Abstract
Background
Age of onset to begin drinking is a known risk factor for alcohol dependence. Factors have been identified that contribute to age of onset to begin regular drinking. These include reduced P300, increased postural sway, and personality variation. A longitudinal study spanning childhood to young adulthood provided the opportunity to determine if these same factors would predict the presence and onset of substance use disorders (SUD).
Methods
Multiplex families were identified through two or more alcohol-dependent brothers. Offspring from these multiplex or control families (n = 133) were followed annually during childhood. Using childhood predictors previously identified as risk factors for age of onset to begin drinking, SUD outcome by young adulthood was modeled.
Results
Familial risk status was a significant predictor of young adult SUD outcome as a main effect and as an interaction with P300 amplitude recorded before the age of 13. In adolescence (age 15), increased postural sway and familial risk predicted the SUD outcome by age 22. Analysis comparing the presence of one or both risk factors showed that those above the median for sway and below the median for P300 amplitude had substantially increased odds of developing SUD (odds ratio = 8.08 [confidence interval = 1.52– 42.83]).
Conclusions
Our findings indicate that among the factors predicting age of onset to begin regular drinking, P300 predicts SUD outcome across an 11-year span. The present findings provide the longest follow-up to date demonstrating that neurobiological factors in childhood are among the most salient predictors of young adult SUD outcome.
Keywords: Adolescents, alcohol dependence, children, genetic factors, high-risk, multiplex, P300, postural sway
Twin, adoption, and family studies have provided ample evidence for genetic mediation of alcohol dependence (AD) susceptibility within families (1–3). Twin studies tend to show greater concordance for AD in monozygotic (MZ) than in dizygotic (DZ) twins (4,5), providing estimates of heritability in the range of .54 to .58 in male subjects (6). Genetic mediation of risk for developing nicotine dependence (7) and cannabis abuse and dependence (8) as well as other psychoactive drugs has also been demonstrated (9,10). The search for genes that may confer increased susceptibility to alcohol dependence has included a number of genes. While progress is being made in identifying such genes (11–13), this endeavor can be accelerated with further identification of childhood risk factors for substance use disorder (SUD) outcome that can be expected to provide endophenotypic information.
Age at Onset to Begin Drinking
A number of clues regarding possible childhood predictors of young adulthood SUD can be obtained by examining predictors of age at onset to begin drinking. Several studies have shown a relationship between age of onset to begin drinking and alcohol dependence outcome. Individuals who begin drinking before the age of 15 are more likely to develop alcohol dependence than those starting after age 20 (14). Other studies suggest that age of onset to begin drinking is familial and heritable in male subjects (15). Offspring from multiplex families who have a higher density of AD (four first- and second-degree relatives on average) have significantly earlier onset to begin drinking regularly than do youngsters from control families (16,17). Analyses of childhood psychopathology in these offspring from multiplex families show increased rates of conduct disorder (CD) and oppositional defiant disorder (ODD) (18).
Age at onset to begin drinking may be symptomatic of an underlying disinhibitory trait because the presence of CD and ODD at age 11 predicts use of alcohol by age 14 (19). P300 amplitude may also represent an indicator of disinhibition (20).
P300, Disinhibition, and Risk for AD
Several early studies established the association between familial risk for alcohol dependence in offspring from alcoholic families and reduced amplitude of the P300 component of the event-related potential (ERP) (21,22), findings also seen in minor offspring from our multiplex for AD families (23,24). P300 is heritable (25–28), with recent biometrical modeling showing substantial influence of genes (29). A meta-analysis based on twin studies with measures of P300 amplitude shows a heritability of 60% (30), further suggesting the likelihood that P300 amplitude is transmitted across generations. How the transmission of reduced P300 amplitude across generations becomes translated into increased risk for AD is currently unknown, though several investigations have addressed possible candidate genes (31,32).
Neurodevelopmental Delays in Motor Performance and Risk for AD
Early observations (33–36) showed that relatives of alcohol-dependent persons exhibit more body sway in a controlled experimental setting. Because postural control becomes better as children become older (37), greater sway for age suggests a developmental delay. Children with multiplex AD history sway more at a given age than control children (38). Currently, it is unclear whether motor delays of childhood are generalized predictors of adult psychiatric outcome or, alternatively, reflect specific diatheses for AD. Comparison of juvenile-versus adult-onset major depressive disorder (MDD) in individuals from a representative birth cohort tracked from childhood to adulthood showed an association between juvenile-onset MDD and delayed motor development (39). Acquisition of motor control is often considered to be part of prefrontal lobe executive cognitive functioning (ECF), so that motor delays of childhood may represent a generalized ECF deficit (40).
Previously, we reported finding that postural sway, P300 amplitude, and the personality trait of extraversion are among the most salient predictors (16,17) of age of onset for regular drinking in multiplex offspring. The present report assesses these variables as predictors of young adult SUD outcome.
Methods and Materials
A total of 225 children between the ages of 8 and 18 years who were either at high or low risk for developing AD have been evaluated at approximately annual intervals (mean = 5.6 assessments) as part of our Longitudinal Cohort Initiative from our family study of relatives of alcoholics (Cognitive and Personality Factors Family Study). Of the 225 subjects tested with repeated assessments during childhood, 133 are being followed during young adulthood (ages 19 to 29 years); the others are maturing into this initiative. Presence or absence of a substance use disorder during young adulthood was determined for these 133 individuals based on repeated evaluations during the young adult period (an average of 2.3 biannual visits).
Demographic characteristics of the 225 youngsters from the Longitudinal Cohort Initiative and the 133 youngsters for whom young adult assessments were completed can be seen in Table 1. A subset of individuals (n = 82) had one or more follow-up visits with a full set of predictor variables collected between the ages of 8 and 13 years; another (n = 127) had one or more visits with predictor variables collected between 14 and 18 years. Some (n = 76) had visits in both developmental periods.
Table 1.
Demographic Characteristics of Samples Used in the Analyses
| Longitudinal Cohort (n = 225) (1117 Visits) | Longitudinal Cohort Annual Follow-up with Young Adult Follow-up (n = 133)a (780 Visits) | Individuals Assessed at Least Once Between Ages 8 and 13 with Young Adult Follow-up (n = 82)a,b,c (626 Visits) | Individuals Assessed at Least Once Between Ages 14 and 18 with Young Adult Follow-up (n = 127)a,b(768 Visits) | |
|---|---|---|---|---|
| Male/Female | 114/111 | 65/68 | 41/41 | 64/63 |
| Age First Assessed | ||||
| Mean (SD) | 12.0 (2.6) | 12.4 (2.7) | 10.6 (1.6) | 10.58 (2.9) |
| Age at Last Evaluation | ||||
| Mean (SD) | 19.7 (5.1) | 23.2 (2.9) | 22.5 (2.0) | 23.3 (2.9) |
| SESd | ||||
| Mean (SD) | 43.1 (10.9) | 41.4 (11.2) | 42.2 (11.8) | 41.3 (11.3) |
| High-Risk/Low-Risk | 124/101 | 78/55 | 50/32 | 75/52 |
SES, socioeconomic status.
The Cox model analyses were based on data from these individuals.
Note some offspring were seen in both developmental periods (n = 76).
Within this group were 70 individuals with P300 collected at age 9 and sway assessment completed at age 15. This group provided data for one of the “no hit” versus “two hit” model analyses. A confirmatory analysis of the no hit versus two hit analysis was performed with an enlarged sample of 86 individuals with P300 data collected between the ages of 8 and 13 years and postural sway completed at age 15. The additional 16 cases were available from a previous study involving both P300 and sway measures.
Socioeconomic status (SES) was determined using the Hollingshead method (A.B. Holllingshead, Four Factor Index of Social Status, unpublished manuscript, 1975), which uses a combination of occupational status and education to determine an SES score. The SES of parents was used to determine the SES of offspring.
High-Risk Multiplex Families
The high-risk families were selected through a pair of alcohol-dependent brothers, an ascertainment scheme that results in multiplex AD families. Families were excluded if recurrent major depression, bipolar disorder, or schizophrenia disorders were present in the proband pair or their first-degree relatives. Additionally, AD must have been diagnosed as occurring at least 1 year before other drug dependence. All proband pairs and their living first-degree relatives (parents and siblings) were interviewed using the Diagnostic Interview Schedule (DIS) (41). Using the DIS, diagnoses of AD and alcohol abuse by DSM-III and DSM-III-R criteria (42,43) were made. In addition, presence or absence of alcoholism by Feighner Criteria (44) was determined. Using the DIS information, a second clinician’s information, and family history reports of all other participating relatives, a best estimate diagnosis was determined. Because a multiplex sampling design was used, the offspring from the proband generation who are being followed as part of the longitudinal effort have an average of four first- and second-degree relatives with AD.
Inclusion and Exclusion Criteria for Low-Risk Families
Control families were selected on the basis of an adult family member volunteering to participate in the study and the family having the same structural characteristics of the high-risk families (two adult brothers). Multiple family members (proband siblings and their parents) were interviewed in person using the DIS to screen for the absence of alcohol or drug dependence, schizophrenia, recurrent major depressive disorder, and bipolar disorder in all first- and second-degree relatives of the adult index case. Low-risk offspring also had diagnostic data for their mothers and her first-degree relatives allowing for determination that offspring came from bilineal low risk for alcoholism pedigrees.
Longitudinal Childhood Evaluation
Because the study design used all available offspring between the ages of 8 and 18 years from the high- and low-risk pedigrees, children entered the childhood evaluation period at differing ages. At each annual evaluation, the children completed a battery of age-appropriate tests that were administered by trained master’s-level clinicians. This included the Child Manifest Anxiety Scale (45), the Coopersmith Self-Esteem Inventory (46), and the Wide Range Achievement Test-Revised (WRAT-R) (47) or Wide Range Achievement Test-Third Edition (WRAT-III) (48) math, reading, and spelling standard scores. The youth form of the Life Stressors and Social Resources Inventory (LISRES) (49) (positive and negative events) was administered to all children who had reached age 13. Additionally, postural sway (Lipscomb and right monopedal stances) and P300 (visual and auditory) were collected at each evaluation and entered into the analysis. At the child’s first visit, the Junior Eysenck Personality Inventory (50) was administered to provide measures of extraversion and neuroticism.
Assessment of Postural Sway
The children were asked to stand on a movement platform (Kistler-Model 9281 B, Kistler, Winterthur, Switzerland) while the output data of amplifiers at each corner recorded changes in pressure throughout the platform, which was digitized and stored at 18 Hz. A total of six trials (three with eyes open and three with eyes closed and blindfolded) in each of two procedures, a bipedal and a monopedal stance previously described (38), were collected. In the monopedal stance, the child was asked to keep the right foot raised while he/she stood on their left foot. A 30-sec intertrial interval and a 1-min interval between tasks were provided in which the child was allowed to get off the platform. Scores obtained from the eyes closed condition were modeled in the present analysis.
Event-Related Potential Assessments
To provide exactly the same recording conditions throughout the longitudinal study, a PDP-11/23 was continuously maintained for stimulus presentation. Electrophysiological data were amplified by 20 k using a Grass Model 12 Neurodata system set to a bandpass of .01 Hz to 30 Hz. Each trial was sampled for 1200 msec at 8-msec intervals beginning with the 200-msec prestimulus baseline. For the visual task, the PDP-11/23 was slaved to coincide with an Atari 130 computer. Each child performed an auditory (choice reaction time) and a visual ERP task with electrodes placed at frontal, vertex, parietal, and occipital locations (Fz, Cz, Pz, Oz, P3, P4). All the active electrodes were referred to linked ears with a forehead ground. Eye blinks were tracked online using an oscilloscope and all trials affected by eye artifact (blinks or eye movements greater than 50 μV) were excluded online. Only the artifact-free trials were averaged according to condition offline.
Auditory ERPs were elicited with high (1500 Hz) and low pitched (800 Hz) tones, presented every 3 sec (70 dBA intensity; 40-msec duration) in a modified oddball paradigm as previously described (23,51,52). The visual task consisted of presentation of a brief (33 msec) target (stick figure head with a nose and only one ear oriented with nose upward or nose downward) or a nontarget (circle) stimulus (modeled after Begleiter et al. 21 [ ]). The subject responded to the position of the ear (left or right) with a button press (left or right). Targets occurred on 80 of the 240 trials presented.
Young Adult Evaluation
Offspring who had completed the childhood portion of the study (had reached 19 years of age) were invited to participate in a young adult initiative. Each young adult was administered the Composite International Diagnostic Interview (CIDI) during each biannual evaluation (53). Substance use disorder in this report is defined as any alcohol abuse/dependence or drug abuse/dependence diagnosed at any evaluation using DSM-IV criteria.
Statistical Analyses
Statistical analyses were planned to investigate the relationship between a set of predictors measured in childhood during two developmental periods (8 to 13 years and 14 to 18 years) and their impact on young adulthood outcome. Outcome was defined by the presence or absence of a SUD diagnosed during any young adult assessment through use of survival curves.
Factor Analysis
Factor analysis was used to reduce the number of variables to be modeled. Five orthogonal factors were obtained by varimax rotation of the principal factors analysis. Factor scores were then created to represent the 13 neurobehavioral variables collected in childhood. To best represent the cross-sectional structure for each age, covariances were obtained for subjects having the same age and then pooled across the ages. Analyses were performed on the correlations that resulted.
Survival Analysis
PHREG (SAS version 9.1, SAS Inc., Cary, North Carolina) was used to perform regression analyses of the survival data. PHREG is based on a Cox proportional hazards model and uses the robust sandwich variance estimator to allow for adjusting for multiple siblings (each family contributed an average of 1.7 siblings). Young adult outcome was determined from the repeated CIDI evaluations performed after age 19 (mean age of 23.2 ± 2.7 years at last follow-up visit).
Main effects and all two-way interactions of each factor with risk status (high or low risk for AD) were tested within one model. Outcome was modeled using the 13 predictor variables (five factors) (Table 2) collected in one of two developmental periods, 8 to 13 years and 14 to 18 years. The earliest record obtained during each period was used.
Table 2.
Results of Factor Analysis for the 13 Childhood Variables
| Factor
|
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| WRAT Math | .576 | ||||
| WRAT Reading | .894 | ||||
| WRAT Spelling | .884 | ||||
| JEPI Extraversion | .325 | ||||
| JEPI Neurotocism | −.621 | ||||
| Self-Esteem Inventory | .125 | .921 | |||
| Manifest Anxiety Score | −.668 | ||||
| Visual P300 | .727 | ||||
| Auditory P300 | .691 | ||||
| LISRES Positive Life Events | .536 | ||||
| LISRES Negative Life Events | −.144 | .843 | |||
| Bipedal Postural Sway (Lipscomb) | .539 | ||||
| Monopedal Postural Sway (Right) | .691 | ||||
Thirteen variables were entered into a factor analysis and five factors resulted using principal axis factoring. Most variables loaded on only one factor. Each factor was scaled with a mean of zero and a standard deviation of 1.0 by derivation. The factors have small correlations in any of the sub-samples tested with standard deviations slightly less than 1.0. Fifty-one percent of the variance could be explained with the five factors.
Factor 3 tested in the 8- to 13-year-old sample was significant as an interaction with risk.
Factor 4 was significant as a predictor of SUD but could not be evaluated in the Cox models involving 8- to 13-year-old data, as the LISRES can only be evaluated in children over the age of 13.
Factor 5 was a significant predictor of SUD outcome along with risk status when tested in a sample of 15-year-olds.
JEPI, Junior Eysenck Personality Inventory; LISRES, Life Stressors and Social Resources Inventory; SUD, substance use disorder; WRAT, Wide Range Achievement Test.
Two variables were of special interest, P300 and postural sway. Developmental changes in P300 and postural sway during childhood and adolescence have been shown to differ by familial risk group status (38,54). Separate analyses were planned to identify the critical age(s) that P300 would predict SUD outcome. Analyses were performed for P300 amplitude recorded at ages 9, 11, 14, 17, and 20 years and membership in either the SUD-positive or SUD-negative groups. Postural sway was evaluated at a single age (age 15) to control for age-related changes.
Results
Factor Scores for Childhood Data
Factor analysis of the 13 childhood variables resulted in the extraction of five factors that preserved the a priori, logically apparent domains (Table 2).
Young Adult SUD Outcome and Derived Factor Scores for 8- to 13-Year-Olds
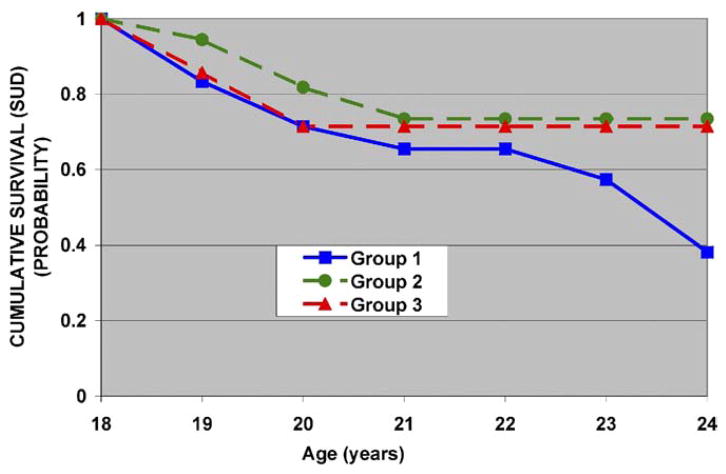
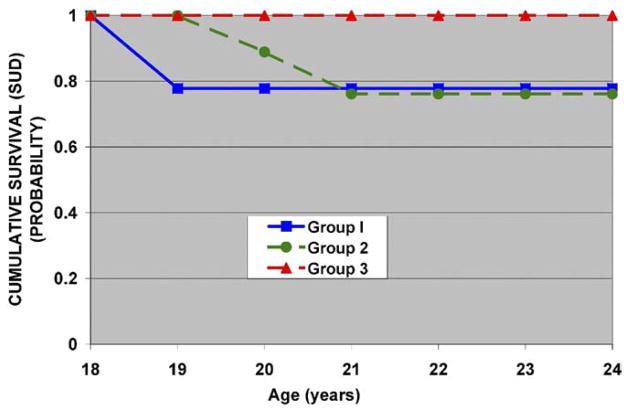
Variables collected during the 8- to 13-year-old developmental period could be linked to young adult outcome in 82 cases. Each of the five factors, risk, and the factor × risk interaction terms were selected for entry into the model, correcting for multiple siblings from the same family. Risk status predicted outcome (χ2 = 6.01, df = 1, p = .01). The interaction of factor 3 scores (P300 amplitude) with familial risk status (χ2 = 5.12, df = 1, p = .02) was also significant, suggesting that SUD outcome predicted from P300 amplitude in childhood has a differing effect by familial risk status. A survival analysis was then performed within risk groups using factor 3 scores grouped into terciles with tercile 1 corresponding to the smallest P300 amplitudes and tercile 3 the largest. Table 3 shows the relative risk associated with each tercile for the entire sample (high- and low-risk groups combined). Within risk groups, tercile placement was an important source of variation predicting SUD outcome (Figures 1 and 2), with better separation seen among high-risk offspring (Figure 1).
Table 3.
Tercile Groups for P300 Amplitude and Postural Sway for the Total Sample (High- and Low-Risk Combined)
| First P300 Evaluation Between Ages 8 and 13 (n = 82)
| ||||
|---|---|---|---|---|
| Tercile | P300 Factor Means (SD) | Relative Risk of Developing SUD | Auditory P300 Amplitude (μV) Mean ± SD |
Visual P300 Amplitude (μV) Mean ± SD |
| 1 | −.77 (.5) | 41% | 12.7 (6.0) | 21.9 (8.3) |
| 2 | .00 (.1) | 25% | 18.5 (6.2) | 32.4 (7.0) |
| 3 | 1.02 (.6) | 15% | 29.9 (10.7) | 41.8 (10.4) |
| Sway at Age 15 (n = 100)
| ||||
|---|---|---|---|---|
| Tercile | Sway Factor Means | Relative Risk of Developing SUD | Distance R Lipscomb (cm) Mean ± SD |
Distance R Monopedal (cm) Mean ± SD |
| 1 | −.69 (.2) | 27% | .55 (.1) | .99 (.1) |
| 2 | −.16 (.1) | 26% | .60 (.2) | 1.08 (.4) |
| 3 | .53 (.5) | 36% | .87 (.2) | 1.48 (.3) |
R, resultant vector of hypothesized area.
Figure 1.
High-risk participants and factor 3 (P300) loadings. Individuals in group 1 with the largest negative factor loadings (−.75 ± .5) have the lowest amplitude, while those in group 2 are in the midrange of factor loading (−.02 ± .1) and group 3 with the positive loadings (1.09 ± .8) represent the largest P300 amplitude. Note that individuals in group 1 with the lowest P300 amplitude show SUD earlier. SUD, substance use disorder.
Figure 2.
Low-risk participants and factor 3 (P300) loadings. Individuals in group 1 with the largest negative loadings (.80 ± .4) have the lowest amplitude, while those in group 2 are in the midrange of factor loading (.05 ± .2) and group 3 with the largest positive loadings (.94 ± .5) represent the largest P300 amplitude. Note that P300 amplitude has only a minimal effect on SUD outcome by age 24 in these individuals. SUD, substance use disorder.
Derived Factor Scores for 14- to 18-Year-Olds and Young Adult SUD Outcome
Survival analyses were repeated, expanding the sample to include childhood data collected for subjects seen at least once between the ages of 14 and 18 years (n = 127 subjects). None of the five factors were found to be statistically significant predictors of young adult SUD outcome in the model that tested risk, the five factors, and their interaction with risk, adjusting for the presence of multiple siblings. Only an effect due to risk was seen (χ2 = 7.63, df = 1, p = .006).
Postural Sway at Age 15
Because there are major developmental changes in postural sway (37,38,55–58), analyses of these data were done using all children at the same age. Maximal data were available at age 15 (n = 100).
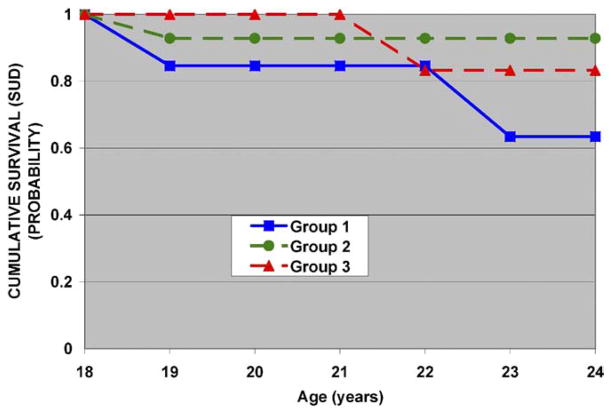
Risk, the five factors, and their interactions with risk were tested, correcting for multiple siblings (Table 2). Using this strategy, risk status was the only significant predictor of young adult outcome (χ2 = 7.36, df = 1, p = .007). Data were also analyzed without adjusting for the contribution of multiple siblings. Here, a factor 5 (sway) × risk interaction was seen (χ2 = 5.51, df = 1, p = .019), a finding that is consistent with previous reports from this laboratory (35,38). Table 3 illustrates the associated risk for developing SUD by tercile for the entire sample (high-risk and low-risk). Substance use disorder outcome among high-risk subjects differed in association with postural sway with those in group 3, with greater sway having reduced SUD survival (Figure 3). For low-risk control subjects, the amount of sway was not associated with a remarkable change in SUD outcome (Figure 4).
Figure 3.
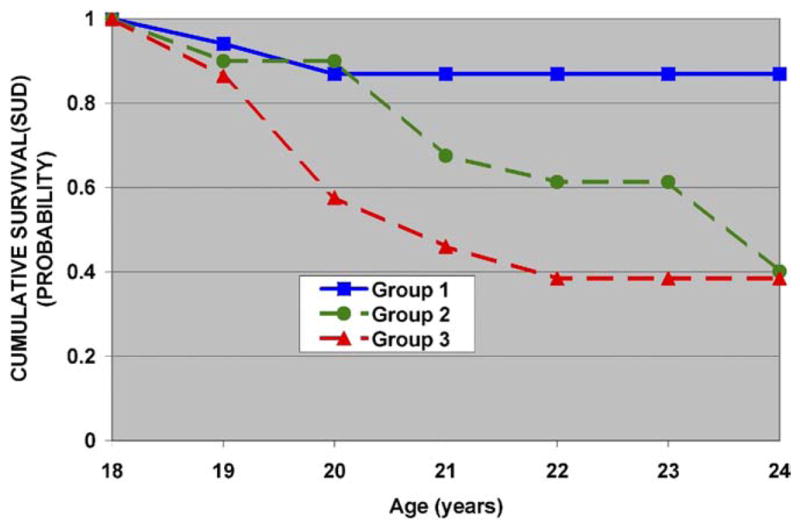
High-risk participants and factor 5 (postural sway) loadings. Individuals in group 1 with the largest negative loadings (−.62 ± .5) have the least amount of postural sway, those in group 2 are in the midrange of factor loading (−.13 ± .1), and group 3 with a positive loading (.55 ± .8) has the largest amount of sway. Those in group 3 with the greatest amount of postural sway develop SUD earlier. SUD, substance use disorder.
Figure 4.
Low-risk participants and factor 5 (postural sway) loadings. Individuals in group 1 with the largest negative loadings (−.76 ± .4) have the least amount of postural sway, group 2 individuals are in the midrange of factor loading (−.24 ± .1), and group 3 with the largest positive loadings (.50 ± .4) have the greatest amount of sway. Among low-risk participants, tercile group placement has minimal effect on SUD outcome by age 24. SUD, substance use disorder.
Comparison of Developmental Periods
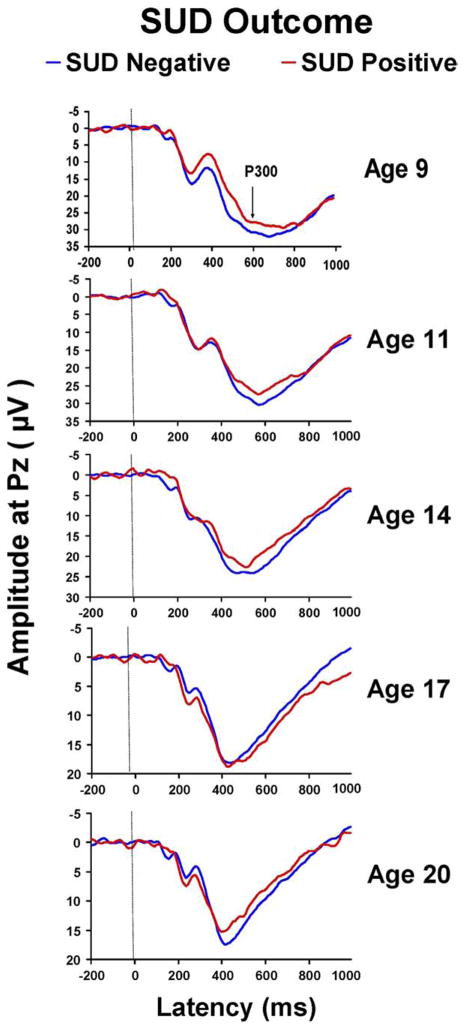
Because the effect of factor 3 (P300 amplitude) was significant in the younger age cohort (ages 8 to 13 years) but not in the age 14 to 18 group, further analyses were performed for selected ages to control for age-related change in P300. As may be seen in Figure 5, there is a clear difference in visual P300 amplitude at age 9 in those who eventually developed SUD versus those who did not, a 7.45 μV difference (29.88 ± 12.3 and 36.43 ± 10.7) that is statistically significant (t = 2.36, df = 80, p = .021). Means and standard deviations for ages 9, 11, 14, 17, and 20 years may be seen in Table 4.
Figure 5.
Grand averages for visual P300 amplitude by SUD outcome groups are illustrated by age at P300 recording. Only the amplitude difference at age 9 differentiates those who will later develop SUD. SUD, substance use disorder.
Table 4.
Visual P300 Amplitude in Association with Development of SUD
| Total n | SUD Positive Mean ± SD (n) |
SUD Negative Mean ± SD (n) |
t | df | p | |
|---|---|---|---|---|---|---|
| Age 9 | 82 | 29.88 | 36.43 | 2.36 | 80 | .021 |
| 12.25 (22) | 10.70 (60) | |||||
| Age 11 | 77 | 29.46 | 33.10 | 1.35 | 75 | ns |
| 11.72 (20) | 9.87 (57) | |||||
| Age 14 | 79 | 24.90 | 27.66 | 1.11 | 77 | ns |
| 7.44 (21) | 10.42 (58) | |||||
| Age 17 | 77 | 21.16 | 21.45 | .12 | 75 | ns |
| 7.88 (20) | 10.25 (57) | |||||
| Age 20 | 55 | 18.04 | 19.06 | .48 | 53 | ns |
| 6.50 (15) | 7.31 (40) |
ns, nonsignificant; SUD, substance use disorder.
Two Hit Model
With our observations that visual P300 amplitude and postural sway were both efficient predictors of young adult outcome by age 24, a two hit model of disease liability was tested in which those with no hits were contrasted with those with two hits (below the median on P300 and above the median on sway). Because of the clear relationship between P300 recorded at age 9 and SUD outcome, an analysis was performed using the 82 offspring with P300 amplitude recorded at age 9 with available sway data obtained at age 15. A total of 70 cases had a P300 record at age 9 and a sway record at age 15. Individuals with P300 amplitude below the median versus those above had an increased odds (odds ratio = 2.80 [confidence interval (CI) = .99–7.87], χ2 = 3.98, df = 1, p = .046) of developing SUD. Also, risk for developing SUD was seen in association with having both P300 amplitude below the median at age 9 and sway above the median at age 15 versus having neither risk factor (odds ratio = 5.09 [CI = .88–29.26], χ2 = 3.70, df = 1, p = .054). This finding was confirmed using a sample of youngsters with any P300 record under age 13 and sway at age 15 (n = 86 cases) (odds ratio = 8.08 [CI = 1.52–42.83], χ2 = 7.22, df = 1, p = .007).
Discussion
Examination of 13 childhood variables revealed five factors that significantly clustered as behavioral indexes for use as predictors. Of these five factors, postural sway and P300 amplitude appear to provide the best prediction of young adult SUD outcome. Using both variables collected in offspring before the age of 15 (age 13 for P300), we find an eightfold increase in risk for developing SUD by age 24. This is substantial given the lesser odds associated with drinking-related behaviors (age of first drink before age 15 confers a fourfold risk in comparison with age after 20).
Early Identification
The importance of measuring these variables at a young age is illustrated for P300. Results of this prospective study of offspring from multiplex families indicate that P300 amplitude collected at an average age of 10.6 years in offspring from multiplex families is a significant predictor of SUD outcome at an average age of 21.8 years, a prediction interval spanning an average of 11.2 years.
Identification of these risk indicators appears important to the search for ways in which those at highest risk can be differentiated for possible intervention. Moreover, identification of risk factors can provide intermediate phenotypes or endophenotypes for genotype analysis. Gottesman and Gould (59) have described criteria for considering a variable an endophenotype, including the requirement that the endophenotype be associated with the illness in the population; that the endophenotype is heritable, state independent, or present even when the person is not currently ill; and that the endophenotype co-segregate with illness in families. Uncovering endophenotypes that are associated with the disease at the time of disease onset are, of course, easier to identify. However, many disorders that do not emerge until adulthood have their antecedents in childhood. Rheumatic heart disease may not emerge until adulthood though infection with Streptococcus bacillus occurs in childhood. The requirement that the endophenotype co-segregate with illness in families would appear to be highly dependent on the age and developmental stage of the individual studied. We view the postural sway and P300 as endophenotypes of this category. These variables may provide a high level of prediction for later development of SUD, though clearly the offspring do not have SUD when evaluated in childhood.
Limitations
Limitations of the present study include the possibility that offspring from multiplex families may not be representative of the general population. This is clearly a possibility because families are purposely selected to be at the extreme end of the distribution for AD. However, multiplex families offer greater economy of search for risk factors and genetic variation that can then be taken to population samples for replication. Many early studies were not population based but did point to P300 as an indicator of AD risk. Among the early studies to demonstrate a relationship was a pilot study conducted in our laboratory in which P300 was recorded in youngsters with an average age of 10.7 years who were followed to an average age of 18.8 years with P300 recorded once again along with clinical status (51). Those with AD by age 18.8 years were more likely to have lower P300 amplitude at both points. Also, Berman et al. (60) found at 4-year follow-up that those with longer P300 latency had a greater likelihood of having SUD.
Community Samples
P300 has been investigated in association with clinical outcome using genetically informative population-based samples. Using a cohort from statewide birth records in Minnesota, Iacono et al. (61) reported that P300 amplitude at age 17 years predicted SUD outcome by age 20 years. Carlson et al. (62), focusing on the status of twin pairs who were stably discordant at both age 17 years and at age 20 years, those newly discordant at age 20 years, and those continuously unaffected, found that P300 amplitude was reduced at age 17 years in both twins where one member of the pair subsequently developed alcohol abuse or dependence. Recent work based on a sample followed to age 24 years (63) shows that reduced P300 is more strongly related to early- than late-onset substance disorders.
Specificity of P300 for Disinhibition and Risk for AD
The issue of whether P300 is specifically related to risk for alcohol use disorders or reflects a more general tendency for disinhibition has recently been addressed (20). Using a sample of 17-year-old twins selected from Minnesota birth records, Iacono and McGue (20) found lower P300 amplitude predicted an earlier age of onset for a variety of adolescent problem behaviors including tobacco, alcohol, or illicit drug use; sexual intercourse; and police contact. However, there is some concern that studies often do not include measures of internalizing disorders in protocols designed to study SUD and other externalizing pathology. In a study using growth curve modeling (54), trajectories of P300 were found to be influenced by internalizing as well as externalizing disorders of childhood.
Postural Sway
Risk group differences in postural sway appear to be the result of age-related differences in acquired postural control, reflecting a slower developmental trajectory of body control in high-risk offspring (38). Associations between motor coordination in childhood and young adult outcome have been seen in large-scale community samples of individuals followed 30 years (39). In that study, adults with major depressive disorder with early onset had been characterized as “clumsy and slow to reach developmental milestones.” It appears that our findings obtained from a sample selected for multiplex AD status is in accord with results obtained from this large-scale community sample with longitudinal follow-up in suggesting that early motor delays are in some way linked to young adult psychiatric status.
Our observations of reduced P300 in offspring from multiplex AD families have been replicated in a number of laboratories when minor age offspring are tested, especially male offspring, and when visual P300 paradigms are used (22). Attempts to use P300 as a predictor of later SUD outcome have not followed individuals as long as the present study, though two previous studies outside of our laboratory followed offspring 3 years (61) and 7 years (64), respectively. Therefore, this study provides evidence that the amplitude of P300 measured in preadolescents can predict SUD 11 years later in young adulthood. Currently, it is unknown if this reduced amplitude is predictive of other types of psychiatric illness in young adulthood or beyond. Nevertheless, P300 amplitude appears to differentiate among those considered high risk because of familial background. Importantly, when postural sway and P300 amplitude data are combined, the predictive odds for SUD outcome appear to be improved.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grants AA 005909, AA 008082, and AA 015168 to SYH.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- 2.Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism: A study of male and female twins. Arch Gen Psychiatry. 1991;48:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- 3.Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- 5.McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: A twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Prescott CA. The genetic epidemiology of alcoholism: Sex differences and future directions. In: Agarwal DP, Seitz HK, editors. Alcohol in Health and Disease. New York and Basel, Switzerland: Marcel Dekker; 2001. pp. 125–149. [Google Scholar]
- 7.Heath AC, Madden PAF. Genetic influences on smoking behavior. In: Turner JR, Cardon LR, Hewitt JK, editors. Behavior Genetic Approaches in Behavioral Medicine. New York: Plenum; 1995. pp. 45–66. [Google Scholar]
- 8.Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS, et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- 9.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, et al. A genome-wide search for genes that relate to low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- 12.Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, Kalmijn J. Genomic scan for loci that lead to low level response to alcohol as established by alcohol challenge. Alcohol Clin Exp Res. 2003;25:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]
- 13.Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome-wide search for alcoholism susceptibility genes. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 15.McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- 16.Hill SY, Yuan H. Familial density of alcoholism and onset of adolescent drinking. J Stud Alcohol. 1999;60:7–17. doi: 10.15288/jsa.1999.60.7. [DOI] [PubMed] [Google Scholar]
- 17.Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high-risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- 18.Hill SY, Locke J, Lowers L, Connolly J. Psychopathology and achievement in children at high risk for developing alcoholism. J Am Acad Child Adolesc Psychiatry. 1999;38:883–891. doi: 10.1097/00004583-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 19.McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- 20.Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 21.Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- 22.Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- 23.Hill SY, Steinhauer SR, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 24.Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- 25.Aston CE, Hill SY. A segregation analysis of the P300 component of the event- related potential. Am J Hum Genet. 1990;47(suppl):A127. [Google Scholar]
- 26.O’Connor S, Morzorati S, Christian JC, Li T-K. Heritable features of the auditory oddball event related potential: Peaks, latencies, morphology and topography. Electroencephalogr Clin Neurophysiol. 1994;92:115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 27.Katsanis J, Iacono WG, McGue M, Carlson SR. P300 event related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 28.Hill SY, Yuan H, Locke J. Path analysis of P300 amplitude of individuals from families at high and low risk for developing alcoholism. Biol Psychiatry. 1999;45:346–359. doi: 10.1016/s0006-3223(97)00525-8. [DOI] [PubMed] [Google Scholar]
- 29.Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Beijersterveldt CE, vanBaal GC. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 31.Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, et al. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 32.Wright MJ, Luciano M, Hansell NK, Montgomery GW, Geffen GM, Martin NG. QTLs identified for P3 amplitude in non-clinical sample: Importance of neurodevelopmental and neurotransmitter genes. Biol Psychiatry. 2008;63:864–873. doi: 10.1016/j.biopsych.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Lipscomb TR, Carpenter JA, Nathan PE. Static ataxia: A predictor of alcoholism? Br J Addict. 1979;74:289–294. doi: 10.1111/j.1360-0443.1979.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 34.Hill SY, Armstrong J, Steinhauer SR, Baughman T, Zubin J. Static ataxia as a psychobiological marker for alcoholism. Alcohol Clin Exp Res. 1987;4:345–348. doi: 10.1111/j.1530-0277.1987.tb01323.x. [DOI] [PubMed] [Google Scholar]
- 35.Hill SY, Steinhauer SR. Postural sway in children from pedigrees exhibiting a high density of alcoholism. Biol Psychiatry. 1993;33:313–325. doi: 10.1016/0006-3223(93)90320-d. [DOI] [PubMed] [Google Scholar]
- 36.McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Family density of alcoholism: Effects on psychophysiologcal responses to ethanol. Alcohol. 1991;8:219–222. doi: 10.1016/0741-8329(91)90870-3. [DOI] [PubMed] [Google Scholar]
- 37.Usui N, Maekawa K, Hirasawa Y. Development of the upright postural sway of children. Dev Med Child Neurol. 1995;37:985–996. doi: 10.1111/j.1469-8749.1995.tb11953.x. [DOI] [PubMed] [Google Scholar]
- 38.Hill SY, Shen S, Locke J, Lowers L, Steinhauer SR, Konicky C. Developmental changes in postural sway in children at high and low risk for developing alcohol-related disorders. Biol Psychiatry. 2000;7:501–511. doi: 10.1016/s0006-3223(99)00175-4. [DOI] [PubMed] [Google Scholar]
- 39.Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin MA. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Arch Gen Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- 40.Barkley RA. The executive functions and self regulation: An evolutionary neuropsychological perspective. Neuropsychol Rev. 2004;11:1–29. doi: 10.1023/a:1009085417776. [DOI] [PubMed] [Google Scholar]
- 41.Helzer JE, Robins LN, McEvoy LT, Spitnagel EL, Stolzman RK, Farmer A, Brockington IF. A comparison of clinical and diagnostic interview schedule diagnoses. Physician reexamination of lay-interviewed cases in the general population. Arch Gen Psychiatry. 1985;42:657–666. doi: 10.1001/archpsyc.1985.01790300019003. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1982. [Google Scholar]
- 43.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 44.Feighner JP, Robins E, Guze SB, Woodruff RA, Jr, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds CR, Richmond BO. What I think and feel: A revised measure of children’s manifest anxiety. J Abnorm Child Psychol. 1978;6:271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed SMS, Valliant PM, Swindle D. Psychometric properties of Coopersmith Self-Esteem Inventory. Percept Mot Skills. 1985;61:1235–1241. [Google Scholar]
- 47.Jastak S, Wilkinson G. Wide Range Achievement Test-Revisited. Wilmington, DE: Jastak Associates, Inc; 1984. [Google Scholar]
- 48.Wilkinson GS. Wide Range Achievement Test. 3. Wilmington, DE: Wide Range, Inc; 1993. (WRAT-3) [Google Scholar]
- 49.Moos RH, Moos BS. Life Stressors and Social Resources Inventory Manual. Odessa, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- 50.Eysenck SBG. Manual for the Junior Eysenck Personality Inventory. San Diego: Educational and Industrial Testing Service; 1963. [Google Scholar]
- 51.Hill SY, Steinhauer SR, Lowers L, Locke J. Eight year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biol Psychiatry. 1995;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- 52.Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. J Stud Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- 53.Janca A, Robins LN, Cottler LB, Early TS. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI field trials B wave II at the St. Louis site. Br J Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- 54.Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 55.Shumway-Cook A, Woollacott MH. The growth of stability: Postural control from a developmental perspective. J Mot Behav. 1985;17:131–147. doi: 10.1080/00222895.1985.10735341. [DOI] [PubMed] [Google Scholar]
- 56.Riach CL, Hayes KC. Maturation of postural sway in young children. Dev Med Child Neurol. 1987;29:650–658. doi: 10.1111/j.1469-8749.1987.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 57.Hayes KC, Riach CL. Preparatory postural adjustments and postural sway in young children. In: Woollacott MH, Shumway-Cook A, editors. Development of Postural and Gait Across the Life Span. Columbia, SC: University of South Carolina; 1989. pp. 97–127. [Google Scholar]
- 58.Schultz AB, Ashton-Miller JA, Alexander NB. What leads to age and gender differences in balance maintenance and recovery? Muscle Nerve. 1997;5(suppl):S60–S64. [PubMed] [Google Scholar]
- 59.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:635–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 60.Berman SM, Whipple SC, Fitch RJ, Noble EP. P300 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- 61.Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- 62.Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who became discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 63.Carlson SR, McLarmon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier-versus later-onset substance-use disorder. J Abnorm Psychol. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- 64.Habeych ME, Charles PJ, Sclabassi RJ, Kirisci L, Tarter RE. Direct and mediated associations between P300 amplitude in childhood and substance use disorders outcome in young adult outcome. Biol Psychiatry. 2005;57:76–82. doi: 10.1016/j.biopsych.2004.09.028. [DOI] [PubMed] [Google Scholar]