Abstract
The purpose of this study is to analyze the safety and clinical efficacy of transcatheter arterial chemoembolization (TACE) combined with portal vein stent and 125I implantation for the treatment of portal vein tumor thrombus (PVTT) in hepatocellular carcinoma. Fifty-six patients from our department diagnosed with advanced hepatocellular carcinoma with PVTT between January 2008 and December 30, 2010 were divided into two groups. Patients in Group A were treated with TACE and portal vein stent; patients in Group B were treated with TACE, portal vein stent and 125I implantation. The success rate of TACE with portal vein stent and 125I implantation was 100%, with no severe surgery-related complications. After an 8 mo follow-up, the total clinical benefit rates were 56.7 and 88.5% for Groups A and B, respectively (p < 0.05). The median survival times (mOS) for the two groups were 5.7 and 8.9 mo, respectively (p < 0.05). The median time of progression (mTTP) of the two groups were 5.3 and 7.9 mo, respectively (p < 0.05). The 2, 6, 8, 12 and 18 mo patency rates in Group A were 100, 93.3, 83.3, 53.3 and 36.6%. Those in Group B were 100, 100, 92.3, 84.6 and 80.7%. The 2, 6 and 8 mo patency rates showed no statistical differences (p > 0.05), but the 12 and 18 mo rates did (p < 0.05). Our results suggest that TACE combined with portal vein stent and 125I implantation are both safe and effective, and 125I implantation can further postpone the restenosis of the portal vein effectively.
Keywords: hepatocellular carcinoma, TACE, thrombus, stent, 125I, treatment, efficacy
Introduction
Portal vein tumor thrombus (PVTT) is one of the most important causes of recurrence, metastasis, and hematogenous dissemination of certain cancer; with a rate of 44–66.2%.1 The prevalence of PVTT is very high even in small-cell hepatocellular carcinoma (HCC).2 The prognosis of HCC combined with PVTT is poor. One report showed that the median objective survival time was 24.4 mo for HCC without PVTT and 2.4–2.7 mo with PVTT.3,4 The main reasons for this are probably as follows: first, extensive intrahepatic metastases may occur because tumor thrombus can spread along the portal vein; second, the portal vein is the main nutrient vessel for normal liver tissue, and tumor thrombus causes partial or total portal vein occlusion, which decreases liver function and can even induce hepatic failure; lastly, tumor thrombus occlusion puts pressure on the portal vein, and the esophageal gastrointestinal bleeding that follows can be lethal.
At present, there are many treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis, including surgical resection, transcatheter arterial chemoembolization (TACE), portal vein stent, radiotherapy, percutaneous ethanol injection (PEI), 125I seed implantation, and laser ablation. They do not all share equal efficacy, however. A standardized, unified treatment option has not yet been created. In theory, surgical resection is the only single method that can cure PVTT, but the requirements of surgical indications and the high rate of recurrence are important restricting factors.5 This leaves TACE as the most common treatment strategy for PVTT, followed surgical resection.6 Due to tumor thrombosis in the hepatic artery, TACE can cause not only lord tumor necrosis, but also tumor thrombosis necrosis. Portal vein stent does not directly treat to PVTT, but it can greatly alleviate the portal hypertension caused by tumor thrombus occlusion, which can reduce the risk of upper gastrointestinal bleeding and acute hepatic failure. The liver is high sensitivity to radiation. The radiation tolerance of a whole liver is 30Gy/3–4 weeks, and even this will decrease when liver is not healthy to start. However, a radiation dose must be above 40Gy to have any clinical effect on hepatic carcinoma. This limits the applications of external radiation therapy.7
We recognized the following from our clinical experience: The problems inherent in TACE combined with portal vein stent for PVTT are their high rate of recurrence and limited stent restenosis. 125I seed implantation has been widely applied in head and neck tumors and in prostate cancer. A long half-life and close contact with the tumorous tissue make it possible to have deliver γ-ray irradiation to the tumor continuously, which greatly reduces the proliferation of tumor cells. In addition, 125I release γ-rays can inhibit hyperplasia of the vascular intima.8-10 The present study retrospectively compared 56 cases of hepatic carcinoma with PVTT. Twenty-six of them were treated with TACE combined with portal vein stent and 125I implantation relative to 30 patients who accepted TACE and portal vein stent alone during the same period.
Results
Tumor response
Eight months after surgery none of the patients in either group rated CR. There were eight PR cases and nine SD cases in Group A, with a benefit rate of 56.7% (17/30). In Group B, there were ten PR cases and 13 SD with a benefit rate of 88.5% (23/26). After the data of the two groups were independently evaluated by χ2, it was concluded that there was a statistical difference (χ2 = 6.899, p < 0.05), which indicated that the short-term efficacy of the treatment given to Group B was better than that of the treatment given to Group A.
Comparison of the cumulative stent patency rates
All cases were successfully followed up, and the patency situations of the patients’ stents were assessed by color ultrasound within our department. Results are displayed in Table 2.
Table 2. The cumulative stent patency rates in Group A and Group B.
| Time of follow-up (months) | Patients of follow-up (months) | Stent blockage (cases) | Cumulative stent patency rates (%) |
|---|---|---|---|
| 2 |
30 (26) |
0 (0) |
100 (100) |
| 4 |
30 (26) |
2 (0) |
93.3 (100) |
| 6 |
28 (26) |
0 (0) |
93.3 (100) |
| 8 |
28 (26) |
3 (2) |
83.3 (92.3) |
| 10 |
25 (24) |
1 (1) |
80.0 (88.4) |
| 12 |
24 (23) |
8 (1) |
53.3 (84.6) |
| 14 |
16 (22) |
2 (0) |
46.7 (84.6) |
| 16 |
14 (22) |
1 (1) |
43.4 (80.7) |
| 18 | 13 (21) | 2 (0) | 36.7 (80.7) |
Inside the brackets is Group B, outside is Group A.
In Group A, the cumulative stent patency rates at 2, 6, 8, 12, and 18 mo were, respectively, 100, 93.3, 83.3, 53.3 and 36.6%. Those in Group B were 100, 100, 92.3, 84.6 and 80.7%. After statistical analysis, the cumulative patency rates at 2, 6 and 8 mo between Group A and Group B showed no statistical difference (p > 0.05). However, at 12 mo (χ2 = 6.249 p = 0.021) and 18 mo (χ2 = 11.062 p = 0.001) there was a statistical difference (p < 0.05).
Overall survival
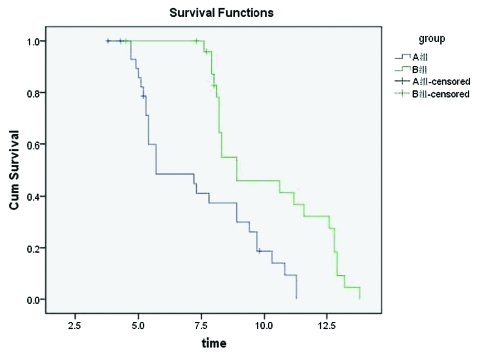
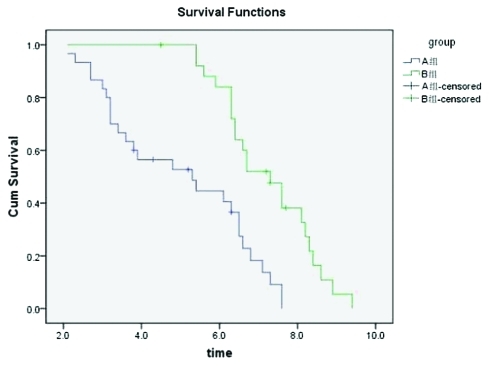
The follow-up rates were 100% for all 56 patients within a period of 4–30 mo. After the collection of clinical data was completed, there were four surviving patients in each group. The survival rates were tested using the Kaplan-Meier method, and survival curves were drawn and accepted via Log-rank testing. We concluded that the mOS in Group A was 8.9 mo (95% CI: 3.4–8.0 mo) with a mean survival time of 7.3 mo (95%CI: 5.4–12.4 mo). The mOS in Group B was 5.7 mo (95% CI: 3.4–8.0 mo), with a mean survival time of 10.2 mo (95% CI: 9.2–11.2 mo). The survival curve was drawn using the Kaplan-Meier method (Fig. 2), then accepted via Log-rank testing. It was then concluded that the two sets of survival rates were statistically different (χ2 = 12.76, p < 0.05). The survival rate was significantly higher in Group B than in Group A. The mTTP in Group A was 5.3 mo (95% CI: 3.0–7.6 mo), and that in Group B was 7.3 mo (95% CI: 6.4–8.2 mo). The curves of progression time were drawn using the Kaplan-Meier method (Fig. 3), and then accepted via Log-rank testing. This indicated that there was a statistical difference between the two groups (χ2 = 16.80, p < 0.05) and that the progression time in Group B was longer than in Group A.
Figure 2.
Comparison of the overall survival rates curve in Group A and Group B (Kaplan-Meier).
Figure 3.
Comparison of progression time cumulative survival rates curves in Group A and Group B (Kaplan-Meier).
Complications
All patients received successful stent and particle implantations with no surgery-related complications such as upper gastrointestinal bleeding or liver failure. No patient accepting 125I implantation was found to have radioactive liver injury. Eighteen patients had varying degrees of granulocytopenia, but after treatment (recombinant human granulocyte colony stimulating factor), they recovered within 3–5 d. Some patients had embolization-related syndrome after TACE, manifesting as fever, embolization-related pain, nausea and vomiting, but after administration of painkillers and antiemetics, they recovered within 2–3 d. Some patients experienced some level of liver dysfunction, manifesting as increased bilirubin, decreased cholinesterase, decreased serum albumin and elevation of transaminase, but they recovered to their preoperative levels liver-protective treatment. The toxicity profiles of the two groups are referenced from CTC2.0, and the results are shown in Table 3.
Table 3. The toxicity profile between Group A and Group B.
| Toxicity | Group A | Group B |
|---|---|---|
| Nausea and vomiting |
16 (53%) |
13 (50%) |
| Surgery |
17 (57%) |
16 (62%) |
| Fever |
21 (70%) |
19 (73%) |
| Transaminase elevation |
20 (67%) |
18 (69%) |
| Granulolytopenia | 8 (27%) | 10 (38%) |
For each characteristic, there is no significant difference in comparison with the TACE stenting group.
Discussion
Primary liver cancer (PLC) is one of the most common malignant tumors worldwide. Global cancer survey statistics for 2008 show that 70−85% of PLCs are hepatocellular carcinomas.12 It is fifth cancer in morbidity in men and seventh in women, and the mortality rate in male and female patients is the second and sixth, respectively. In 2008, 748,000 primary liver cancer patients were newly diagnosed, and 695,900 of these patients died. Half of the newly diagnosed and newly deceased patients were in China. At the beginning, PLC is insidious and has limited clinical symptoms, so 80–90% of the patients who come in for treatment are at advanced stages. PVTT is one of the major complications of PLC during. It has high morbidity even in small-cell hepatic carcinoma.1 Chau studied 37 resected specimens whose diameters were less than 2 cm, and 40.5% of these specimens showed vein tumor embolisms under light microscopy.2 At the same time, the prognosis of hepatic carcinoma with PVTT is very poor. Because most patients are advanced stages when they seek treatment, surgeries for PVTT have a low resection rate and a high reoccurrence rate. Fan found that 56.7% of patients with PVTT experienced recurrence or metastasis, and intrahepatic metastasis was commonest, occurring 78.57% of the time.13 Some scholars also tried systemic chemotherapy in patients with PVTT, but no significant survival benefit was observed.14
In our department, we mainly treat PVTT with TACE and portal vein stent. PVTT was once thought to be a contraindication of TACE, but recent studies have shown that the development of PVTT is a gradual process.6 Because of self-compensation, collateral circulation cannot form around the thrombus. Studies on the portal vein blood supply showed that some of the blood supply for tumor thrombi actually came from the hepatic artery. For these patients, if there were no severe ascites and passable liver function, TACE could be performed. Qi Liu divided 128 patients with hepatic carcinoma with PVTT into three groups.15 Group A (18 patients) accepted only TACE, Group B (28 patients) accepted TACE and lipiodol embolization, and Group C (82 patients) accepted TACE with lipiodol embolization and gel foam embolization. The survival rates after one year were 25, 28.52 and 41.76, respectively, with a mean survival time of 7.2, 8.4 and 10.3 mo.
Portal vein stent combined with TACE effectively reduced the risk of upper gastrointestinal bleeding and acute liver failure.16 Gao-quan Gong compared the portal vein pressure in 12 PVTT patients before and after the stent, and the results showed that, after surgery, all of the pressure in portal vein (PV), branches of the portal vein (PVB), splenic vein (SV), and superior mesenteric vein (SMV) had decreased significantly (p < 0.05). A study by Wang JH showed that TACE combined with potal vein stent was reasonably safe and offered a mean survival time of 242 d and mOS of 113 d, which was consistent with our own findings.17 However, in our clinical work, it was found that portal vein stent combined with TACE for PVTT had some problems such as high rates of reoccurrence and restenosis. There were two possible reasons for this. One is that TACE for PVTT was merely a palliative treatment and it could result in residual tumor tissue, which would lead to reoccurrence and restenosis. The other is intimal hyperplasia after surgery, induced by the stent.
125I seeding can release continuous low doses of x- and γ-rays, leading to a total dose of 160–180 Gy to local tissure.18 γ-rays can damage the DNA in the nuclei of the tumor cells, depriving them of the ability to proliferate. 125I seeds have a half-life of 59.6 d and a radiation diameter of 1.7 cm. This long half-life and close contact with the tumor tissue make it possible to deliver γ-ray irradiation to the tumor continuously, which greatly reduces the proliferation of tumor cells. Some studies have shown that β- or γ-rays can inhibit neointimal hyperplasia.9,10 Que-lin Mei reported that, 1 mo after 237Mbq 125I particles were implanted into normal rabbit liver tissue, a large hepatic coagulation necrosis appeared within 5 mm of the particles, and at this 5 mm boundary, necrotic cells and normal liver cells formed an apparent zone of apoptotic cells.19 Jiang-tao Wang performed 125I implantation in eight patients.20 At the 6 mo follow-up, CT indicated that PVTT had been controlled to varying degrees. There were five patients whose PVTT had disappeared, two whose cases had partially shrunk, and one whose case remained stable. The short-term effect was satisfactory. In addition, 125I can effectively kill cancer cells without destroying normal tissue. Because of these advantages, 125I brachytherapy has been widely used in the treatment of solid tumors like prostate cancer, lung cancer, head and neck tumors and pancreatic cancer.21-24
Based on the fact that the γ-rays released by 125I can be effective for PVTT, this study was designed as follows: portal vein stent and 125I implantation combined with TACE was performed to treat PVTT, and it was hoped that this would solve the problem of recurrence and restenosis known to occur after TACE combined with stent. We used retrospective analysis to assess the survival time and cumulative stent patency rates in two groups of patients. The results showed that both the mean survival time and mOS in Group B were significantly longer than those in Group A (p < 0.05), the mTTP was 5.3 mo in Group A, and 7.3 mo in Group B, which were statistically different (p < 0.05). This means that the progression time in Group B was longer than that in Group A. The 2, 6 and 8 mo patency rates of Groups A and B showed no statistically significant difference (p > 0.05), but at 12 and 18 mo, there were significant differences (χ2 = 11.062, p = 0.001) (p < 0.05).
In summary, portal vein stent can reduce pressure within the portal the incidence of non-neoplastic-related death. 125I implantation can prolong the time of reoccurrence after TACE and inhibiting intimal hyperplasia and improve long-term portal vein stent patency. The combination of the three for hepatic carcinoma was shown to be efficient, and the duration of patient lifespan was satisfactory. However, because of the similarity of the distribution of tumor thrombus between the two groups, it is not clear whether the difference between the types of tumor thrombus influence the clinical efficacy of this method.6 A further controlled clinical study is required.
Materials and Methods
Patient selection
All procedures performed in this study were approved by the Sun Yat-sen University Cancer Center and Guangdong General Hospital Committee of Ethics. All patients provided written informed consent before treatment. The patients involved in this study were clinically or pathologically diagnosed with hepatic carcinoma with portal vein tumor thrombus (PVTT) in our department between January 2008 and December 2010. Among these, 40 patients were male and 16 female. Their ages ranged from 27−73, with a mean of 50.3. Seventeen patients had main portal vein thrombus (mPPTV), 25 patients had right portal vein thrombus (17 of them also had mPPTV), 14 patients had left portal vein thrombus (11 also had mPPTV); 44 patients also had hepatitis B, and three patients also had hepatitis A.
Eligibility criteria
Study eligibility criteria were as follows: (1), after clinical or pathological diagnosis of advanced hepatic carcinoma, indication via enhanced CT or MRI that PVTT was also present but that at least one branch of the portal remained patent and at least one lesion was measurable; (2) a Child-Pugh score of at least A or B; (3) leucoctye count over 3.5 × 109/L before surgery or capability for the count to be elevated to that level after support treatment; (4) no extrahepatic metastasis (including lymph node metastasis).
Exclusion criteria
Study exclusion criteria were as follows: I, mPPTV combined with left/right portal vein thrombus; II, Child-Pugh scores of C; III, dysfunction in any major organ, such as the heart, liver or kidneys.
Test group
According to these criteria, 56 patients entered this study. They were divided into two groups: Group A (30 patients) were treated with TACE combined with portal vein stent, Group B (26 patients) were treated with TACE combined with portal vein stent and 125I implantation. The clinical data of the two groups is listed in Table 1. Before treatment, all patients demonstrated a Karnofsky Performance Score (KPS) of greater than 70. As shown via routine laboratory of blood, urine, stool, electrolytes, liver function, renal function and electrocardiogram (ECG), all patients were received Child-Pugh scores under 9.
Table 1. General information of the patients.
| Characteristic | TACE stenting group | TACE stenting-125 I seed group | ||
|---|---|---|---|---|
| No. of patients |
30 |
26 |
||
| Mean age (y) |
51 ± 2.3 |
48 ± 1.6 |
||
| Female/male patients |
7/23 |
9/17 |
||
| Position of lesion |
|
|
||
| Left lobe |
10 |
11 |
||
| Right lobe |
15 |
12 |
||
| Both lobes |
5 |
3 |
||
| Poison of thrombosis |
|
|
||
| Main portal vein |
7 |
10 |
||
| Main or/and right/left branch |
23 |
16 |
||
| Hepatitis |
|
|
||
| HAV |
1 |
2 |
||
| HBV |
21 |
23 |
||
| Serum AFP ≥ 400ng/ml |
26/30 |
20/26 |
||
| Child-pugh classification |
|
|
||
| Class A |
17 |
13 |
||
| Class B |
13 |
11 |
||
| Class C |
0 |
0 |
||
| Arteriovenous shunt | 5/30 | 3/26 | ||
For each characteristic, there is no significant difference in comparison with the TACE stenting group.
Transcatheter arterial chemoembolization treatment
Femoral artery puncture was performed using the Seldinger method, which delivered the tip of the catheter into the hepatic artery for angiography. Diagnostic angiography of the celiac trunk and liver artery was performed selectively with a 5F RH catheter. With the guidance from the digital signature algorithm (DSA), the tumor’s main feeding artery was superselected by microcatheter. The following drugs were used: 135 mg/m2 oxaliplatin (Sanofi-Aventis France), 30–40 mg/m2 epirubicin (Pfizer Inc.), 10–20 ml lipiodol ultra liquid (Guerbet France). Once the microcatheter was in place, oxaliplatin and lipiodol ultra liquid administered in cream form to embolize the artery. The embolic material was applied under fluoroscopic guidance until the plow became visibly decreased. If DSA indicated that the embolism was not complete, 500–710 μm gelatin sponge particles were used to reembolize the artery. Ateriovenous fistulas was embolized by rims before surgery. Thirty to forty-five days were allowed to elapse between the two procedures. The precise number of days was determined according to each patient’s specific AFP level, lipiodol deposition, tumor cell survival, reoccurrence and metastasis situation.
Portal vein stent
In Group A, portal vein stents were implanted one week after TACE so long as liver function had been restored. A 21-gauge needle was delivered into an uninvaded branch of portal vein under CT guidance with slice width of 5-mm. Then, after insertion of the 7F sheath, a 5F pigtail catheter was delivered into the splenic or superior mesenteric vein for angiography, which could be used to gauge any vein filling, collateral circulation, or stomach vein filling defect, collateral circulation, stomach coronary vein, and measure the pressure in the portal vein. The inner diameter and stenosis of the portal vein were assessed to select the proper stent, and then the stent was placed inside the stenosis. The angiography of the stomach coronary vein was re-performed to assess the location and patency of the stent, and pressure in the portal vein was re-measured.
125I Brachytherapy instruments and planning
In Group B, portal vein stents and 125I were implanted one week after TACE, when liver function had been restored (Fig. 1). The particle type was 6711, the activity was 0.6–0.8 mCi, the half life period was 59.4 d, the average energy was 27–32 KeV, and the matched peripheral dose (MPD) was 110–150 Gy. The CT and MRI images were input into a radioactive particle treatment planning system (TPS), and, according to the diameter in three vertical planes, the average energy, amount, and distribution of particles were calculated. Then the point and direction of puncture were determined. Under CT guidance, an 18G particle needle was implanted into tumor thrombus, and 125I particles were released as it was withdrawn, with an interval of 0.5–1.0 cm. The particle penetration of 125I was about 1.7 cm. CT scan was re-performed after particle implantation was completed to check for drop-out or particle displacement. In that case, replantation was performed. Finally, portal vein stent stent was performed under CT as described previously.
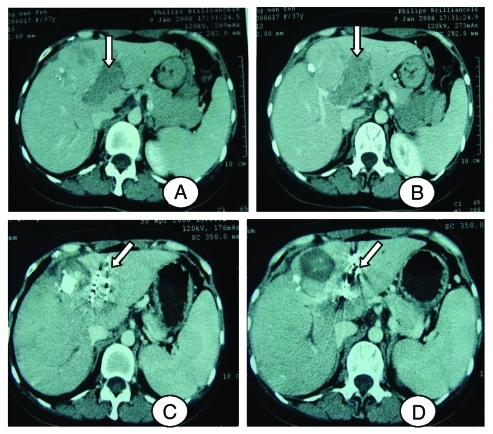
Figure 1.
A 38 y old female patient. (A and B) Computer tomography shows tumor thrombus in left branch of portal vein. (C and D) Computer tomography shows that tumor thrombus was largely lessened for the same patient, and the left branch was more patency than before.
Follow-up
After treatment, all patients were required to undergo laboratory tests every two weeks and CT or MRI every mouth. A single nurse was assigned to collect statistics. A follow-up was arranged 30–45 d after the first treatment, and survival time was calculated by the day that the stent or particles were implanted. Survival time, ECOG score, laboratory test results (routine blood test, AFP, clotting index, liver, and kidney function), imaging results (enhanced CT or MRI), and B ultrasound evaluations of stent patency were collected.
Evaluation criteria
Effectiveness was evaluated using RECIST-based criteria: 1) complete response (CR): disappearance of all target lesions; 2) partial response (PR): at least a 30% decrease in the sum of the longest diameter of the target lesion compared with the baseline sum of the longest diameter; 3) stable disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD when compared with the smallest sum of the longest diameter measured prior to treatment; 4) progressive disease (PD): at least a 20% increase in the sum of the longest diameter of the target lesions compared with the smallest sum of the longest diameter recorded before the start of treatment or the appearance of one or more new lesions.11 For each case, a total treatment response rate (RR) value was determined as follows: (case of CR + case of PR) / (number of cases).
Statistical analyses
Follow-up archives were assembled for each patient with SPSS16.0 software. The survival rate was calculated using the Kaplan-Meier method. Survival curves were drawn and accepted via a log-rank test. The short-term effect, cumulative stent patency rates, and general clinical data from the two groups (such as the positive rate of AFP, tumor situations, thrombus distribution, and complications of hepatitis) were inspected by χ2 test. Values of p < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/17676
References
- 1.Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, et al. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397–400. doi: 10.1007/s004320050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau GY, Lui WY, Wu CW. Spectrum and significance of microscopic vascular invasion in hepatocellular. Surg Oncol Clin N Am. 2003;12:25–34. doi: 10.1016/S1055-3207(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 3.Pawarode A, Voraved N, Sriuranpong V, Kullavanijava P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386–91. doi: 10.1097/00000421-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Lee HS, Kim JS, Choi LJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcathether arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–94. doi: 10.1002/(SICI)1097-0142(19970601)79:11<2087::AID-CNCR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. Surgical Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Ann Surg Oncol. 2010;17:2073–80. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 6.Shuqun C, Mengchao W. Therapeutic effects of transcatheter arterial chemoembolization on different types of tumor in portal vein in patients with hepatocellular carcinoma. Chin J Hepatocellular Surg. 2004;10:386–8. [Google Scholar]
- 7.Dawson LA, Ten Haken RK, Lawrence TS. Partial irradiation of the liver. Semin Radiat Oncol. 2001;11:240–6. doi: 10.1053/srao.2001.23485. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FJ, Li CX, Jiao DC, Zhang NH, Wu PH, Duan GF, et al. CT guided 125iodine seed implantation for portal vein tumor thrombus in primary hepatocellular carcinoma. Chin Med J (Engl) 2008;121:2410–4. [PubMed] [Google Scholar]
- 9.Amols HI. Review of endovascular brachytherapy physics for prevention of restenosis. Cardiovasc Radiat Med. 1999;1:64–71. doi: 10.1016/S1522-1865(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 10.Sidawy AN, Weiswasser JM, Waksman R. Peripheral vascular brachytherapy. J Vasc Surg. 2002;35:1041–7. doi: 10.1067/mva.2002.123751. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline. Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;6:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Zhou J, Wu ZQ, Qiu SJ, Wang XY, Shi YH, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2005;11:1215–9. doi: 10.3748/wjg.v11.i8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman MA. Primary hepatocellular cancer - present results and future prospects. Int J Radiat Oncol Biol Phys. 1983;9:1841–50. doi: 10.1016/0360-3016(83)90352-8. [DOI] [PubMed] [Google Scholar]
- 15.Qi L, Yu-cheng J, Jia H, Zhen-tang W, Jian-ming T, Hua Y, et al. Chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Zhonghua Fang She Xue Za Zhi. 1995;4:239–42. [Google Scholar]
- 16.Gong G, Wang X, Wang J, Yan Z, Cheng J, Qian C, et al. The portal venous pressure change due to metallic stents implanted into portal vein in HCC patients. J Intervent Radiol. 2007;3:159–61. [Google Scholar]
- 17.Zhang XB, Wang JH, Yan Z, Qian S, Liu R. Hepatocellular Carcinoma Invading the Main Portal Vein: Treatment with Transcatheter Arterial Chemoembolization and Portal Vein Stenting. Cardiovasc Intervent Radiol. 2009;32:52–61. doi: 10.1007/s00270-008-9454-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang FJ, Li CX, Zhang L, Wu PH, Jiao DC, Duan GF. Short-to mid-term evaluation of CT –guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol Ther. 2009;7:585–90. doi: 10.4161/cbt.8.7.7902. [DOI] [PubMed] [Google Scholar]
- 19.Mei QL, Liu PC, Yang JY. DU RM, Chen ZZ. Liu Pengcheng, Yang Jianyong, Du Rimin, Chen Zaizhong. Safety evaluation of iodine-125 seed implantation in rabbit liver tissue. Nan Fang Yi Ke Da Xue Xue Bao. 2007;5:675–8. [PubMed] [Google Scholar]
- 20.Zhang FJ, Li CX, Jiao DC, Zhang NH, Wu PH, Duan GF. ZHOU shengli.The primary study of 125I seeds on portal vein tumor thrombus. Chin Med J (Engl) 2009;10:1287–9. [Google Scholar]
- 21.Johnson M, Colonias A, Parda D, Trombetta M, Gayou O, Reitz B, et al. Dosimetric and technical aspects of intraoperative I-125 brachytherapy for stageInon-small cell lung cancer. Phys Med Biol. 2007;52:1237–45. doi: 10.1088/0031-9155/52/5/002. [DOI] [PubMed] [Google Scholar]
- 22.Peretz T, Nori D, Hilaris B, Manolatos S, Linares L, Harrison L, et al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys. 1989;17:931–5. doi: 10.1016/0360-3016(89)90138-7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang FJ, Wu PH, Zhao M, Huang JH, Fan WJ, Gu YK, et al. CT guided radioactive seed 125I implantation in treatment of pancreatic cancer. Zhonghua Yi Xue Za Zhi. 2006;86:223–7. [PubMed] [Google Scholar]
- 24.Ebara S, Katayama N, Tanimoto R, Edamura K, Nose H, Manabe D, et al. Iodine-125 seed implantation (permanent brachytherapy) for clinically localized prostate cancer. Acta Med Okayama. 2008;62:9–13. [PubMed] [Google Scholar]