Abstract
Norcantharidin (NCTD) has been reported to induce tumor cell apoptosis. However, the underlying mechanism behinds its antitumor effect remains elusive. We have previously shown that TR3 expression is significantly decreased in metastatic melanomas and involved in melanoma cell apoptosis. In this study, we showed that NCTD inhibited melanoma cell proliferation and induced apoptosis in a dose related manner. NCTD induced translocation of TR3 from nucleus to mitochondria where it co-localized with Bcl-2 in melanoma cells. NCTD also increased cytochome c release from mitochondria to the cytoplasm. These changes were accompanied by increased expression of Bax and cleaved caspase-3 along with decreased expression of Bcl2 and NF-κB2. The effects of NCTD were inhibited by knockdown of TR3 expression using TR3 specific shRNA in melanoma cells. Furthermore, NCTD significantly decreased tumor volume and improved survival of Tyr::CreER; BRAFCa/+; Ptenlox/lox transgenic mice. Our data indicates that NCTD inhibits melanoma growth by inducing tumor cell apoptosis via activation of a TR3 dependent pathway. These results suggest that NCTD is a potential therapeutic agent for melanoma.
Keywords: norcantharidin, apoptosis, TR3, melanoma
Introduction
Malignant melanoma represents a significant and growing public health burden worldwide. The incidence of metastatic melanoma cases is increasing significantly faster than any other cancer in the US.1,2 At the current rate, 1 in 74 Americans will develop melanoma during his or her lifetime.3 Until recently there were few treatment options for advanced melanoma.4 However, anti-CTLA4 and mutant BRAF-targeted therapies have shown great promise for advanced melanoma.5,6 Unfortunately, only a subset of patients respond to these treatments and drug resistance may occur within a short period of time.7-10 There is an urgent clinical need to develop new therapeutic modalities that may be used alone or in combination with these therapies to treat melanoma.
Programmed cell death (apoptosis) plays an important role in development, homeostasis and anticancer protection of multicellular organisms.11-13 Apoptosis is characterized by distinct morphological and biochemical changes that take place upon the activation of a family of serine proteases known as caspases.14 TR3 (also called Nur77 or NGFI-B) is an immediate-early response gene and an orphan member of the nuclear receptor super family and was originally recognized for its role in the regulation of cell survival and differentiation.15,16 More recently it has been shown to induce apoptosis in a number of cell lineages exposed to proapoptotic stimuli by directly targeting the mitochondria and inducing cytochrome c release.17-19 It plays an important role during cell apoptosis by inducing conformation changes of Bcl-2.20,21 We previously showed that TR3 expression was significantly decreased in melanomas comparing to benign nevi.19 Overexpression of wild type TR3 or mutant TR3 lacking the DNA binding domain resulted in massive apoptosis in melanoma cells.19 Thus, the TR3 dependent pathway plays an important role in the control of melanoma cell apoptosis and can be used as a target pathway for screening biologically active substances in the treatment of melanoma.
Norcantharidin (NCTD) is the demethylated analog of cantharidin (CTD), a 7-oxabicyclo [2.2.1] heptane-2, 3-dicarboxylic acid derivative isolated from natural blister beetles.22 CTD has been used as a medicinal agent listed under the name of Mylabris for over 2,000 y to treat abdominal masses, rabies, as well as an abortifacient.23 CTD has antitumor activity and at the same time causes Ieukocytosis, however, it is very toxic and a strong irritant for urinary system.23,24 NCTD can be easily synthesized from furan and maleic anhydride via the Diels-Alder reaction and it has significantly less side effect.24 NCTD has been reported to induce cell apoptosis in oral, breast, liver cancer and melanoma in vitro25-29 and prolong the life of mice carrying hepatoma in vivo.30 However, the underlying mechanism by which NCTD exerts its effects remains unclear.
In this study, we investigated the effect of NCTD on melanoma in vitro and in vivo. We found that NCTD can suppress melanoma growth by inducing tumor cell apoptosis. We discover a new mechanism that NCTD exerts its apoptotic effects through TR3 mitochondria translocation. The effect of NCTD depends on the expression of TR3 in melanoma cells. The result suggests that NCTD is potential therapeutic agent for melanoma treatment.
Results
NCTD induces melanoma cell apoptosis
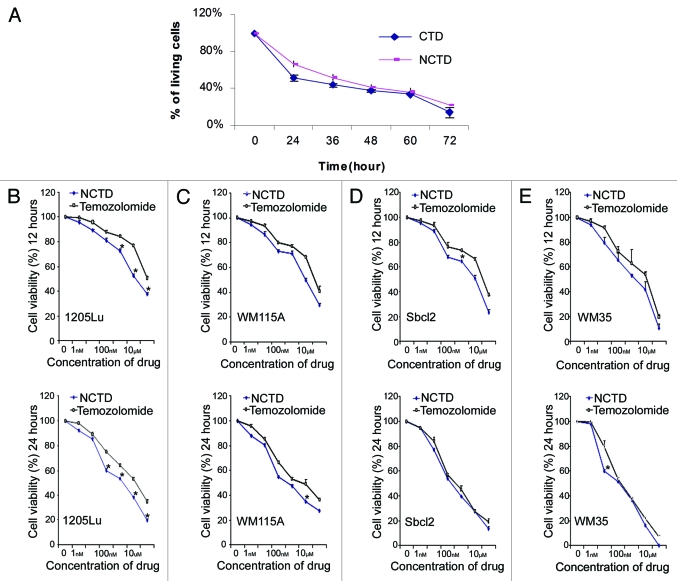
We first compared the effect of NCTD (6.5 µM) with CTD (6.5 µM) on melanoma growth using MTT assays. They showed similar inhibitory effect on melanoma cell proliferation 24 h after treatment (Fig. 1A). Then we compared the effect of NCTD with temozolomide, a chemotherapeutic drug used clinically to treat melanoma. Four different melanoma cell lines (1205Lu, WM115A, Sbcl2 and WM35) were used in the MTT assays. Tumor cells were treated with NCTD or temozolomide for 12 or 24 h at different concentrations (0, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM and 100 µM). NCTD and temozolomide induced dose dependent inhibition of melanoma cell proliferation. Twenty-four hour treatment induced more inhibition of cell proliferation than 12 h treatment. NCTD had significantly better or comparable effect to temozolomide in suppressing of melanoma growth (Fig. 1B–E). Similar results were found when we compared NCTD with cisplatin (data did not show).
Figure 1.
NCTD inhibits melanoma cell survival. (A) Inhibitory effects of CTD and NCTD on 1205Lu cells. The cytotoxicity of CTD and NCTD was evaluated using the MTT assay. Three independent experiments were performed. (B–E) Effect of NCTD on 1205Lu, WM115A, Sbcl2 and WM35 cells. The cytotoxicity of NCTD and temozolomide was evaluated using the MTT assay. Melanoma cells were treated for 12 or 24 h in the presence of various concentrations of NCTD or temozolomide as indicated. * Indicates p < 0.05, compared with corresponding temozolomide treated melanoma cells.
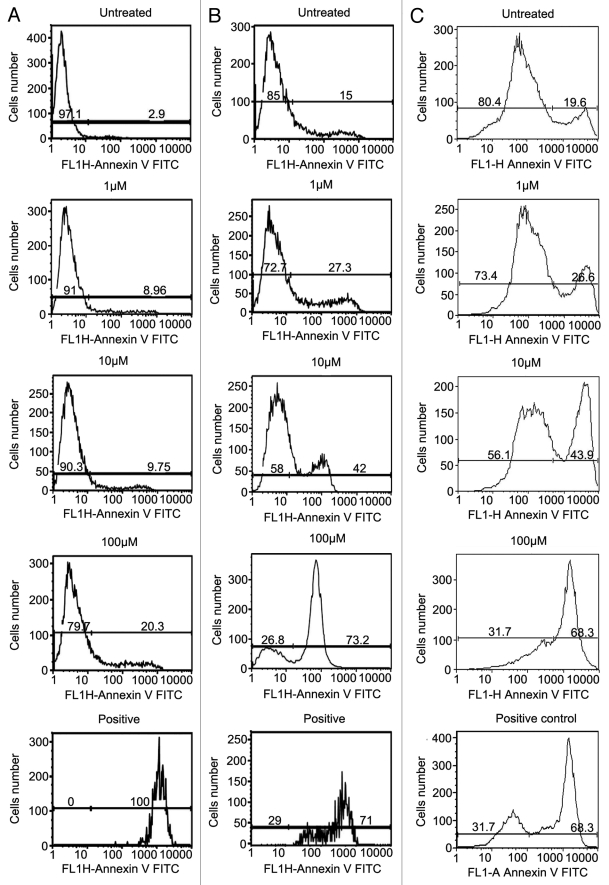
We then tested whether the antitumor effect of NCTD is due to its ability to induce cell apoptosis. We compared the effect of NCTD on foreskin derived normal melanocyte, WM35, and 1205Lu melanoma cell apoptosis using Annexin V staining and FACS analysis. Tumor cells were treated with NCTD (concentration of 0, 1 µM, 10 µM, and 100 µM) for 24 h. Cells treated with DMSO were used as negative control. Positive control was induced by incubation of the tumor cells with 5% ethanol for 60 min.31 NCTD induced dose-dependent increase of melanocyte, WM35 and 1205Lu cell apoptosis (Fig. 2). The percentage of apoptotic melanocytes after NCTD treatment (1 µM, 8.96 ± 1.39%; 10 µM, 9.75 ± 2.75%; 100 µM, 20.3 ± 1.98%) was less than that of WM35 (1 µM, 27.3 ± 2.33%; 10 µM, 42 ± 7.22%; 100 µM, 73.2 ± 4.69%) or 1205 Lu (1 µM, 26.6 ± 3%; 10 µM, 43.9 ± 6.98%; 100 µM, 68.3 ± 7.32%).
Figure 2.
NCTD induces apoptosis in melanoma cells. Melanocyte, WM35 and 1205Lu cells were treated with different concentrations of NCTD (0, 1, 10 and 100 µM) for 24 h, stained with Annexin V and analyzed by FACS analysis. Column (A) percentage of apoptotic melanocytes after NCTD treatment; Column (B) percentage of apoptotic WM35 cells after NCTD treatment; Column (C) percentage of apoptotic 1205Lu cells after NCTD treatment. Three independent experiments were performed.
NCTD induces TR3 translocation to mitochondria
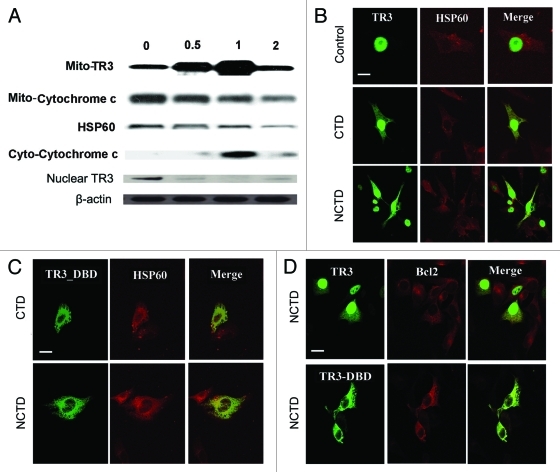
We first examined whether NCTD increases TR3 expression. Melanoma cells (1205 Lu) were harvested 0.5, 1, and 2 h after exposure to NCTD (6.5 µM). Mitochondria, cytosolic and nuclear fractions of treated cells were isolated and subjected to protein gel blot analysis. There was a rapid increase in mitochondria TR3 protein levels, with a concomitant decrease in nuclear TR3 peaking at 1 h after treatment. This change was accompanied by a decrease of mitochondrial cytochrome c and an increase of cytoplasmic cytochrome c peaking at 1 h after treatment (Fig. 3A).
Figure 3.
NCTD induces TR3 mitochondrial translocation in melanoma cells. (A) Melanoma cells were treated with NCTD (6.5 µM) for 0–2 h. Mitochondrial (for TR3 and cytochrome C), cytoplasmic (for cyto-cytochrome C) or nuclear (for TR3) proteins were isolated from the melanoma cells and analyzed by protein gel blot analysis. HSP60 was used as a loading control for mitochondrial proteins. β-actin was used as a loading control for total protein. Representative blot from three repeats was shown. (B) Melanoma cells were transfected with TR3-GFP and then treated with PBS, CTD or NCTD for 3 h. HSP60 staining was used to visualize the mitochondria in the cytoplasm. Representative fluorescent images of tumor cells after treatment were shown (n = three repeats). Bars indicate 10 μm. (C) Melanoma cells were transfected with TR3-GFP/ΔDBD and then treated with CTD or NCTD for 3 h. HSP60 staining was used to visualize the mitochondria in the cytoplasm. Representative fluorescent images of tumor cells after treatment were shown (n = three repeats). Bar indicates 10 μm. (D) Melanoma cells were transfected with either GFP-TR3 or GFP-TR3-ΔDBD, and then treated with NCTD for 3 h. BCL-2 staining was performed. Representative fluorescent images of tumor cells after treatment were shown (n = three repeats). Bar indicates 10 μm.
To confirm that NCTD induces TR3 mitochondrial translocation, 1205Lu cells were transfected with wild type GFP-TR3 plasmid as previously described,19 and these cells were treated with CTD, NCTD or DMSO (carrier control). In DMSO-treated melanoma cells, GFP-TR3 exhibited an exclusive diffuse nuclear staining pattern consistent with previously reported results.32 Exposure to CTD or NCTD (6.5 µM) induced a rapid, but incomplete translocation of TR3 to the mitochondria in melanoma cells. Within 3 h of exposure, we observed a punctuate GFP-TR3 staining pattern in the cytoplasm (Fig. 3B). Co-staining with an antibody against the mitochondrial associated protein Hsp60 revealed an overlap between GFP-TR3 and Hsp60 in the cytoplasm after CTD or NCTD treatment.
To study whether the effect of NCTD is mediated through the TR3 DNA binding domain (DBD), 1205Lu cells were transfected with GFP-TR3 without DNA binding domain (GFP-TR3/ΔDBD). GFP-TR3/ΔDBD was detected exclusively in the cytoplasm (Fig. 3C) similarly to that was reported in LNCaP cells transfected with TR3/ΔDBD.33 GFP-TR3/ΔDBD co-localized with mitochondrial Hsp60 3 h after exposure to CTD or NCTD (6.5 µM). These results indicate that the effect of NCTD does not require the TR3 DBD.
TR3 co-localizes with Bcl-2 after NCTD treatment
It has been shown that TR3 localized to mitochondria induces a Bcl-2 conformational change that exposes its BH3 domain, resulting in conversion of Bcl-2 from an inhibitor of apoptosis to an apoptosis inducer.16 We studied whether NCTD can induce TR3 co-localization with Bcl-2. TR3-GFP transfected 1205Lu melanoma cells were treated with NCTD (6.5 µM) for 3 h. Bcl-2 was visualized by immunostaining followed by confocal microscopy. Indeed, TR3 co-localized with Bcl-2 in melanoma cells after treatment (Fig. 3D, upper panel). TR3/ΔDBD transfected cells showed similar co-localization with Bcl-2 after treatment, indicating that interaction of TR3 and Bcl-2 does not require DBD (Fig. 3D, lower panel).
Effect of NCTD on TR3 protein expression in melanoma
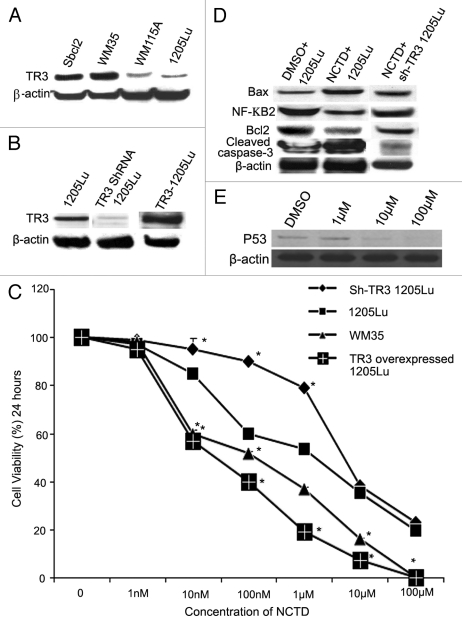
We first examined the expression level of TR3 protein in human melanoma cell lines derived from various stages of tumor progression by protein gel blot. TR3 protein expression was detectable in all cell lines examined. TR3 expression levels were significantly higher in the cell lines derived from radial growth phase melanomas (WM35 and Sbcl2) than those expressed in cell lines derived from metastatic melanomas (1205Lu and WM115A) (Fig. 4A).
Figure 4.
TR3 mediates the effects of NCTD on melanoma cells. (A) TR3 expression in melanoma cells. Radial growth phase (Sbcl2 and WM35) melanoma cells expressed higher levels of TR3 than metastatic (WM115A and 1205Lu) melanoma cells. β-actin was used as a loading control (representative blot from three experiments). (B) TR3 knockdown or overexpression. 1205Lu cells were transfected with TR3 shRNA or overexpression vectors. Protein gel blots were performed to confirm the levels of TR3 expression in these cells. β-actin was used as a loading control (representative blot from three experiments). (C) Effect of NCTD depends on TR3 expression level in melanoma cells. 1205Lu cells with TR3 knockdown, overexpression or control as well as WM35 cells were treated with different concentrations of NCTD for 24 h and cell survival was evaluated using the MTT assay. * Indicates p < 0.05, compared with 1205Lu control cells. (D) NCTD activates TR3 dependent apoptotic pathway. 1205Lu and 1205Lu with TR3 knockdown cells were treated with NCTD or vehicle for 24 h and cell lysates were subjected to protein gel blot with antibodies to Bax, Bcl2, cleaved caspase-3 or NF-κB2. β-actin was used as a loading control (representative blot from three experiments). (E) p53 expression after NCTD treatment. 1205Lu cells were treated with different concentrations of NCTD and then cell lysates were subjected to protein gel blot with an antibody to P53.
To further study the role of TR3 in NCTD induced apoptosis, we transfected 1205Lu melanoma cells with vectors containing shRNA to TR3 or TR3 overexpressing vectors. The knockdown and overexpression of TR3 protein was confirmed by protein gel blot (Fig. 4B). Cell survival in response to NCTD was assessed by MTT assay. The time- and dose-dependence of the cytotoxic effect on the four cell lines are shown in Figure 4C. The median inhibitory concentration (IC50) for 1205Lu cells was 6.5 ± 1.27 µM; TR3 knockdown 1205Lu cells, IC50 is 11.73 ± 0.95 µM; TR3 overexpressed 1205LU cells, IC50 is 87.23 ± 17.38 nM; WM35 cells, IC50 is 235.24 ± 27.5 nM. These results suggest that the effects of NCTD depend on the TR3 expression levels in melanoma cells.
To further study the underlying mechanism of NCTD on melanoma cells, we examined important proteins involved in apoptosis. NCTD treatment of 1205Lu cells resulted in increased expression of Bax and cleaved caspase-3, which was accompanied by decreased expression of Bcl2 and NF-κB2 (Fig. 4D). The effects of NCTD on these proteins were diminished after knockdown of TR3 expression (Fig. 4D). In addition, we also found that higher doses of NCTD (10 and 100 µM) inhibited the p53 expression (Fig. 4E).
NCTD inhibits melanoma growth in vivo
Mutational activation of BRAF is the most common genetic alteration in human melanoma. Here, we used a transgenic mouse model in which expression of BRAFV600E combined with Pten tumor suppressor gene silencing elicited development of melanoma with 100% penetrance34 in order to study the role and the mechanism of NCTD-mediated cytotoxicity toward melanoma in vivo.
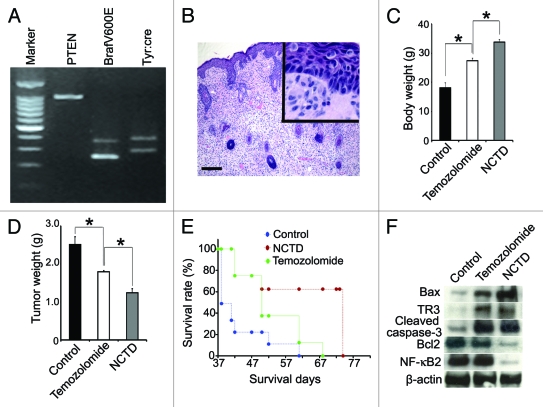
First we confirmed the genotype of these mice by PCR. As we previously reported,34 these mice showed typical genotype of Tyr::CreER; BrafCA/+;Ptenlox/lox (Fig. 5A). After 4-TH induction, the mice developed melanoma and histology showed pagetoid proliferation of tumor cells in the epidermis and the dermis was occupied by the epithelioid and spindled melanoma cells (Fig. 5B). We then determined the effect of NCTD on melanoma growth by treating the transgenic mice with NCTD, temozolomide or vehicle. We examined the tumor weight and mouse body weight 40 d after melanoma induction with 4-HT. As depicted in Figure 5C and D, we found that there was a significant decrease in tumor volume accompanied by an increase in body weight in transgenic mice treated with NCTD comparing to either vehicle control or temozolomide. These results suggest that NCTD exerts an inhibitory effect on melanoma tumor growth and cachexia.
Figure 5.
NCTD inhibits melanoma growth and cancerous cachexia in vivo. Melanoma was induced by applying 4-TH on Tyr::CreER; BrafCa/+; Ptenlox/lox mice. Ten mice were used in each group. (A) Typical genotype of the Tyr::CreER; BrafCa/+; Ptenlox/lox transgenic mice. (B) Histology shows pagetoid proliferation of tumor cells in the epidermis and epithelioid/spindled tumor cells in the dermis after induction (H&E), bar indicates 100 μm. (C) Effect of NCTD on body weight of melanoma bearing mice. The transgenic mice were treated with NCTD, temozolomide or DMSO and all the mice were euthanized 40 d after tumor induction and weighted. * indicates p < 0.05. (D) Effect of NCTD on tumor weight. The transgenic mice were treated with NCTD, temozolomide or DMSO and all the mice were euthanized 40 d after tumor induction and the tumors were dissected and weighted. * indicates p < 0.05. (E) Effect of NCTD on mouse survival. The transgenic mice were treated with NCTD, temozolomide or DMSO and they were euthanized according to the standard score of body condition. Kaplan-Meier survival analysis showed that NCTD prolonged the lifespan of melanoma bearing mice better than temozolomide. (F) NCTD activates TR3 dependent apoptosis pathway in vivo. Melanoma tissues were harvested from treated mice and tissue lysates are subjected to immunoblotting with antibodies to TR3, Bax, Bcl-2, cleaved caspase-3 or NF-κB2. β-actin was used as a loading control. Representative blot from three repeats is shown.
In parallel with the previous experiment, we treated the transgenic mice with NCTD, temozolomide or vehicle until they had to be euthanized according to a standard body conditioning score. The Kaplan-Meier survival curve showed that the transgenic mice treated with NCTD had a significantly long life span compared with either the control or temozolomide treatment groups (Fig. 5E).
To determine whether there was a correlation between the expression of apoptotic proteins and the effects of NCTD in vivo, we examined the expression of apoptosis related protein expression in the melanoma tissue from treated transgenic mice. We found that both temozolomide and NCTD increased the expression of Bax, TR3 and cleaved caspases-3 in melanoma tissues. NCTD induced more Bax expression than temozolomide treatment. NCDB treatment also led to decreased expression of Bcl-2 and NF-κB2 (Fig. 5F). These results suggest that additional effects of NCTD seen in comparison to temozolomide may be attributed to its ability to induce more apoptotic protein expression in vivo.
Disscussion
In this study, we have demonstrated that NCTD suppresses melanoma proliferation in vitro and in vivo. The treatment efficacy of NCTD is superior to temozolomide in the models we tested. NCTD induces melanoma cell apoptosis in a dose-dependent manner. Mechanistically, NCTD induces translocation of TR3 from the nucleus to the mitochondria with increased release of cytochrome c from mitochondrial membrane into the cytoplasm. Knockdown of TR3 expression levels decreased the effect of NCTD on melanoma. Taken together, these results indicate that NCTD inhibits melanoma proliferation by activating the TR3 pathway.
It has previously been shown that NCTD inhibits the proliferation of certain cancer cells, such as HL60, K562, Bel-7402, MCF-7, Colo205, HT-29, SW480 and A375 melanoma cells.28 CTD and NCTD are known to inhibit protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A).35 This activity appears necessary for the growth inhibition activity of these compounds.22,36 A more recent mRNA expression study using the 60 NCI cell lines showed that many of genes whose expression changed in response to CTD are in one way or another involved in DNA damage response, DNA repair and/or apoptosis.37 Indeed, it has been shown that NCTD can interrupt DNA synthesis and upregulate cell surface expression of CD95 and CD95L on colon cancer cells.38 CTD or NCTD treatment led to mitochondrial dysfunction and activation of caspases involved in the intrinsic (mitochondrial) pathway of apoptosis.37,39 However, the specific mechanism responsible for activation of mitochondrial pathway was still unclear. To our knowledge, this is the first time that NCTD is shown to induce TR3 mitochondrial translocation. The significance of TR3 as an effector of NCTD treatment is underscored by experiments showing that NCTD efficacy can be modulated by TR3 expression levels in the cells.
NCTD not only induces apoptosis in vitro, it also inhibits melanoma growth in vivo as seen in an inducible melanoma transgenic mouse model. The effect of NCTD treatment is significantly better than temozolamide and results in significantly longer survival time of the melanoma bearing mice. Examination of the tumors excised from the NCTD and temolozide treated mice, revealed that the protein expression levels of Bax, TR3 and cleaved caspase-3 were increased in NCTD treated tumors as compared with temozolozide treated tumors. Interestingly, NCTD but not temozolozide treatment led to decreased expression of Bcl-2 and NF-κB2. These results are in accordance with the in vitro studies.
In summary, our present study demonstrates that TR3 is a critical regulator for melanoma cell apoptosis. NCTD can induce melanoma apoptosis and inhibit melanoma growth in vivo. NCTD exerted these effects in part due to activation of the TR3 dependent pro-apoptotic pathway. NCTD is a new potential candidate chemotherapeutic agent for treatment of melanoma patients.
Materials and Methods
Materials
We obtained temozolomide from TOCRIS Biosciences. Cisplatin was bought from Ben Venue Laboratories Inc. NCTD, CTD and other chemicals were purchased from Sigma. GFP-TR3, GFP-TR3 lacking of DNA binding domain (GFP-TR3/DBD), as well as a control vector (pEGFPN2) were kindly provided by Dr. Xiaokun Zhang at The Burnham Institute.32
Cell culture
The melanoma lines WM115A, WM35, Sbcl2 and 1205LU were cultured in a MCDB153/L15 medium (v/v: 4/1) supplemented with 2% FBS, insulin (5 mg/ml), 2 mM CaCl2 and 100 U/ml penicillin and 100 mg/ml streptomycin (2% tumor medium).40 Cells were cultured in a 5% CO2 incubator at 37 °C. Cells were switched to a serum-free medium 24 h prior to performing the experiments.
Transfection of melanoma cells
Melanoma cells were seeded at 2×105 per well in 6-well plates, incubated overnight and transfected with two micrograms of GFP-TR3, GFP-TR3/ DBD, or N2 vectors using Lipofectamine (Invitrogen) according to manufacturer’s instructions. For protein co-localization studies, melanoma cells were seeded on 8-well chamber slides and transfected with the above plasmids. After 16 h, cells were washed with phosphate-buffered saline (PBS) and treated with various reagents for 3 h.
Knock down of TR3 expression by RNA interference
The short hairpin RNA (shRNA) cassette for TR3 (5′-AGA CTA CAC AAA TCT GTG CAC TCC CCC AAG TCG GTG TTT CGT CCT TTC CAC AAG-3′) was synthesized and cloned into a pSilencer expression vector under the control of the human H1 promoter (Ambion) as previously described.32 A scrambled shRNA showing no homology to any known human gene was used as a negative control. The plasmids were transfected into melanoma cells using Lipofectamine 2000. Transfected melanoma cells were selected using 200 mg/mL G418 for 48 h.
Immunoflorescence analysis
Cells were fixed in 4% paraformaldehyde for 15 min, and permeabilized with 0.5% Triton X-100/phosphate buffered saline (PBS, pH 7.4) for 5 min. To detect mitochondria, a goat polyclonal Hsp60 antibody was used (1:200 dilution), followed by staining with donkey anti-goat Alexa Fluor 594 secondary antibody (Invitrogen, 1:500 dilution). Bcl-2 was detected using a mouse monoclonal anti-Bcl-2 IgG (1:200), followed by exposure to a donkey anti-mouse Alexa Fluor 594 conjugated secondary antibody (Invitrogen, 1:500) for 1 h at room temperature. Staining was observed by a Nikon E600 fluorescence microscope or Leica TCS SP2 confocal microscope.
Isolation of mitochondria
Mitochondria were isolated using the Mitochondria Isolation Kit for Cultured Cells (Pierce) according to the manufacture’s instruction. Briefly, 1×107 cells were seeded in 100 mm tissue culture plates, cultured in regular medium overnight and then transferred into serum-free media for 24 h. Following exposure to NCTD (6.5 µM) for the times indicated, cells were collected and processed for mitochondria isolation. Mitochondrial protein concentration was determined using a Bradford Protein Assay (Bio-Rad).
Isolation of nuclear fraction
Nuclear fraction was isolated following the manufacturer’s manual (Nuclear Extract Kit, Active Motif).
Protein gel blot
Primary antibodies specific for Bax, cleaved caspase-3, NF-κB2 p65 and β-actin were purchased from Cell Signaling Technology, Inc., antibodies to p53 primary antibody (DO-1), Bcl-2, TR3 and HSP-60 were obtained from Santa Cruz. Anti-cytochrome C antibody was from BD Biosciences. Protein concentration assay kit was obtained from Bio-Rad. Fifty milligrams of mitochondrial or nuclear proteins were separatedon a Nu-PAGETM (4–12% Bis-Tris Gel, Invitrogen) and subsequently transferred to a PVDF membrane (HybondTM-P, Amersham Biosciences). Proteins were detected using following antibodies: TR3 (M-210, 1:200) and cytochrome c (1:200). As a loading control, a goat polyclonal antibody to HSP60 (N-20, 1:500) was used. Bcl-2 (1:500), cleaved caspase-3 (1:500), NF-κB2 (1:250), Bax (1:500), and β-actin (1:2,000) were used. Membranes were incubated with the primary antibodies overnight at 4 °C, followed by incubation with HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Bio-Rad). Immunoreactive bands were visualized using chemiluminescence (ECLTM Western Blotting Detection System, Amersham Biosciences). Bands were scanned and intensities were quantified using a ChemiDoc XRS system (Bio-Rad).
MTT assay
The number of viable cells was assessed using a modified MTT assay (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega) according to the manufacturer’s instructions. Briefly, 1205Lu cells were seeded in 96-well plates at 5,000 cells per well in a total volume of 100 μL and then incubated for 24 h. Cells were switched to serum free media for 24 h and then exposed to CTD/NCTD (6.5 µM) for 24/36/48/60/72 h. 1205Lu, WM115A, Sbcl2 and WM35 cells were seeded in 96-well plates at 5,000 cells per well in a total volume of 100 μL and then incubated for 24 h. Cells were switched to serum free media for 24 h and then exposed to NCTD (0–100 µM) for 12/24 h.WM35, TR3 knock down, TR3 overexpressing or control 1205Lu cells were seeded in 96-well plates at 5,000 cells per well in a total volume of 100 μL and then incubated for 24 h. Cells were switched to serum free media for 24 h and then exposed to NCTD (0–100 µM) for 24 h. For color development, 20 μL of the dye solution was added to each well and plates were incubated at 37 °C for 4 h. At the end of the incubation period with dye, 100 ml of solubilization/stop solution were added to each well. Plates were kept at 4 °C overnight and absorbance at 570 nm wavelength was recorded using a microplate reader (Bio-TEK, Winooski, Vermont). Each experimental condition was performed in triplicate.
Evaluation of apoptosis
Apoptosis was assessed using an Annexin V assay (Invitrogen). The positive control was induced by incubation with 5% ethanol for 60 min.31 Cells were grown in 6-well plates, treated with NCTD at the concentration of 1, 10 and 100 µM as described above and assayed in triplicate. After 24 h of exposures, both attached and floating cells were harvested, washed in cold PBS, and resuspended at a concentration of 1 × 105 cells/mL in Annexin-binding buffer containing 10% (v/v) annexin V conjugate and 0.2% trypan blue solution (Sigma). After 15 min at room temperature, the cells were washed once with annexin-binding buffer and analyzed using a FACSCalibur flow cytometer. Post-analysis was performed using FloJo™ software.
Mouse breeding and treatment
The Tyr::CreER; BrafCA/+;Ptenlox/lox transgenic mice were originally generated by Drs. Marcus Bosenberg (Yale) and Martin McMahon (UCSF). Transgenic mice were handled according to the criteria specified by the NIH. The protocol has been approved by IACUC at the University of Pennsylvania. Genotyping was performed as they described.34 Topical administration of 4-hydroxytamoxifen (4-HT) was performed by preparing a 65–130 mM solution of 4-HT (70% Z-isomer, Sigma) in DMSO. Adult (6–8 weeks of age) mice were treated topically with a small paint brush on their right flank, ear and tail containing 2 μl of 5mM 4-HT to wet right flank with a small paint brush for 3 d. NCTD was dissolved in DMSO and injected intraperitoneally at a dose of 2 mg/kg, three times each week, for 3 weeks starting 2 d after application of 4-HT.41 Temozolomide was also dissolved in DMSO and administered i.p. at 100 mg/kg, one time each week for 3 weeks.42 Control animals in the melanoma prevention studies were administered with the relevant solvent. Tissues were prepared for analysis as previously described.43,44
We used 60 mice for the experiment; these 60 mice were separated to two groups. In one group, we used ten for control (treated with DMSO), ten for temozolomide treatment group and another ten for NCTD treatment group; we sacrificed all these mice 40 d after 4-HT tumor induction. We dissected tissue for histology to confirm melanoma formation. We measured the weight of the induced tumors and the body weights of mice to evaluate for cachexia. As for another group, we used ten for control (treated with DMSO), ten for temozolomide treatment group and another ten for NCTD treatment group; the mice were euthanized until they reach the standard body condition score. Freshly isolated melanoma tissue was used for analyzing apoptotic protein expression.
Statistical Analysis
Student’s t-test or one way ANOVA was used to analyze gene expression and cell viability. Statistical significance was determined if two-sided p < 0.05.
Acknowledgments
We thank Drs. Xiaokun Zhang at The Burnham Institute, La Jolla, CA for GFP-TR3, GFP-TR3/DBD, and a control vectors; Meenhard Herlyn at the Wistar Institute, Philadelphia, PA for the melanoma cell lines, and Shanshan Feng for preparation of this manuscript. This work was supported by the grants CA-116103, CA-093372 and AR-054593 from National Institute of Health to X.X.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/18380
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, et al. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 3.Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50:215–36, quiz 37-40. doi: 10.3322/canjclin.50.4.215. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–36. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solit DB, Rosen N. Resistance to BRAF inhibition in melanomas. N Engl J Med. 2011;364:772–4. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 10.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–61. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–54. doi: 10.1016/S0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 13.Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–81. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–42. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 15.Liang B, Song X, Liu G, Li R, Xie J, Xiao L, et al. Involvement of TR3/Nur77 translocation to the endoplasmic reticulum in ER stress-induced apoptosis. Exp Cell Res. 2007;313:2833–44. doi: 10.1016/j.yexcr.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–43. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 17.Chen HZ, Zhao BX, Zhao WX, Li L, Zhang B, Wu Q. Akt phosphorylates the TR3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis. 2008;29:2078–88. doi: 10.1093/carcin/bgn197. [DOI] [PubMed] [Google Scholar]
- 18.Zhan YY, Wu Q. [Translocation of orphan receptor TR3 from nuclei to mitochondria induced by staurosporine] Ai Zheng. 2004;23:1593–8. [PubMed] [Google Scholar]
- 19.Yu H, Kumar SM, Fang D, Acs G, Xu X. Nuclear orphan receptor TR3/Nur77 mediates melanoma cell apoptosis. Cancer Biol Ther. 2007;6:405–12. doi: 10.4161/cbt.6.3.3755. [DOI] [PubMed] [Google Scholar]
- 20.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 21.Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta. 2006;1757:1301–11. doi: 10.1016/j.bbabio.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Liao HF, Chen YJ, Chou CH, Wang FW, Kuo CD. Norcantharidin induces cell cycle arrest and inhibits progression of human leukemic Jurkat T cells through mitogen-activated protein kinase-mediated regulation of interleukin-2 production. Toxicol In Vitro. 2011;25:206–12. doi: 10.1016/j.tiv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Moed L, Shwayder TA, Chang MW. Cantharidin revisited: a blistering defense of an ancient medicine. Arch Dermatol. 2001;137:1357–60. doi: 10.1001/archderm.137.10.1357. [DOI] [PubMed] [Google Scholar]
- 24.Oaks WW, Ditunno JF, Magnani T, Levy HA, Mills LC. Cantharidin poisoning. Arch Intern Med. 1960;105:574–82. doi: 10.1001/archinte.1960.00270160072009. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59:201–8. doi: 10.1007/s10616-009-9210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, et al. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol. 2003;39:19–26. doi: 10.1016/S1368-8375(01)00129-4. [DOI] [PubMed] [Google Scholar]
- 27.Sun ZX, Ma QW, Zhao TD, Wei YL, Wang GS, Li JS. Apoptosis induced by norcantharidin in human tumor cells. World J Gastroenterol. 2000;6:263–5. doi: 10.3748/wjg.v6.i2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An WW, Wang MW, Tashiro S, Onodera S, Ikejima T. Norcantharidin induces human melanoma A375-S2 cell apoptosis through mitochondrial and caspase pathways. J Korean Med Sci. 2004;19:560–6. doi: 10.3346/jkms.2004.19.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK, Kuo YS, et al. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett. 2005;217:43–52. doi: 10.1016/j.canlet.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 30.Chen YN, Cheng CC, Chen JC, Tsauer W, Hsu SL. Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells. Br J Pharmacol. 2003;140:461–70. doi: 10.1038/sj.bjp.0705461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Kis A, Zettl A, Bertozzi CR. A cell nanoinjector based on carbon nanotubes. Proc Natl Acad Sci USA. 2007;104:8218–22. doi: 10.1073/pnas.0700567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–64. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 33.Wilson AJ, Arango D, Mariadason JM, Heerdt BG, Augenlicht LH. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–7. [PubMed] [Google Scholar]
- 34.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fathi AR, Krautheim A, Lucke S, Becker K, Juergen Steinfelder H. Nonradioactive technique to measure protein phosphatase 2A-like activity and its inhibition by drugs in cell extracts. Anal Biochem. 2002;310:208–14. doi: 10.1016/S0003-2697(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 36.McCluskey A, Ackland SP, Bowyer MC, Baldwin ML, Garner J, Walkom CC, et al. Cantharidin analogues: synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg Chem. 2003;31:68–79. doi: 10.1016/S0045-2068(02)00524-2. [DOI] [PubMed] [Google Scholar]
- 37.Rauh R, Kahl S, Boechzelt H, Bauer R, Kaina B, Efferth T. Molecular biology of cantharidin in cancer cells. Chin Med. 2007;2:8. doi: 10.1186/1749-8546-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, et al. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002;128:223–30. doi: 10.1007/s00432-002-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efferth T, Rauh R, Kahl S, Tomicic M, Bochzelt H, Tome ME, et al. Molecular modes of action of cantharidin in tumor cells. Biochem Pharmacol. 2005;69:811–8. doi: 10.1016/j.bcp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Kumar SM, Yu H, Edwards R, Chen L, Kazianis S, Brafford P, et al. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res. 2007;67:3177–84. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- 41.Chen YJ, Shieh CJ, Tsai TH, Kuo CD, Ho LT, Liu TY, et al. Inhibitory effect of norcantharidin, a derivative compound from blister beetles, on tumor invasion and metastasis in CT26 colorectal adenocarcinoma cells. Anticancer Drugs. 2005;16:293–9. doi: 10.1097/00001813-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 42.McConville P, Hambardzumyan D, Moody JB, Leopold WR, Kreger AR, Woolliscroft MJ, et al. Magnetic resonance imaging determination of tumor grade and early response to temozolomide in a genetically engineered mouse model of glioma. Clin Cancer Res. 2007;13:2897–904. doi: 10.1158/1078-0432.CCR-06-3058. [DOI] [PubMed] [Google Scholar]
- 43.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–84. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, et al. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44:262–7. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]