Abstract
Oncolytic viruses with their capacity to specifically replicate in and kill tumor cells emerged as a novel class of cancer therapeutics. Rat oncolytic parvovirus (H-1PV) was used to treat different types of cancer in preclinical settings and was lately successfully combined with standard gemcitabine chemotherapy in treating pancreatic ductal adenocarcinoma (PDAC) in rats. Our previous work showed that the immune system and particularly the release of interferon-gamma (IFNγ) seem to mediate the anticancer effect of H-1PV in that model. Therefore, we reasoned that the therapeutic properties of H-1PV can be boosted with IFNγ for the treatment of late incurable stages of PDAC like peritoneal carcinomatosis. Rats bearing established orthotopic pancreatic carcinomas with peritoneal metastases were treated with a single intratumoral (i.t.) or intraperitoneal (i.p.) injection of 5x108 plaque forming units of H-1PV with or without concomitant IFNγ application. Intratumoral injection proved to be more effective than the intraperitoneal route in controlling the growth of both the primary pancreatic tumors and peritoneal carcinomatosis, accompanied by migration of virus from primary to metastatic deposits. Concomitant i.p. treatment of H-1PV with recIFNγ resulted in improved therapeutic effect yielding an extended animal survival, compared with i.p. treatment with H-1PV alone. IFNγ application enhanced the H-1PV-induced peritoneal macrophage and splenocyte responses against tumor cells while causing a significant reduction in the titers of H1-PV-neutralising antibodies in ascitic fluid. Thus, IFNγ co-application together with H-1PV might be considered as a novel therapeutic option to improve the survival of PDAC patients with peritoneal carcinomatosis.
Keywords: parvovirus H-1, interferon γ, pancreatic cancer, peritoneal carcinomatosis, metastasis
Introduction
Pancreatic cancer is an aggressive malignancy with one of the worst outcomes among all cancers. For all stages combined, the 5-y relative survival rate is only 5%.1 The radical surgery (Whipple’s operation) is the only curative option in this aggressive tumor but can be offered to less than 20% of PDAC-patients. Chemotherapy can be used as adjuvant to surgery or in advanced stage pancreatic cancer where, in a small group of patients, it offers real benefit in terms of survival and quality of life.2 Nevertheless, the therapeutic options for PDAC patients, especially these with peritoneal carcinomatosis, are lacking.
Novel virus-based anticancer therapies involve the use of viruses either as replicating oncolytic agents, or as recombinant vectors for gene transfer.3 The autonomous parvoviruses MVMp and H-1 belong to a group of small (~5 kb) non-integrating single-stranded DNA viruses. Their oncotropic and oncotoxic properties make them promising candidates for both types of applications.4 Recently we demonstrated that applying H-1PV as mono-therapy or as second-line treatment after gemcitabine chemotherapy, caused the reduction of tumor growth, prolonged the survival of rats bearing pre-established pancreatic tumors and led to the suppression of metastases.5 Furthermore, we found that immunological mechanisms are involved in the anticancer activity of H-1PV with a strong correlation between the therapeutic effect of the virus and IFNγ expression in the draining lymph nodes of pancreatic tumors.6
IFNγ is a cytokine with pleotropic functions, acting on virtually all immune cells and both innate and adaptive immune responses.7 In contrast to interferon α and interferon β, that can be expressed by all cell types, IFNγ, also known as immune interferon, is secreted mainly by T-helper (type 1) lymphocytes and NK cells. Interferon γ increases the antigen presentation by macrophages and activates antigen presenting cells in general, promoting Th1 differentiation and suppressing Th2 cell activity.8 Due to its antitumor and anti-infection activities IFNγ has been tested in several clinical trials in the past 20 y, where its tolerability and pharmacology have been determined.9 Concerning macrophage function, it was recently shown that IFNγ can redirect monocyte differentiation from tumor associated macrophages (TAM/ M2) into M1-polarized immunostimulatory cells, overcoming TAM-induced immunosuppression and lack of effectors’ T-cell generation.10 Intraperitoneal application of interferon γ has been shown to achieve surgically documented responses as both second- and first-line therapy in randomized phase III clinical trials for ovarian cancer.11
Our previous data, suggested a link between IFNγ expression in draining lymph nodes and the parvoviral oncosuppressive effect in PDAC upon early intratumoral inoculation.6 Therefore, we decided to extend further our studies and (i) evaluate the role of this cytokine in the parvovirus anticancer effect and (ii) eventually improve the latter through a combination of both treatments in PDAC complicated with peritoneal metastatic involvement. Using a previously reported model of orthotopic PDAC in Lewis rats5, we first established that the depletion with a neutralizing antibody (αIFNγ) or addition of recombinant interferon gamma (recIFNγ) had respectively negative or positive impacts on virus-modulated immunological functions of peritoneal macrophages and splenocytes. Modifying our PDAC animal model to mimic peritoneal carcinomatosis of the late stage PDAC, we found that in this area (the peritoneum) rich in macrophages, IFNγ can locally improve the therapeutic effect of H-1PV against metastasis upon intraperitoneal application, and reduce the titers of virus neutralizing antibodies in ascitic fluid.
Results
RecIFNγ contributes to the immunomodulating anticancer effect of H-1PV
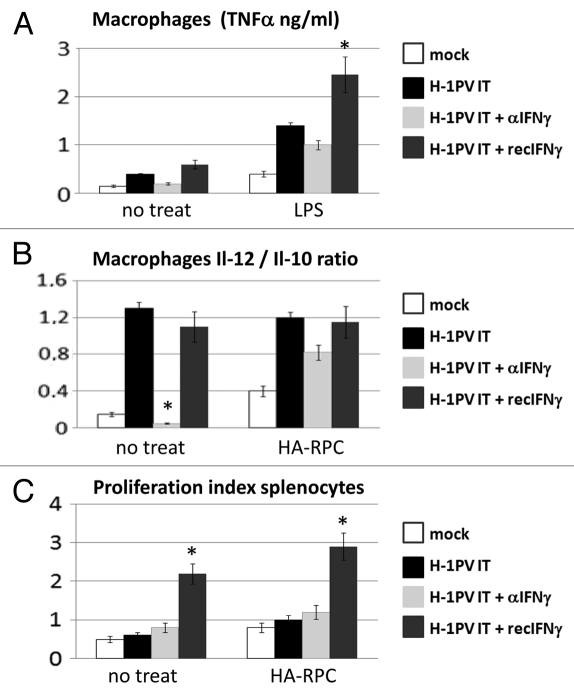
As a first step of our work we wanted to establish the potential impact of interferon γ on the immunomodulating features of parvovirus H-1PV in pancreatic cancer. Therefore, we applied H-1PV in tumors raised orthotopically through injection of HA-RPC cells in the pancreatic tail of three groups of rats using a PBS-treated group as control. Virus treatment was also combined either with intraperitoneal recombinant rat interferon (recIFNγ) or with a neutralizing antibody against it (αIFNγ). Three days later animals were sacrificed to perform immunological profiling of splenocytes and peritoneal macrophages. The cells were cultured for 48 h either alone (no treat), together with HA-RPC rat pancreatic cancer cells (the cell line used for initiating the tumors) or LPS. We analyzed different parameters related to the anticancer immune response, like the production of TNFα (Fig. 1A), the IL-12/IL-10 ratio (Fig. 1B) of cytokines released by macrophages, as well as the proliferation capacity of splenocytes (Fig. 1C). The supernatants of macrophages isolated from rats, in which H-1PV was combined with recIFNγ contained up to 1 ng more TNFα, compared with those obtained from animals treated with virus only. The lowest levels of TNFα release were measured either in the non-treated mock (0.2 and 0.5 ng) or when rats were treated with a neutralizing antibody against interferon gamma (0.3 and 1 ng). A similar pattern of effects of recIFNγ or αIFNγ was detected when comparing the IL-12/IL-10 ratios of the different macrophage cultures. In addition, the combination of H-1PV with recombinant interferon gamma caused a significant 2-fold increase in the proliferative potential of splenocytes both spontaneously and in the presence of tumor cells.
Figure 1.
Impact of IFNγ addition or depletion on H-1PV immunomodulating activity. (A) Macrophages were isolated from the peritoneal cavity of four groups (n = 3) of tumor bearing Lewis rats treated either with PBS (mock) or with an intratumoral injection of 5 × 108 pfu/rat of H-1PV (H-1PV IT) combined either with an antibody against IFNγ (H-1PV IT + αIFNγ) or recombinant IFNγ (H-1PV IT + recIFNγ). Cells were plated in 48-well plates at a density of 5 × 105 cells per well and stimulated or not with LPS. TNFα production in the supernatants was measured 24 h later by ELISA. Average values and standard deviations are shown. (B) Peritoneal macrophages (5 × 105/well) from the same groups of rats were cocultured or not with 1 × 105 HA-RPC cells at a 5:1 ratio in 48 well plates and the release of interleukins -10 and -12 was measured by ELISA. Mean cytokine ratios and standard deviations are presented. (C) Single cell suspensions of rat splenocytes were labeled with CFSE, plated in 24 well plates at 1 × 106 cells/ well and cocultured or not with 2 × 105 HA-RPC cells at a 5:1 ratio. 48 h later cells were harvested and processed for FACS analysis of proliferation. All data were median from three animals from triplicate wells. Differences were considered significant at p values below 0.05.
These data pointed that H-1PV application alone can activate peritoneal macrophages or combined with recIFNγ i.p. introduction could change the activation status of immune cells both in spleen and in the peritoneal cavity leading to predominance of immuno-stimulatory cytokines (TNFα, IL-12) over the immunosuppressive factors (IL-10). The decrease of the above-mentioned immunological parameters upon depletion of IFNγ, especially in the case of peritoneal macrophages, confirmed our assumption that this cytokine plays a role in stimulating the innate immune system as part of the immunomodulating effect of oncolytic H-1PV.
RecIFNγ improves the therapeutic potential of H-1PV for the treatment of PDAC peritoneal carcinomatosis
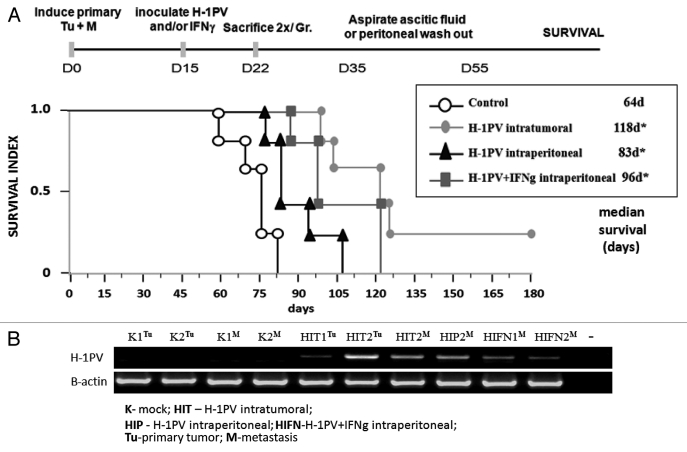
The results obtained encouraged us to use a combination of recombinant IFNγ and H-1PV parvovirus for the treatment of one of the most lethal complications of pancreatic cancer in humans, namely the spread of the tumor to the peritoneal cavity. To mimic this situation we induced in parallel tumors both in the pancreas and in the peritoneal cavity of Lewis rats. Two weeks later, the rats were randomly divided into four groups, in which H-1PV was applied through two different routes (intratumoral or intraperitoneal). In one group the virus i.p. inoculation was combined with recIFNγ using the same route (Fig. 2A, protocol). Animal survival was followed (Fig. 2A) confirming that H-1PV intratumoral injection was still most effective to protect rats against PDAC with two animals remaining tumor free more than six months after treatment.5 H-1PV could significantly improve the survival of rats upon peritoneal application compared with the control group but was still less effective in comparison to the i.t. route. Notably, the combination with recIFNγ could significantly improve the effect of the i.p. injected virus, extending the median survival from 83–96 d (Fig. 2A).
Figure 2.
Therapeutic effects of H-1PV+IFNγ combination and virus distribution. (A) Lewis rats (n = 28) bearing simultaneously induced orthotopic tumors and peritoneal metastases were divided into four groups (n = 7) and either left untreated (control), inoculated i.t. (H-1PV intratumoral) or i.p (H-1PV intraperitoneal) with H-1PV in the absence or presence of recIFNγ (H-1PV+IFNγ intraperitoneal). After the sacrifice of two animals 1 week after treatment, the survival of five rats from each group was followed up to six months after tumor induction when animals were sacrificed. Median values were considered significant at p values below 0.05. (B) Two animals per group were sacrificed 1 week after H-1PV and/or IFNγ treatments. Total RNA was extracted from visible tumors and metastases, converted to cDNA and subjected to RT-PCRs to evaluate the presence of H-1PV DNA/ unspliced mRNA and β-actin transcripts, using respective primers. The abbreviations for the route and type of treatment are indicated on the figure. The source of the material (Tumor or Metastasis) is indicated in superscript.
Two animals per group were sacrificed one week after treatment to analyze virus presence by RT-PCR (Fig. 2B). The distribution of viral DNA signals showed that (i) the virus could migrate from the primary tumor after intratumoral application (HITTu) to metastasis (HITM) in the peritoneal cavity, (ii) it can infect metastasis upon intraperitoneal inoculation (HIPM), and (iii) that upon i.p. combination with H-1PV IFNγ does not change significantly the virus levels in metastases (compare HIPM and HIFNM).
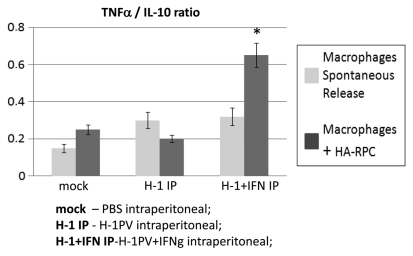
Isolation of peritoneal macrophages from mock, H-1PV or H-1PV with IFNγ intraperitoneally treated rats showed that the ratio between TNFα and IL-10 produced was significantly increased in the presence of recombinant IFNγ when macrophages were cocultured with HA-RPC cells, speaking in favor of phagocytes’ activation (Fig. 3).
Figure 3.
Macrophage activation after H-1PV+IFNγ combined treatment. Macrophages were isolated from the peritoneal cavity of the H-1PV and H-1PV+IFNγ intraperitoneally injected rats, plated at 5 × 105/well in 48 well plates and cocultured or not with 1 × 105 HA-RPC cells. The ratios of TNFα and IL-10 in supernatants were determined 24 h later by ELISA.
In conclusion, intratumoral application of H-1PV seems to have a superior effect compared with intraperitoneal inoculation for the treatment of PDAC. In the case where intraperitoneal inoculations of the virus are performed at the stage of advanced metastatic disease, a combination with IFNγ can be very favorable.
RecIFNγ cotreatment reduces the titers of H-1PV neutralizing antibodies in ascitic fluid
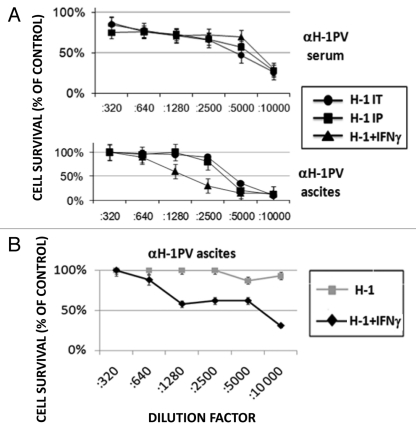
One of the major functions of IFNγ is its ability to prime the cellular (through Th1 cells/ cytokines) and to down-modulate the humoral (through Th2 cells/cytokines) immune response. Therefore, we assumed that the combination of IFNγ and oncolytic H-1PV may also reduce the titers of neutralizing antibodies produced against the virus. In order to address this hypothesis, we collected serum from peripheral blood and ascitic fluid from the peritoneal cavity of rats participating in the above-mentioned experiment and determined the titers of αH-1PV antibodies, using a cytotoxicity protection assay on virus-sensitive cells. We found that in the first experiment performed, no evident difference could be detected in the titer of αH-1PV in animal sera irrespective of the virus inoculation route and IFNγ treatment (Fig. 4A, upper panel). Similarly, the inoculation route had no impact on the antiviral titers in ascitic fluid collected in the time-frame (20–40 d) after virus treatment. On the other hand, co- application of recIFNγ together with H-1PV caused a significant reduction (from 1:5000 to 1:1280) in the titers of αH-1PV in the ascitic fluid of the animals most probably due to the stronger effect of i.p. applied IFNγ.
Figure 4.
Influence of IFNγ application on the generation of virus neutralizing antibodies. (A) Serum and ascitic fluid were collected from all groups of virus-treated rats (Fig. 2) and the titers of virus neutralizing antibodies (αH-1PV) were determined using cytotoxicity protection assay on NB324K cells. The titers are expressed as the percentage of antivirus protection offered by serum or ascitis dilutions compared with mock infected cells. (B) Two groups of metastasis bearing rats were treated with two intraperitoneal injections of H-1PV (3 × 108 pfu/injection per animal) spanning four weeks between them, with or without intermediate recIFNγ i.p. inoculation. Titers of αH-1PV in ascitic fluids were determined 10–30 d after the second H-1PV i.p. injection and expressed as indicated above.
We then attempted, in a modified experimental setting to test whether the IFNγ-provoked drop in antiviral antibodies within ascites would increase the levels of H-1PV DNA in metastases when this cytokine is applied before a second virus inoculation. We noticed first that, the titers of αH-1PV in ascitic fluid collected within 10–30 d after this second H-1PV i.p. injection (Fig. 4B) were much higher than the ones induced by a single H-1PV i.p. application (Fig. 4A, lower panel). This effect was most probably due to boosting of the immune system related to the repeated virus application. Interestingly, when IFNγ was applied before the second H-1PV inoculation (H-1+IFNγ) we noticed that αH-1PV titers remained similar to the ones observed in fluids from animals subjected to a single virus inoculation (compare Fig. 4B with 4A, lower panel), suggesting that the cytokine has inhibited the overproduction of αH-1PV triggered by the second virus injection.
We also evaluated the H-1PV transduction level of metastasis in the two groups of rats after the second virus application (Fig. S1). Unfortunately, IFNγ treatment had no positive impact on the amounts of viral DNA in metastasis despite the reduction of antiviral antibodies (Fig. 4B) suggesting that this reduction was not sufficient to overcome the antibody pressure in ascitic fluid.
Rec IFNγ can improve the effect of H-1PV to stimulate the human innate immune system
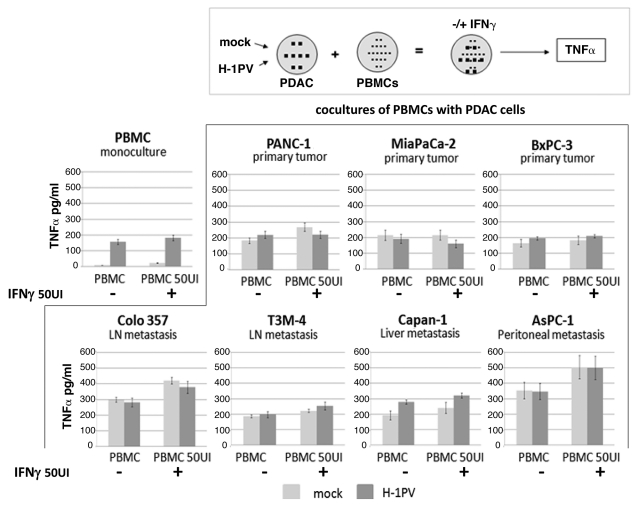
In search of clinical relevance of the obtained data, we continued our studies using human PDAC cell lines and peripheral blood monocytes derived from healthy donors, aiming to find out whether the latter can be activated more efficiently with a combination of virus and IFNγ. We previously reported that H-1PV infection leads to a limited but significant activation of human PBMCs as indicated by their TNFα release. The latter effect was largely masked in the case when PBMCs were cocultivated with pancreatic cancer cells irrespective of their infection status.6 Considering the fact that PDAC cells can express IFNγ receptors12,13 we evaluated first of all the lethal effect of H-1PV and IFNγ combination on pancreatic cancer cells. We established that IFNγ does not change H-1PV-induced toxicity on human PDAC cells (Fig. S2). In a next step, PBMCs were cocultured with pancreatic cancer cells that had been previously infected (or not) with H-1PV, and used the release of TNFα as a read-out for innate immune cell activation. As already previously reported, the direct infection of PBMCs with H-1PV resulted in an increased release of TNFα at 48hpi (Fig. 5, PBMC monoculture).6 Addition of relatively low dose IFNγ (50 UI/ml) to the cultures did not significantly enhance TNFα production. The same was the case when PDACs were pre-infected with H-1PV before coculturing them with the PBMCs. However, in general, in the presence of IFNγ, PBMC cocultures with Panc-1, T3M4, Capan-1 and especially Colo357 and AsPC-1 produced approximately 100–150 pg/ml more TNFα, corresponding to a higher level of activation of innate immune cells. Interestingly, despite the fact that the fluctuations of TNFα were not statistically significant, a tendency could be observed that PDAC cells deriving from metastatic (lymph node, liver or peritoneal) pancreatic cancer seemed to be more potent stimulators of PBMCs in the presence of IFNγ than the lines established from primary PDAC tumors. In general, despite the lack of significance such an observation may still merit broader investigation if not in the context of H-1PV oncolytic therapy then for the treatment of advanced metastatic disease with IFNγ.
Figure 5.
Release of TNFα from PDAC and PBMC cocultures after H-1PV+IFNγ treatment.The indicated pancreatic cancer cell lines were seeded into 10 cm2 dishes at 1.5 × 106 cells/ dish and infected or not with H-1PV at an MOI of 10 pfu/cell. Twenty-four hpi cells were harvested and plated onto pre-isolated PBMCs in 48 well plates at a ratio of PDAC:PBMC 1:5. The cocultures were treated or not with 50 UI/ml of human recombinant IFNγ and the release of TNFα was measured in supernatants 24 h later by ELISA. Mock or H-1PV infected (MOI 10) monocultures of PBMCs served as controls. The indicated values are average of at least three independent experiments. SD values are shown.
Discussion
In view of our previous data identifying IFNγ as one of the immune mediators of H-1PV anticancer effects in pancreatic cancer therapy, we continued our preclinical studies by attempting to treat peritoneal carcinomatosis as one of the most common complications marking the pre-terminal stages of PDAC. Considering the fact that intraperitoneally applied IFNγ has gained acceptance as therapy for ovarian cancer, we assumed that this beneficial effect may be due to the enrichment of the peritoneal cavity with macrophages that represent one of the major targets of IFNγ stimulatory effects, thus being converted into potent direct antitumor effectors. Latest reports show that macrophages might be key players in successful immunotherapies against PDAC.14 We found that the survival of rats bearing both primary tumors and peritoneal metastasis could be prolonged by H-1PV intraperitoneal application. Recently, oncolytic reovirus was also successfully used to treat the dissemination of pancreatic cancer cells to the peritoneum.15 In our preclinical model, using parvoviruses we found that this treatment regimen could be further improved by the concomitant inoculation of recombinant interferon gamma. The therapeutic effects were accompanied by modulation of macrophage and splenocyte immune functions especially when IFNγ was present in the treatment protocol. Interestingly, the best therapeutic result was still obtained when H-1PV was inoculated directly into the tumors leading to about 40% long-term survivors. A plausible explanation for this could be the fact that from this location the virus could block both the local development of the primary malignancy preventing vessel obstructions and other complications and, as we could demonstrate, also migrate to metastatic deposits. However, it is also likely that still the cellular milieu present within the primary tumor allows for better priming of the immune system than the environment characteristic for metastasis. In general, one has to consider that the rats used in our studies had an intact immune system and under these conditions H-1PV has already demonstrated its ability to trigger certain level of IFNγ expression, which could explain why we could not improve further the therapeutic effect of H-1PV intratumoral inoculation by concomitant IFNγ treatment (data not shown). Therefore, this system does not faithfully reflect the immuno-depressed state of cancer patients where the additional application of IFNγ may contribute much more to the therapeutic effect of H-1PV, bringing also a more pronounced benefit in terms of immunomodulation, local tumor control and overall survival rates compared with our animal experiments. In addition to that, IFNγ may additionally foster antigen presentation by increasing the expression of MHCII molecules on the surface of macrophages and dendritic cells.
The observed reduction in the titers of virus neutralizing antibodies induced by IFNγ represents a very interesting phenomenon in the frame of oncolytic virotherapy. It is in agreement with the changes observed in the Il-12/Il-10 cytokine ratio secreted from macrophages pointing to a shift in the Th1/Th2 balance in the peritoneal cavity. Probably, an additional modification of the IFNγ treatment protocol or its combination with certain immunosuppressive agents, recently reported in oncolytic virotherapy may improve the described effect and reduce the antibodies to levels permitting repeated virus applications and metastasis transduction.16
Treatment of peripheral blood mononuclear cells with H-1PV could prime the release of TNFα, a cytokine that represents one of the main products secreted upon macrophage activation possessing also strong antitumor properties. However, coculturing PBMCs with pancreatic cancer cell lines deriving from different metastatic organ locations caused a generalized increase in TNFα levels that seemed to almost completely mask the effect of H-1PV pre-infection of PDAC cells. IFNγ could serve as an additional stimulator of TNFα production mostly in the case of cocultures between PBMCs and metastatic PDAC cancer lines. Notably, this effect was most pronounced for AsPC-1, a cell line deriving from a clinical case of peritoneal metastasis, therefore giving stronger credibility to our results obtained in animal experiments with peritoneal carcinomatosis.
In conclusion, we consider that the combination of an oncolytic virus with a powerful imunomodulating cytokine like IFNγ may represent a promising strategy for cancer therapy. In view of the forthcoming clinical applications of H-1PV as an oncolytic agent, a therapeutic protocol involving co-treatment with the two modalities has potential to improve the outcome in terminal stage patients with pancreatic cancer.
Materials and Methods
Cells and reagents
Human pancreatic carcinoma cell lines from primary (Panc-1, MiaPaCa-2, BxPC-3) or metastatic (Capan 1, T3M4, AsPC-1, Colo357) tumors, were obtained from ATCC and grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). The HA-RPC cell line derived from a chemically induced pancreatic ductal adenocarcinoma in Lewis rats was grown in DMEM supplemented with 10% FCS. Human NB324K cells used for cytotoxicity protection assays were cultured in MEM medium with 5% FCS. All media were supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml). Lyophilized recombinant rat and human IFNγ were obtained from Biomol GMBH and reconstituted in sterile deionized water. The mouse monoclonal antibody clone DB-1 with specificity against murine IFNγ (αIFNγ) was produced in bulk amount by NatuTec GmbH. Where indicated, in some experiments cells were stimulated using LPS at final concentration of 5 μg/ml.
For the isolation of peritoneal macrophages rats received an i.p. injection of 4 ml of 4% Thioglycolate solution in PBS three days before sacrifice. After sacrificing the animals, 40 ml of sterile PBS were instilated in the peritoneal cavity and recovered using a syringe. The cells were collected by centrifugation and plated in DMEM containing 10% FCS and antibiotics.
Peripheral blood mononuclear cells (PBMC) were isolated from the heparinized blood of randomly selected healthy donors by differential centrifugation over Histopaque (Sigma) and cultured in RPMI with 10% FCS and antibiotics. Peripheral blood macrophages were enriched by adherence to plastic surface. Buffy coats were obtained from the blood bank of IKTZ Heidelberg.
Virus-neutralizing antibody detection
Serial dilutions of the sera of experimental animals were made in MEM and mixed with an equal volume of H-1PV virus suspension (corresponding to 2 × 104 pfu/well). After incubation for 30 min at 37°C, the mixture was inoculated onto NB324K cells plated in 96-well plates (2 × 103 cells/well). The cell survival rates were assessed after 72 h using a MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] assay.
Semi-quantitative RT-PCR
For RT-PCR total RNA was extracted from pancreatic tumors or metastatic nodules of treated animals, reverse transcribed into cDNA, and PCRs for H-1PV and β-actin were performed using previously described primer sequences and conditions.6
ELISA
Measurement of rat TNFα; IL-10, IL-12 and human TNFα release was done using commercially available ELISA kits from eBioscience as described by manufacturer.
FACS determination of splenocytes’ proliferation index
Rat spleens were pressed against a mesh to obtain single cell suspensions and splenocytes were adjusted to a concentration of 5 × 106/ml in PBS. The stock 5mM CFSE solution was diluted at 1/1000 in PBS (a final concentration of 5 µM), added to lymphocytes pellet and mixed rapidly. After incubation for 5 min at room temperature ten volumes of PBS containing 5% FSC were added and the cells were centrifuged. Washes in PBS/FCS were repeated three times. Labeled splenocytes were co-cultured with HA-RPC cells or alone as a control. After 72 h of incubation, cells were collected, washed and measured for CFSE fluorescence using FACSCalibur (BD). The proliferation index was calculated based on the level of reduction in fluorescence intensity of the cultures.
Animal studies
The orthotopic rat model using HA-RPC cells has been previously described.5 For the induction of metastasis a cell suspension was prepared in phosphate-buffered saline (PBS) out of subcutaneous tumors performed by implantation of HA-RPC cells and injected intraperitoneally to Lewis rats at 3 × 106 cells in 500 μl per animal.
Rat recIFNγ was injected in 3 consecutive weeks at 30 000 UI/week i.p. in a 100 µl volume for a total dose of 90 000 UI/ animal. The αIFNγ antibody was applied at the same times at 0.8 mg/ animal for a total dose of 2.4 mg. Ascitic fluid was obtained using a peritoneal puncture under aerosol anesthesia at the time before animal sacrifice.
Statistical methods
Means and standard deviations were calculated from at least three animals and in triplicate wells for in vitro experiments. Statistical differences were assessed using Student t-test and Wilcoxon test. For in vivo mortality data assessment, experimental groups were compared with log-rank test.
Supplementary Material
Supplementary PDF file supplied by authors.
Acknowledgment
This study was partially supported by a grant from the German Research Council, (DFG- GI 802/1–1, RA 1891/2–1) and ORYX GmbH, representing a potential conflict of interest. We are grateful to S. Rüffer from the Department of Surgery, University Hospital Heidelberg for his technical support.
Glossary
Abbreviations:
- IFNγ
interferon gamma
- PBMCs
peripheral blood mononuclear cells
- PDAC
pancreatic ductal adenocarcinoma
- i.p.
intraperitoneal
- i.t.
intratumoral
- pfu
plaque forming units
Disclosure of Potential Conflicts of Interest
The study was supported by ORYX GmbH.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/17678
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M. Cancer Statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Fleming JB, Lee JE, Pisters PW. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010;15:1205–13. doi: 10.1634/theoncologist.2010-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics. Mol Med Today. 1996;2:519–27. doi: 10.1016/S1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis JJ, Haag A, Kornfeld C, Balboni G, Dege A, Neeb B, et al. Autonomous parvovirus vectors In: Cid-Arregui A, Garcia-Garrancá A, eds Viral Vectors: Basic Science and Gene Therapy Natick, MA: Eaton Publishing 2000;97–118. [Google Scholar]
- 5.Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res. 2009;15:511–9. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 6.Grekova S, Aprahamian M, Giese N, Schmitt S, Giese T, Falk CS, et al. Immune cells participate in the oncosuppressive activity of parvovirus H-1PV and are activated as a result of their abortive infection with this agent. Cancer Biol Ther. 2011;10:1280–9. doi: 10.4161/cbt.10.12.13455. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 8.Toporovski R, Morrow MP, Weiner DB. Interferons as potential adjuvants in prophylactic vaccines. Expert Opin Biol Ther. 2010;10:1489–500. doi: 10.1517/14712598.2010.521495. [DOI] [PubMed] [Google Scholar]
- 9.Miller CH, Maher SG, Young HA. Clinical Use of Interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E. al. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer. 2009;125:367–73. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 11.Windbichler GH, Hausmaninger H, Stummvoll W, Graf AH, Kainz C, Lahodny J, et al. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82:1138–44. doi: 10.1054/bjoc.1999.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raitano AB, Scuderi P, Korc M. Binding and biological effects of tumor necrosis factor and gamma interferon in human pancreatic carcinoma cells. Pancreas. 1990;5:267–77. doi: 10.1097/00006676-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 14.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano S, Etoh T, Okunaga R, Shibata K, Ohta M, Nishizono A, et al. Reovirus inhibits the peritoneal dissemination of pancreatic cancer cells in an immunocompetent animal model. Oncol Rep. 2009;21:1381–4. doi: 10.3892/or_00000364. [DOI] [PubMed] [Google Scholar]
- 16.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–88. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.