transplant centers, Dubin et al. reported that 28 patients received ICDs while awaiting transplantation (16 had DCM). Of these, 42% had an appropriate discharge; however, the incidence of inappropriate ICD discharge was 25% (19). The model presented here provides evidence that ICD implantation is not indicated for all children presenting with DCM, and demonstrates that those at highest risk can be identified. However, the successful identification of the children at highest risk of SCD, a relatively rare event, lacks specificity, with 26 implants required to prevent one SCD. Therefore, recommendations for ICD placement in children must be considered in conjunction with the concomitant anxiety related to inappropriate shocks, as well as an increased likelihood of complications such as lead fractures and the need for frequent lead and defibrillator replacement, and body size issues that necessitate epicardial rather than transvenous systems in smaller children (20). Clinical decision-making is a continuous process, and utilizes cumulative evidence regarding a patient’s condition that is garnered across multiple follow-ups. If a child who was diagnosed prior to age 14 years consistently meets all of the criteria that indicate a high risk of SCD (abnormal LV ESD z-score and LVPWT:EDD ratio <0.14), ICD implantation should be considered. Definitive criteria for ICD use in children must await clear evidence of improved survival based on these criteria.
Limitations
This analysis has some limitations. First, 56 of 280 deaths did not have an identifiable cause of death. Although some of these deaths may have been sudden rather than due to CHF, we hypothesize that for most it is a random sample of deaths with missing information. Therefore, we reported a secondary estimate of SCD incidence that incorporated 16% of the deaths with unknown cause into the event rate estimate. The estimated 5-year rate was still only 3%.
Another limitation is the lack of information about the incidence of ventricular arrhythmias in this population. Ventricular arrhythmias are a risk factor for SCD in adults with DCM. However, only 9 patients out of 538 with therapy data available had undergone an ICD placement; thus, it is unlikely that ventricular arrhythmias were common in this population. Furthermore, unlike in adult patients, invasive electrophysiologic stimulation is not performed routinely in children to assess risk for sustained monomoprhic ventricular tachycardia. Therefore, it is possible that the components of the risk stratification model would differ if this information were available.
Similarly, electrocardiogram and Holter data were not included in our multivariable analyses, as they were collected on <15% of the SCD cases and less than one-third of the patients without SCD. Some of the missing data are due only to the timing of the data collection protocol and thus are possibly missing at random; however, other causes for not undergoing an ECG or Holter that may render those with data to be a non-representative subset cannot be dismissed.
We chose to use CART methodology, due in part to the visual appeal of the classification tree and ease of interpretation and application for clinicians. However, we note as a limitation that CART has been shown to have similar and not necessarily superior predictive power relative to other risk stratification methods, such as logistic regression and machine learning approaches (21). If our data were analyzed using a different approach, it is possible that a different set of discriminatory factors might be identified. The final limitation is that the risk stratification models presented have not been validated using an independent dataset. Application of these models to other DCM cohorts will provide needed evidence of its accuracy and reliability.
CONCLUSIONS
The 5-year incidence of SCD in children with DCM does not exceed 3%, a rate much lower than in adults. Independent predictors of SCD include echocardiographic features of both LV thinning and dilation, the LVPWT:EDD ratio, use of antiarrhythmic therapy within one month of diagnosis, as well as age at diagnosis before ages 13 to 14 years. SCD can be predicted with 86% sensitivity, although lower specificity (57%), and requires 26 implants to prevent one SCD. Our data support the concept that universal implantation of ICDs is probably not warranted. However, risk stratification is possible and is strengthened by a patient’s condition meeting all of the high-risk criteria for an extended period. In such situations, ICD placement should be considered for pediatric patients with DCM.
Acknowledgments
Grant support: Supported by grants from the National Heart, Lung, and Blood Institute HL53392 and the Children’s Cardiomyopathy Foundation. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI.
Abbreviations and Acronyms
- CART
Classification and Regression Tree
- CHF
congestive heart failure
- DCM
dilated cardiomyopathy
- ICD
internal cardiac defibrillator
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVPWT:EDD
left ventricular posterior wall thickness to end-diastolic dimension ratio
- NYHA
New York Heart Association
- PCMR
Pediatric Cardiomyopathy Registry
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures.
Clinical Trials Registration # NCT00005391
REFERENCES
- 1.Johnson RA, Palacious I. Dilated cardiomyopathy of the adult. N Engl J Med. 1996;334:493–9. [Google Scholar]
- 2.Kadish A, Dyer A, Daubert JP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 3.Bänsch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: the Cardiomyopathy Trial (CAT) Circulation. 2002;105:1453–8. doi: 10.1161/01.cir.0000012350.99718.ad. [DOI] [PubMed] [Google Scholar]
- 4.Bardy G, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, et al. American Association for Thoracic Surgery, Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 7.Dimas VV, Denfield SW, Friedman RA, et al. Frequency of cardiac death in children with idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;104:1574–7. doi: 10.1016/j.amjcard.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Friedman RA, Moak JP, Garson A., Jr. Clinical course of idiopathic dilated cardiomyopathy in children. J Am Coll Cardiol. 1991;18:152–6. doi: 10.1016/s0735-1097(10)80233-5. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 10.Grenier M, Osganian SK, Cox GH, et al. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139(2 Pt 1):S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 11.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 12.Tai BC, Machin D, White I, et al. Competing risks analysis of patients with osteosarcoma: a comparison of four different approaches. Stat Med. 2001;20:661–84. doi: 10.1002/sim.711. [DOI] [PubMed] [Google Scholar]
- 13.Breiman L. Classification and Regression Trees. Wadsworth International Group; Belmont, CA: 1984. [Google Scholar]
- 14.Rhee EK, Canter CE, Basile S, et al. Sudden death prior to pediatric heart transplantation: would implantable defibrillators improve outcome? J Heart Lung Transplant. 2007;26:447–52. doi: 10.1016/j.healun.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Shekha K, Ghosh J, Thekkoott D, et al. Risk stratification for sudden cardiac death in patients with non-ischemic dilated cardiomyopathy. Indian Pacing and Electrophysiol J. 2005;5:122–38. [PMC free article] [PubMed] [Google Scholar]
- 16.Kirk R, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung transplantation: twelfth official pediatric heart transplantation report-2009. J Heart Lung Transplant. 2009;28:993–1006. doi: 10.1016/j.healun.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Assomul RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Lanzarini L, Fontana A, Lucca E, et al. Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am Heart J. 2002;144:1087–94. doi: 10.1067/mhj.2002.126350. [DOI] [PubMed] [Google Scholar]
- 19.Dubin AM, Berul CI, Bevilacqua LM, et al. The use of implantable cardioverter defibrillators in pediatric patients awaiting heart transplantation. J Card Fail. 2003;9:375–9. doi: 10.1054/s1071-9164(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 20.Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;17:1685–91. doi: 10.1016/j.jacc.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Columbet I, Ruelland A, Chatellier G, Gueyffier F, Degoulet P, Jaulent MC. Models to predict cardiovascular risk: comparison of CART, multilayer perceptron and logistic regression. Proc AMIA Symp. 2000:156–160. [PMC free article] [PubMed] [Google Scholar]